Abstract
Aims: The aims of this review are to identify and evaluate studies exploring the cost-effectiveness of primary angioplasty (PPCI) vs. thrombolysis (TL) for treating acute myocardial infarction (AMI).
Methods and results: A comprehensive free-text searching identified economic evaluation studies that were reviewed with respect to their effectiveness data, identification, measurement and valuation of resource data, measurement and valuation of health outcomes (clinical and QALYs) and uncertainty analysis. A total of 14 studies were included in the review: seven were economic evaluations alongside RCTs, two community-based studies or registries and five decision-analytical models. PPCI was found to be cost-effective when compared with TL in eight studies, cost-saving in three, cost-neutral in one, and not significantly different in terms of both cost and benefits in two studies.
Conclusions: The cost-effective evidence available is mainly derived from RCTs with stringent inclusion criteria using established catheter laboratories for providing PPCI treatment; these two components might restrict the generalisability of their “for managing patients with STEMI in hospital” settings. In order to aid policy makers on the real costs and benefits of the PPCI and TL, it is necessary to conduct more analyses with data from the real world in which there are more strategies evaluated for delivering PPCI than merely those in established catheter laboratories.
Abbreviations
AMI: acute myocardial infarction
CVD: cardiovascular diseases
Non-STEMI: non-ST-elevation myocardial infarction
PPCI: primary angioplasty
QALYs: quality adjusted life years
RCT: randomised controlled trial
STEMI: ST-elevation myocardial infarction
TL: thrombolysis
Introduction
Cardiovascular diseases (CVD) are the world’s largest killer, claiming 17.3 million lives in 2008, with forecasts estimating 19.4 million deaths in 2015, and likely to reach the exorbitant figure of 23.6 million in 20301. In 2006, overall, CVD were estimated to cost European countries up to 192 billion euros a year, of which 57% were directly linked to health care, 21% to productivity losses, and 22% to informal care costs. More recently, the financial per capita burden of CVD in Europe was estimated to be 391 euros in 20062. This figure is considerably higher for the UK with an estimated overall expenditure of 30.7 million pounds sterling while maintaining a similar proportion with respect to key cost components, i.e., 47%, 27% and 26% for health care, productivity losses and informal care costs, respectively, which translates to a per capita cost of 508 pounds sterling3. The US projections for 2010 to 2030 are daunting, with over 40% of the population having some form of CVD by 2030, tripling the medical costs from 273 billion US dollars to 818 billion US dollars and doubling productivity losses from 172 billion US dollars to 276 billion US dollars (US dollars 2008)4.
Among CVD, the leading cause of death in high-income countries is ischaemic heart disease, accounting for 15.6% of deaths in 20085. Acute myocardial infarction (AMI) is a common presentation of ischaemic heart disease. This is further classified into ST-segment elevation myocardial infarction (STEMI), the more severe type, and non-ST-elevation MI (non-STEMI), with primary angioplasty (PPCI) and thrombolysis (TL) used as alternative treatment options. A considerable amount of information from randomised controlled trials (RCTs) and meta-analyses is currently available that has examined the effectiveness of these two treatments, including their cardiovascular outcomes and long-term outputs6-8. Nevertheless, in an area of growing needs and scarce health care resources, decisions concerning the choice of treatment should be the result of analysing the clinical health outcomes in combination with the impact on resource utilisation. Economic evaluation studies, if rigorously conducted, provide the necessary evidence for aiding policy makers to take informed decisions. The objective of this paper is to complement the clinical evidence available by reviewing and assessing the economic evaluation studies that have evaluated PPCI and TL for AMI. Particular attention was paid to incorporate also those studies in which health benefits were combined as a composite measure in terms of quality and quantity of life, i.e., quality adjusted life years (QALYs).
