Abstract
Aims: The aim of this study is to use real-world data from West London to compare the cost-effectiveness of a contemporary primary angioplasty (PPCI) service to thrombolysis which it superseded over a time horizon of one year. Previous studies have depended on randomised trials and economic modelling.
Methods and results: Resource and outcome data were collected on 400 consecutive patients treated for ST segment elevation myocardial infarction (STEMI) at the hub and two spoke sites over three years. After the first 200 received thrombolysis, the PPCI service was introduced providing treatment for the next 200 cases. The incidence of major adverse cardiac events was significantly less in the PPCI group at 30 days (46.2% versus 7.0%, adjusted odds ratio (AOR) 12 p<0.001) and one year (57.4% versus 13.2%, AOR 8.6 p<0.001) driven by reductions in mortality and ischaemia driven revascularisations. Mean index and one year cumulative costs did not differ significantly between thrombolysis and PPCI (£7,016 versus £6,802; p=0.653 and £8442 versus £7,731; p=0.213 respectively). Initial angioplasty costs were significantly higher in the PPCI group offset by reduced hospital stay (8.5 versus 4 days; p<0.001).
Conclusions: This model of PPCI delivery is associated with larger than expected benefits and is cost-neutral when compared to thrombolysis.
Introduction
Primary angioplasty (PPCI) has been shown to be superior to thrombolysis (TL) for the treatment of ST segment elevation myocardial infarction (STEMI) in randomised controlled trials. This has led to the development of comprehensive PPCI services replacing TL throughout the United Kingdom and elsewhere.1
The applicability of the results of randomised trials comparing the two reperfusion strategies to real world PPCI services has been questioned.2 It cannot be assumed that the results of trials involving highly selective patient populations translate to contemporary clinical practice. Health economic analyses utilise trial data to calculate the cost effectiveness of these reperfusion strategies leading to similar concerns as costs associated with excluded patients may be disproportionately weighted.
The aim of this study is for the first time to use a novel approach based on actual costs to determine the cost effectiveness of a contemporary PPCI service compared to TL.
The data was collected from the West London service which replaced TL as the standard reperfusion treatment for STEMI in 2003 and is based on the European Hub and Spoke model. By providing only one service at a time and including consecutive patients the design of this study arguably provides a more complete dataset for cost analysis.
Methods
The National Research Ethics advised that the study was an evaluation of service delivery and therefore did not require formal ethical review. Informed consent was obtained from all patients contacted.
The sample size was calculated by undertaking a pilot study of the first 10 patients in each group. To achieve 84.5% power to detect a difference between the two groups at a significance level (alpha) of 0.05 using a two-sided Mann-Whitney test at least 150 patients in each treatment arm were required.
Patients were recruited from one hub site and two spoke sites: Hammersmith Hospital, Charing Cross Hospital and West Middlesex University Hospital. The Myocardial Infarction National Audit Project (MINAP) and local hospital databases were used to retrospectively identify 200 consecutive patients who presented with suspected STEMI and received TL from 2002 until immediately prior to the commencement of the PPCI service in October 2003. No patients in the thrombolytic arm received pre-hospital TL.
The first 200 consecutive patients referred to the PPCI service were identified prospectively from the commencement of the service in October 2003 until February 2005. No patients who were accepted for referral were excluded from the study.
Health economic data was gathered on the index admission and any subsequent admissions due to a cardiac cause over the subsequent year. This information was obtained from hospital records, computer database and telephone follow-up with the patient. Detailed data regarding baseline demographics, pre-hospital treatment, admission clinical findings, length of stay, cardiac interventions and thrombolytic and Glycoprotein 2b/3a receptor inhibitor (GP2b3a) use was recorded.
The cost analysis was performed from a health care provider perspective with a time horizon of one year. Costs reported were limited to the medical costs of patient care incurred by the institutions delivering the care. All costs are expressed in United Kingdom pounds sterling (£) and the price year is 2005. Table 1 provides a breakdown of the unit costs.