Methods
A comprehensive free-text searching was used in Ovid Medline (1980 to week 4, Dec 2011); EMBASE (1966 to 2011) and NHS EED (1997 to 2011) in order to identify economic evaluation studies that had used PCI vs. TL for the treatment of AMI. The free-text searches included: myocardial infarction, STEMI, angioplast, primary angioplasty, PCI or pci or percutaneous coronary intervention, thrombolysis, economics, health economics, costs, quality adjusted life years, quality of life, wellbeing or well-being, hrqol or qol or euroqol or health utility. These searches were complemented with hand searches for additional references from those retrieved articles. The review considered only economic evaluation articles, including decision-analytical models, written in English, Italian or Spanish that evaluated PCI and TL. Editorial, letters, comments and non-economic evaluation studies were excluded from the review.
Study details were extracted regarding the year of publication, country in which the study was conducted, type of study, diagnosis, number of patients included, treatment option and thrombolytic agent (Table 1). For the economic models only the year, country, diagnosis and type of thrombolytic agent were extracted (Table 2). In addition, the economic evaluation checklist9 (data extraction not presented in this article) was used to describe and assess the quality of the studies in terms of methods for deriving effectiveness data, identification, measurement and valuation of resource data, measurement and valuation of health outcomes (clinical and QALYs) and uncertainty analysis. All references were exported to the Reference Manager database, Version 11/12, © 2009, The Thomson Corporation.
Results
The bibliographic electronic searches provided 268 hits, of which 107 were duplicates. From the 161 remaining, 147 were rejected and only 14 articles were retrieved for full review. A total of seven articles were economic evaluations alongside RCTs, two community-based studies or registries, and five were decision-analytical models. The majority of studies (5/9) were performed in Europe and focused on STEMI, while the four remaining studies were performed in the US (3/9) and Canada (1/9) focusing on STEMI (2/9) and on AMI (2/9). Concerning the models, the majority considered European countries’ settings (4/5), half of which focused on AMI and half on STEMI; the only model performed in the US focused on AMI (further details are provided in Table 1 and Table 2).
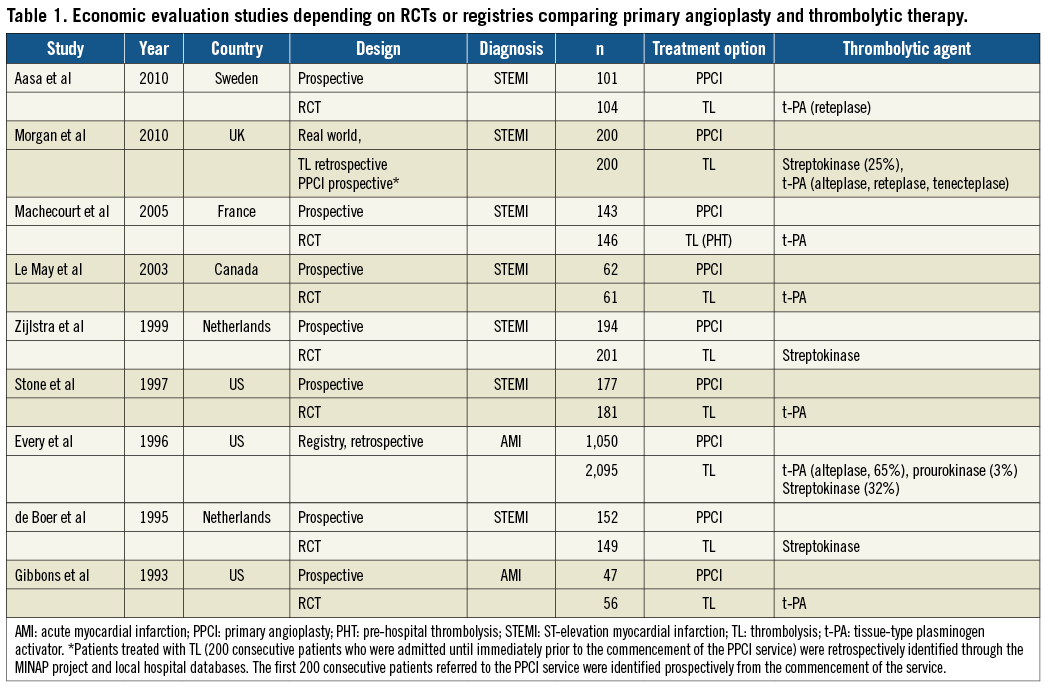
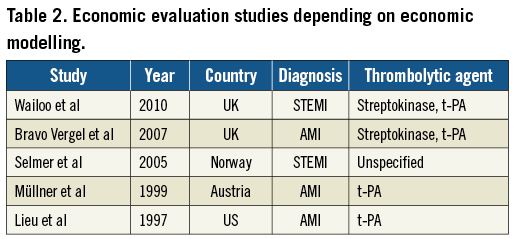
Most of the studies reported an established catheter laboratory as an important factor for delivering an effective PPCI treatment10-20. For those studies that specified the administered thrombolytic agents10,11,13-20,21, tissue-type plasminogen activator (t-PA) was reported as the main thrombolytic agent, with the exception of two 1990s Dutch studies15,18 in which patients received streptokinase, a first-generation thrombolytic agent. However, it is important to mention that in the two community-based studies11,19 the choice of thrombolytic agent reflected the hospital policy at the time of the study (streptokinase and t-PA [alteplase, reteplase, tenecteplase, prourokinase]). The UK models used as thrombolytic agent streptokinase and t-PA12,22, while the modelling study in Norway23 opted for an unspecified thrombolytic agent.
EFFECTIVENESS
Those studies that reported clinical outcomes as their measure of effectiveness selected major adverse cardiovascular events (e.g., death, non-fatal reinfarction, non-fatal stroke, recurrent ischaemia rates and revascularisation procedures [i.e., balloon, stent, intra-aortic balloon pump]) as their primary outcome measures11,13-15,17-19,21,23. In the Norwegian study23 the health outcome measure used was life expectancy after the first STEMI, while the primary endpoint of the oldest study20 was the change in the size of perfusion defect as assessed at admission and discharge.
PPCI resulted in better clinical outcomes when compared to TL11,13,15,17,18, specifically lower incidence of revascularisation procedures (32% vs. 56%, p<0.001) in the first year. The same holds if we consider the incidence of recurrent infarction and death or stroke (8% vs. 29%, p<0.001)18. PPCI also reduced rates of in-hospital mortality (2.3% vs. 7.2%, p=0.03), reinfarction (2.8% vs. 7.2%, p=0.06), recurrent ischaemia (11.3% vs. 28.7%, p<0.0001), stroke (0% vs. 3.9%, p=0.02) and non-fatal reinfarction (6% and 22%) when compared to TL17. Conversely, no significant difference in health outcomes between PPCI and TL was found in older studies with the exception of Aasa and colleagues10,14,19,20.
QALYs were estimated in four of the 14 articles retrieved: of these three were decision models12,16,22 and only one was a prospective RCT conducted in Sweden10. The Swedish study obtained health states from patients using the EQ-5D24 and used the UK EQ-5D utilities tariff25 in order to estimate QALYs. Utilities differed in definition and values. Aasa and colleagues estimated the mean quality-adjusted survival to be 0.759 and 0.728 for PCI and TL respectively, but the difference between groups was not found statistically significant10. Wailoo11 used the utilities obtained by Bravo Vergel22 whose health states utility values for angioplasty were obtained from a literature review for long-term health states but with a single utility score for stroke weighted by the probability that the event led to disability or not. The utility values derived were myocardial infarction (MI) state (year 1) (0.683), after MI state (≥2 years) (0.718), non-disabled stroke (0.740), disabled stroke (0.380), and combined stroke (0.612)22. For both studies PPCI gained more QALYs than TL. Lastly, the study by Lieu and co-authors16 used a modified Delphi panel using published26, unpublished data and conference abstracts for deriving utility values that corresponded to the Canadian Cardiovascular Society classes (Class I and II 0.970; Class III and IV 0.87527 and non-fatal disabling stroke 0.1028) to show that PPCI produced more QALYs than TL.