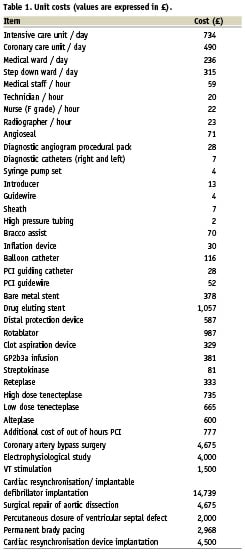
A Hospital Trust accountant was consulted to determine the hospital stay costs. This included nursing and equipment costs and was stratified according to the type of ward setting (normal ward, coronary care [CCU], step down or intensive care [ICU]). The cost of individual percutaneous coronary intervention (PCI) procedures was calculated based on the number and type of personnel involved, duration of procedure and equipment used. An out of hours surcharge was added as appropriate. Overheads charges were added to reflect the cost of facilities used in providing the service as advised by a health economist.
Telephone follow-up was undertaken at least one year following the index event. Details of any further healthcare resources were obtained from the relevant hospital’s notes and database. In addition the patient’s case notes were reviewed at the relevant hub and spoke site.
The major adverse cardiac events (MACE) of interest were myocardial infarction (MI), cerebrovascular accident (CVA), ischaemia driven revascularisation (IDR) and death. MI was defined as a history consistent with acute ischaemia and rise in troponin or creatine kinase (CK) above the reference limit for the biochemistry laboratory. CVA was defined as a persistent focal neurological deficit present for greater than 24 hours with associated new lesion on brain imaging. IDR was defined as either a PCI or coronary artery bypass grafting (CABG) operation precipitated by an unplanned hospital admission due to a cardiac cause, including after failed PCI or TL, with ongoing symptoms, electrocardiographic (ECG) changes or a positive myocardial stress test. Non-IDR was defined as planned or staged PCI or planned CABG following angiography ± PCI with no ongoing ischaemic symptoms or ECG changes or unplanned cardiac admissions.
Statistical analysis
The chi squared test was used to compare categorical variables and Fishers exact test was used when the numbers were small. Continuous data were tested for normality using the Kolmogorov-Smirnov test.
Costs were not normally distributed and comparisons of unadjusted costs were made using the non-parametric Mann-Whitney U test. Outcome and cost data were corrected to account for differences between groups.
Index hospital stay and total costs up to one year were log transformed to produce a normal distribution allowing costs to be modelled using analysis of covariance (ANCOVA) after removing any outliers. Univariate analysis was used to identify factors predicting costs allowing development of a model using ANCOVA to predict costs. Only index admission and cumulative costs to one year were modelled as individual costs remained skewed even after log transformation.
Univariate analysis was also undertaken to identify variables which were significantly associated with outcomes. The outcomes of interest were death at 30 days and one year and the combined endpoint of death, CVA, MI and IDR at 30 days and one year. Univariate analysis was performed for variables in each group to avoid confounding. A stepwise logistic regression model was then created to predict the binary outcomes of death and MACE at 30 days and one year and presented as an odds ratio (OR).
A p value of less than 0.05 was considered to be significant.
Results
Follow up was 98.5% at 30 days and 96.25% at one year. Both groups were well matched as illustrated in Table 2. Significant covariates were incorporated into models to adjust costs and outcome. The delay to presentation did not differ significantly between groups and was therefore not used in any more of the analyses. Killip class did differ significantly but only Killip class 3 and 4 were strongly predictive for outcome. Individual Killip Classes and various combinations of Killip grouping were analysed but the numbers were too small to be incorporated into the outcome model.
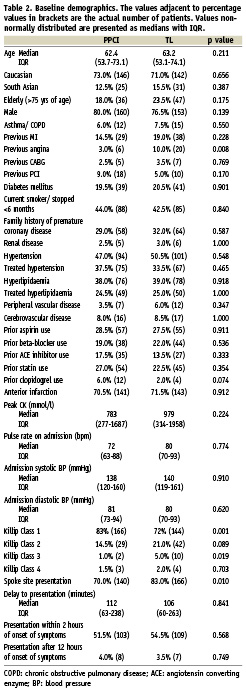
There was a significantly lower incidence of prior history of angina in the PPCI group. This factor only predicted outcome in the TL group and was incorporated in the outcome model for this group. Spoke site presentation did not predict outcome and was not incorporated in the outcome model.
In the TL group the median door to needle time was 22 minutes. The mean time from referral for PPCI to balloon inflation was 88 minutes. Median ischaemic times were 244 minutes in the PPCI group and 142 minutes in the TL group. There was no significant difference in the delay to presentation between groups.