RESOURCE UTILISATION
The public health care providers’ perspective was adopted in four studies10,16,22,23. Two studies stated using the societal perspective10,14, only one used the viewpoint of the health insurance21, while in six studies the viewpoint was unclear13,15,17-20. All the studies included hospitalisation costs10 as the main cost component21. However, studies differ in the comprehensiveness of the resources identified and included for estimating hospitalisation costs, for example whether the studies included revascularisation11,14, type of ward for hospitalisation, i.e., standard care, coronary or postoperative wards10,15,16, staff costs, subdivided by type of healthcare providers, i.e., medical staff, nurse, technician11 or overall13. The studies also varied with respect to the type of laboratory tests, interventions and drugs included11, transportation costs16,23 or if cost of meals13 was also considered. Only two articles included outpatient costs10,15. Only one study10 that stated it was using the societal perspective estimated productivity losses through the number of days of sick leave (from a self-reported patient questionnaire) while Gibbons20 stated that indirect costs were also composed by those patients returning to work (proportion of patients returning to employment who had been employed before their myocardial infarction) but provided no cost estimate disaggregate figure for patients returning to work.
The valuation of resource consumption also differed among studies with charges used in the American studies16,17,19,20, national reference costs in the UK ones11,12,22, DRG prices in Norway23 , annual running ward costs in Sweden10, ancillary micro-costing in France14, province budget records in Canada13, hospital administration data in the Netherlands15,18, and lastly from pooled data between the Austrian Medical Association and insurance companies21. For those articles that provided disaggregated total costs by PPCI or TL10,13,14,18, length of hospital stay was the main cost driver for those receiving TL (33%10 to 49%14). Nevertheless, productivity losses were also a cost driver in the article by Aasa and colleagues accounting for 38% and 36% of the total cost in the PPCI and TL arms, respectively10. In terms of total cost of PPCI and TL the evidence is mixed, i.e., no statistical difference between the two treatment options11,13,18,20, TL having statistically significant lower total costs than PPCI11,19,22,23, and PPCI being statistically significantly cheaper than TL10,14,15,17.
COST-EFFECTIVENESS
PPCI was found to be cost-effective when compared to TL in eight studies10,12,15,16,18,21-23. Aasa provided a more detailed analysis by using the joint distribution of incremental costs and quality-adjusted survival using a nonparametric bootstrap approach, concluding that PPCI was cost-effective in 67% of the cases10. The analysis by Wailoo and colleagues reported a 90% probability of PPCI being cost-effective with a lower threshold (i.e., 20,000 pounds sterling per QALY gained) than the one assumed by Aasa. The results by Stone and colleagues17 showed that although PPCI resulted in lower hospital costs, these were offset by higher staff costs (i.e., physician fees) after receiving PPCI; however, the authors concluded that PPCI is more cost-effective for non-high-risk patients (<70 years old and no previous MI). Other studies showed that PPCI was cost-saving in three studies13,14,19, cost-neutral11, or that there was no statistical difference between PPCI and TL20.
SENSITIVITY ANALYSIS
The robustness of PPCI as the cost-effective intervention was tested and confirmed in eight studies. The parameters that were varied in the sensitivity analyses were: the specification of the distribution of the data8; the probabilities used for bootstrapping (80%, 88% and 89%); the thresholds (0, 50,000 US dollars and 100,000 US dollars)10; higher rates of revascularisations and increasing the time for treatment delay associated with PCI12, excluding patients who experienced coronary artery bypass graft, major bleed and stroke13. Also the effectiveness of PPCI was varied, the type of hospitals that offered the services, the volume of services and the time to treatment16; increasing the time delay for receiving treatment (30, 60 and 90 minutes)22, using the same long-term risk of developing symptomatic coronary diseases for PPCI and TL; performing the analysis in younger individuals or using worse health outcomes for PPCI23, and lastly by varying additional costs and effectiveness associated with PPCI21. This last study was sensitive with respect to changes of additional costs.
Discussion
The economic evidence derived from the randomised controlled trials10,13-15,17,18,20, due to their stringent inclusion criteria, might restrict the generalisability of their findings for managing patients with STEMI in hospital settings. The only recent study11 that used real-world data from UK hospitals demonstrated that primary angioplasty when compared to thrombolytic therapy was associated with a dramatic reduction of major adverse cardiovascular events; however, no statistically significant difference was found in costs.
Another strong limitation for the generalisability of results is that all the studies assumed an established catheter laboratory offering PPCI treatment which ignores the fact that in some hospitals a considerable monetary investment in facilities and human capital would be necessary in order to provide this service. This point is also supported by the conclusions of Aasa10, Le May13 and de Boer18, in which the authors stated that their results were obtained from already equipped hospitals with catheterisation laboratories operating 24 hours a day, seven days a week, with trained operators. This limitation opens up two questions: (1) will investing in catheterisation laboratories for providing PPCI be cost-effective; and (2) which will be the most cost-effective strategy, i.e., implementing a network based on the hub-and-spoke model or building new facilities and training staff? In order to provide more informed evidence for aiding policy makers to decide on providing PPCI services it is necessary to conduct observational studies that will take into consideration these questions and that also include out-of-reach localities. In addition, assessing the organisational impact of implementing a new intervention such as PPCI will be essential to guarantee a quality-assured and sustainable service provision29.
The study by Concannon30 elucidates the previous point since the authors evaluated the comparative effectiveness of STEMI at regional level. Their base case strategy (i.e., standard resources, transport provision, no new investment in building or staffing) was compared with different scenarios in which hospital PCI capacity was expanded and with one scenario in which emergency service was used to transport all STEMI suspect patients to existing operating PCI treatment hospitals. Their results suggested that the emergency service strategy was more effective and less costly than hospital-based strategies, including those with new buildings and increased staffing.
An additional caveat of current economic evaluation studies is the exclusion of productivity losses and carer’s costs. Selmer and colleagues23 justified the omission of productivity losses in their study since the age of their sample reflected that individuals were in retirement or near to the age of retirement. This might also be the implicit justification by other studies11-15,17,19,20. However, given the increase of retirement ages in Europe, productivity losses may increase in importance and should be considered and measured as well as carer’s costs.
Most of the cost-effectiveness studies reviewed here have ignored in their conclusions untreated STEMI patients10,11,13-15,17-23. Lieu and colleagues16 were the only ones who considered in their analysis patients not receiving interventions since they were attending hospitals without a catheter laboratory; however, the analysis of cost and health outcomes was limited to the PPCI and the TL treatments. While Wailoo12 and co-authors acknowledged untreated patients, the evidence in terms of health outcomes and costs for these patients was unavailable to include in their analysis. However, a study31 using data from the Euro Heart Survey on Acute Coronary Syndromes II demonstrated that no reperfusion was given to 39% of European STEMI patients while the proportion of untreated patients in the US was 30%, despite the fact that reperfusion therapies were available and there were no contraindications32. In the future these figures need to decline and economic evaluation studies have to consider the health outcomes and resource consumption of these patients.
None of the cost-effectiveness analyses have taken account of the costs for implementing a network for treating AMI. It would be advisable that future research fills this gap since implementing these networks might be a prerequisite to improving patient’s access to treatment and to guaranteeing a timely reperfusion strategy according to international guidelines, thereby reducing mortality and morbidity and keeping within cost constraints33.
Funding
This study was funded by the Italian Society of Invasive Cardiology (SICI-GISE).
Conflict of interest statement
The authors have no conflicts of interest to declare.