A second or third generation thrombolytic was used in 73.5% of the TL group. During the index admission 57.5% of the patients in the TL group went on to have invasive investigation. Due to failure of TL, 13.5% required rescue angioplasty. A further 9% underwent urgent cardiac catheterisation while 35% underwent non-urgent in-patient cardiac catheterisation. The median time from admission to urgent catheter was two days (inter-quartile range (IQR) 1-8 days) and from admission to non-urgent in patient catheter 16 (IQR 6.75-26) days. Patients who had evidence of ongoing ischaemia as evidenced by ongoing symptoms, ECG changes or stress testing underwent invasive investigation. Invasive investigation was also performed on patients with no demonstrable ischaemia at the discretion of the referring physician and influenced by age and comorbidity. The costs incurred from non-invasive ischaemia tests were not included in the analysis as preliminary analysis found the sums involved too small to be influential.
Of the patients in the PPCI group, 81% went on to have PPCI, 2% had emergency CABG, 5.5% underwent coronary angiography and were found to have significant coronary disease not requiring intervention, 0.5% were refused on medical grounds and 11% had no evidence of obstructive coronary disease on angiography.
Of the patients undergoing PPCI, 93% underwent coronary artery stenting with 25% receiving one or more drug eluting stent. 79% received GP2b3a. All patients received Clopidogrel.
In the TL group, all patients referred for either rescue or urgent PCI, and 96% of patients undergoing a non-urgent PCI had at least one coronary stent deployed. Fourteen per cent (14%) of patients in the TL group undergoing index admission coronary intervention had at least one drug eluting stent deployed. Adjuvant GP2b3a use was 89% for rescue PCI, 78% for urgent PCI and 47% for non-urgent index admission PCI. Clopidogrel was used in all procedures.
There was a large and significant reduction in the incidence of MACE at 30 days (46.2% vs. 7.0%, adjusted OR 12; p<0.001) sustained at one year (57.4% vs. 13.2%, adjusted OR 6.6; p<0.001) in favour of PPCI (Figure 1). This was driven by a reduction in IDR and mortality (Tables 3 and 4). Mortality rates were significantly reduced at 30 days (13.1% versus 3.6%, adjusted OR 3.8; p=0.017) and one year (19.5% versus 5.8%, adjusted OR 3.5; p=0.003) in favour of PPCI (Figure 2). The adjusted OR are summarised in Figure 3. When the additional endpoints of cardiac transplant, aortic dissection repair, ventricular septal defect (VSD) closure and device implantation are included in the definition of MACE at one year the superiority of PPCI persists (60.7% vs. 20.8% p<0.001).

Figure 1. Thirty day and one year MACE rates by reperfusion strategy.
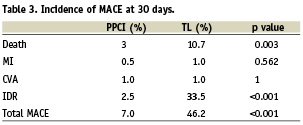
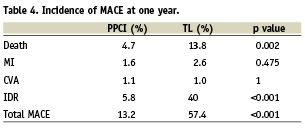
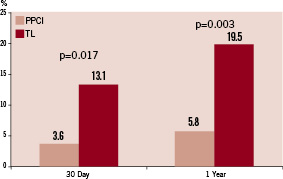
Figure 2. Thirty day and one year death rates by reperfusion strategy.
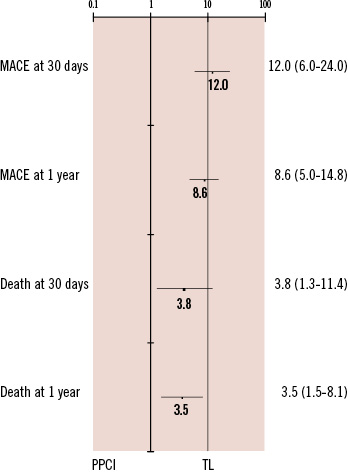
Figure 3. Adjusted odds ratios of outcomes with 95% confidence intervals.
There was no significant difference in the mean adjusted costs of the index admission and cumulative costs accrued at one year between groups (Table 5).

As PPCI was associated with significant improvements in outcome with no additional costs there was no requirement for further economic analysis.
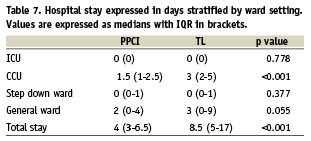
The significantly higher costs associated with PCI in the PPCI group were more than offset by a reduction in index hospital stay (Table 6) equating to a median reduction of 4.5 days (Table 7).
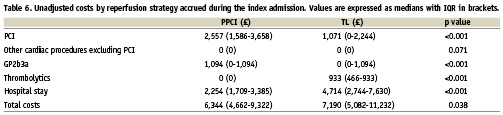
Over one third of patients in the TL group did not undergo cardiac catheterisation during the index admission. Fewer than 40% of PPCI procedures were performed within normal working hours. In the TL group most PCI procedures were non-urgent procedures undertaken within normal hours in over 90% of cases. Only 36% of urgent procedures were performed out with working hours. These differences explain in part the higher PCI costs in the PPCI group.
Follow-up costs over the first year were skewed and remained so even after log transformation making individual cost comparison inappropriate. However analysis of the mean adjusted cumulative one year costs showed no significant difference between groups (Table 5).
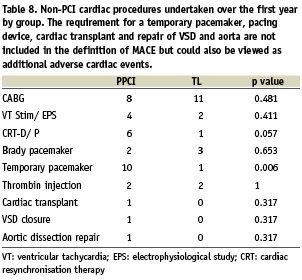
Table 8 illustrates further non-PCI procedures performed over the course of the first year. The strong trend towards increased utilisation of cardiac resynchronisation therapy and implantable defibrillators is likely due to the greater number of surviving patients in the PPCI group eligible for these therapies.
Discussion
This study demonstrates that the West London PPCI service is associated with dramatic early and sustained reductions in MACE and is cost neutral when compared to the TL service which it replaced.
Over the past 15 years PPCI has been shown to be the superior reperfusion strategy compared to TL.3,4 A meta-analysis of these studies has demonstrated small but significant reductions in MACE at 4-6 weeks.1 PPCI remains a superior strategy even when this necessitates transfer to an interventional centre.5-7
There were higher attrition rates in the TL group and greater than expected benefit associated with PPCI than have been previously reported, although the results are similar to some of the more recent randomised trials.8 This may be due to the inclusion of higher risk patients who have been shown to derive the greatest absolute benefit from PPCI.9 The elderly4,10, those with cardiogenic shock or significant comorbidities such as diabetes are often excluded or underrepresented in randomised trials.11-15
The mortality rate of patients presenting with cardiogenic shock in the context of STEMI is high and trial data suggests the benefits of PPCI are greater compared to a strategy of initial medical treatment.16
The evidence for PPCI in the elderly is limited, with studies indicating this group to have greater baseline risk but also greater absolute benefit from PPCI although this benefit may be abolished in the very old.17 In a meta-analysis of 11 trials comparing reperfusion strategies, the number needed to treat to save one life with PPCI over thrombolysis was eight in the over 70 group compared to 23 in patients under the age of 70 years.18 PPCI is not of proven benefit in elderly patients presenting with cardiogenic shock.16
There is conflicting evidence regarding the benefit of PPCI in patients with diabetes. A meta-analysis has demonstrated greater absolute benefits while a substudy of the DANAMI trial has suggested that diabetes may abolish the benefit of PPCI.19,20
The incidence of anterior infarction is higher than has been reported in some previous trials, but is similar to that reported by the London Ambulance Service across the city.21 The reason for this difference is unclear but may be due to both anterior and antero-lateral infarctions being included in this category. The authors of the Primary Angioplasty versus Immediate Thrombolysis (PRAGUE)-2 also comment that there may have been a tendency to not randomise patients with anterior infarction but rather proceed directly with PPCI in view of the perceived benefits.5 The design of our study eliminates this physician bias in the decision regarding treatment choice for presenting patients.
PPCI is much less time dependant than TL and the inclusion of patients presenting late is likely to contribute to the greater than expected benefits.22
The TL group in this study represents a historical control and some of the benefits of PPCI may be due to the development of more advanced cardiac services and infrastructure rather than PPCI as a reperfusion strategy per se. A more contemporary TL cohort may have higher rates of invasive investigation leading to better outcomes although the rate of invasive investigation remains consistent with current practice and that seen in previous trial data.5,23
The increased utilisation of drug eluting stents in the PPCI group may also contribute to its superiority by reducing the incidence of IDR although trial data indicates that this would not explain the mortality benefits.24
The choice of thrombolytic in this study reflected the policy of the treating hospital at that time and just over a quarter of patients were treated with streptokinase, whose use has been superseded by more thrombin specific agents such as tenecteplase. These newer agents are associated with a lower incidence of serious bleeding complications in particular intracranial haemorrhage and may be associated with improved rates of infarct arterial artery patency. However the rate of intracranial haemorrhage in this study was already low and meta-analysis of various thrombolytics has failed to demonstrate significant differences in outcome.25
This study demonstrates that a contemporary PPCI service can be cost neutral and is consistent with the results of previous studies based on trial data.
The Zwolle trial which originally reported significant reductions in both MACE and costs in favour of PPCI in the pre-stent era found that at one year there was no significant difference in costs.26 The Primary Angioplasty In Myocardial Infarction (PAMI) trial based in the United States (US) looking at balloon angioplasty alone showed large reductions in admission MACE rates with no difference in initial costs. The distribution of costs in this study highlighted important differences in the US healthcare system with a significant proportion of costs being allocated to professional fees.27 The strongest predictor of costs was hospital stay as is the case for this study.
Other studies have extended these findings to show stenting and GP2b3A use to be cost effective strategies with only slight increases in overall costs at one year.28,29
A more contemporary Canadian study found PPCI to be associated with improved outcomes when compared to TL with large reductions in both in-hospital and 6 month MACE and significant reductions in admission costs in favour of PPCI ($6,354 vs. $7,893; p<0.001).30 This cost reduction extended to the follow-up period of six months ($7,100 vs. $9,559; p=0.001) driven by the reduced hospital stay both at initial presentation and over the subsequent six months.
The lack of inclusive contemporary costing data comparing reperfusion strategies has led to the development of economic models within both Scandinavian and United Kingdom (UK) healthcare systems based on trial data.31-33 Both suggest improved outcomes and reduced lifetime costs associated with PPCI compared to TL.
The National Infarct Angioplasty Project (NIAP) was set up to determine the feasibility of undertaking PPCI within the UK. It recruited a number of interventional centres in a variety of settings and published its final report concluding that PPCI can be delivered within acceptable treatment times in a variety of settings.34 It did not set out to make any direct comparisons between the two reperfusion strategies based on the data collected.35 However resource utilisation from the initial admission of patients undergoing PPCI and TL from NIAP was incorporated into existing economic models to provide a more accurate estimate of cost effectiveness. PPCI had an incremental cost effectiveness ratio of £4,520 per quality adjusted life year gained.36 This analysis still depended on results from previous trials to estimate the probability of MACE rates up to six months with longer term benefits estimated from registry data. Additionally cost data was limited to the index admission only.
In this and other studies the length of hospital stay was the most important predictor of cost. PPCI was associated with significantly shorter stays. Fewer IDR in the PPCI group allowed patients to be discharged more quickly and patients in the TL group tended to experience delays as they waited for transfer for invasive investigation prior to discharge. A more efficient TL service could help reduce hospital stay costs associated with this strategy but is unlikely to have the same emphasis as early discharge after PPCI which has been documented in many studies. In addition there were patients, particularly the more elderly, where physicians were reluctant to refer for early catheterisation, but who were referred late after they had demonstrated ischaemia on mobilisation. Another less likely factor is physician bias in the timing of discharge of patients in the PPCI group. The study design was known to treating physicians and the importance of timely discharge on costs had already been shown in previous studies.
The results of this study cannot be extrapolated to different infrastructures such as where pre-hospital TL is used, where long ambulance times exist or where there is only a small volume PCI centre. As this economic model is sensitive to small variations in length of hospitalisation the applicability of these observed results to the general population may not be accurate.
The superiority of PPCI over TL has been proven beyond doubt but the challenge remains in providing a comprehensive service in a timely fashion which improves on existing TL while minimising costs. This study complements the results of previous analyses but is unique in that it is the first to present actual resource utilisation costs and outcomes over a one year time horizon in a real world setting. It demonstrates that PPCI can be successfully implemented within a European healthcare system using the hub and spoke model and is highly cost effective when compared to existing TL services.
Acknowledgements
The authors wish to thank the other members of the research team for their help, advice and support: Clare Sheehy, Cas Shotter, Phillip Eardley and Ezzat Mostefavi.
The authors also acknowledge the contribution of Elena Kulinskaya of the Imperial College statistical support group for developing the statistical analysis for this study.
The results of the study reflect the excellence of the clinical staff involved in the delivery of the service especially the staff of the cardiac catheter laboratory. Their diligence in collecting data made a major contribution to the study. Dr. K. Morgan received an unrestricted fellowship from Boston Scientific to undertake this study.

