Abstract
Aims: Large inequalities in the use of primary percutaneous interventions (PPCI) for ST-elevation myocardial infarction (STEMI) are evident. In order to understand how we can help to implement best practice for STEMI patients, we investigated the variation in PPCI utilisation in 120 regions in 10 EU countries and the association with economic, organisational and demographic characteristics.
Methods and results: We performed an ecological study using mixed effects regression models in the following 10 countries: Austria, Belgium, Denmark, England and Wales, Germany, Italy, Portugal, Spain, Sweden, and Northern Ireland. The main finding was the annual number of PPCI per million inhabitants from 2003 through 2008. Overall, the annual increase in PPCI utilisation was 1.15 (95% CI: 1.12, 1.19) per million per year. Regional-level rates varied from 0.74 (95% CI: 0.42, 1.30) to 1.90 (95 % CI: 1.01, 3.55) per million per year. At a regional level, significant positive associations with PPCI utilisation were the number of physicians per 100,000 inhabitants; the number of nurses and midwives per 100,000 inhabitants; and the proportion of the region’s population aged 50 to <70 years. At a country level, significant positive associations with utilisation were the year of STEMI treatment, population density per km2; number of general hospital beds per 100,000 inhabitants; and the number of physicians per 100,000 inhabitants.
Conclusions: Between 2003 and 2008, PPCI utilisation increased significantly in the ten European countries studied, but there was a great variation within country regions. Regional variation in PPCI rates were associated with both demographic and supply factors, revealing substantial opportunities to improve PPCI utilisation across Europe at national and regional levels.
Introduction
Prompt reperfusion optimises outcomes of ST-elevation myocardial infarction (STEMI)1,2. Although programmes for delivering timely primary percutaneous coronary intervention (PPCI) in experienced centres have been implemented in some European countries, large inequalities in the use of PPCI for STEMI across Europe have been reported3.
General research into healthcare inequality has focused on drivers for healthcare costs and attitudes towards implementation in groups of healthcare staff, and has often been restricted to a single country4,5. Patterns of PPCI utilisation in Europe according to economic, demographic and institutional context, and locality within countries have been sparsely examined. In order to understand how we can help to implement best practice for STEMI therapy, international comparative studies performed at a regional level in European countries may identify opportunities to reduce inequalities. Given the severity of STEMI, knowledge of factors hampering patient access to best evidence-based treatment would be the first step in reducing an unacceptable inequality with regard to life-saving treatment for STEMI patients in Europe.
Therefore, we conducted a study in order to: 1) quantify the variation in the use of PPCI among 120 regions in 10 European countries; 2) identify factors associated with such variation; and 3) investigate whether common factors associated with PPCI utilisation exist across regions, or whether these factors differ among regions. Our study therefore provides further insights into the factors affecting the implementation process of PPCI.
Methods
DESIGN AND SOURCE POPULATION
We conducted an ecological study of data aggregated on a regional level. The research was restricted to ten EU countries: Austria, Belgium, Denmark, England and Wales, Germany, Italy, Northern Ireland, Portugal, Spain, and Sweden.
DEPENDENT VARIABLE
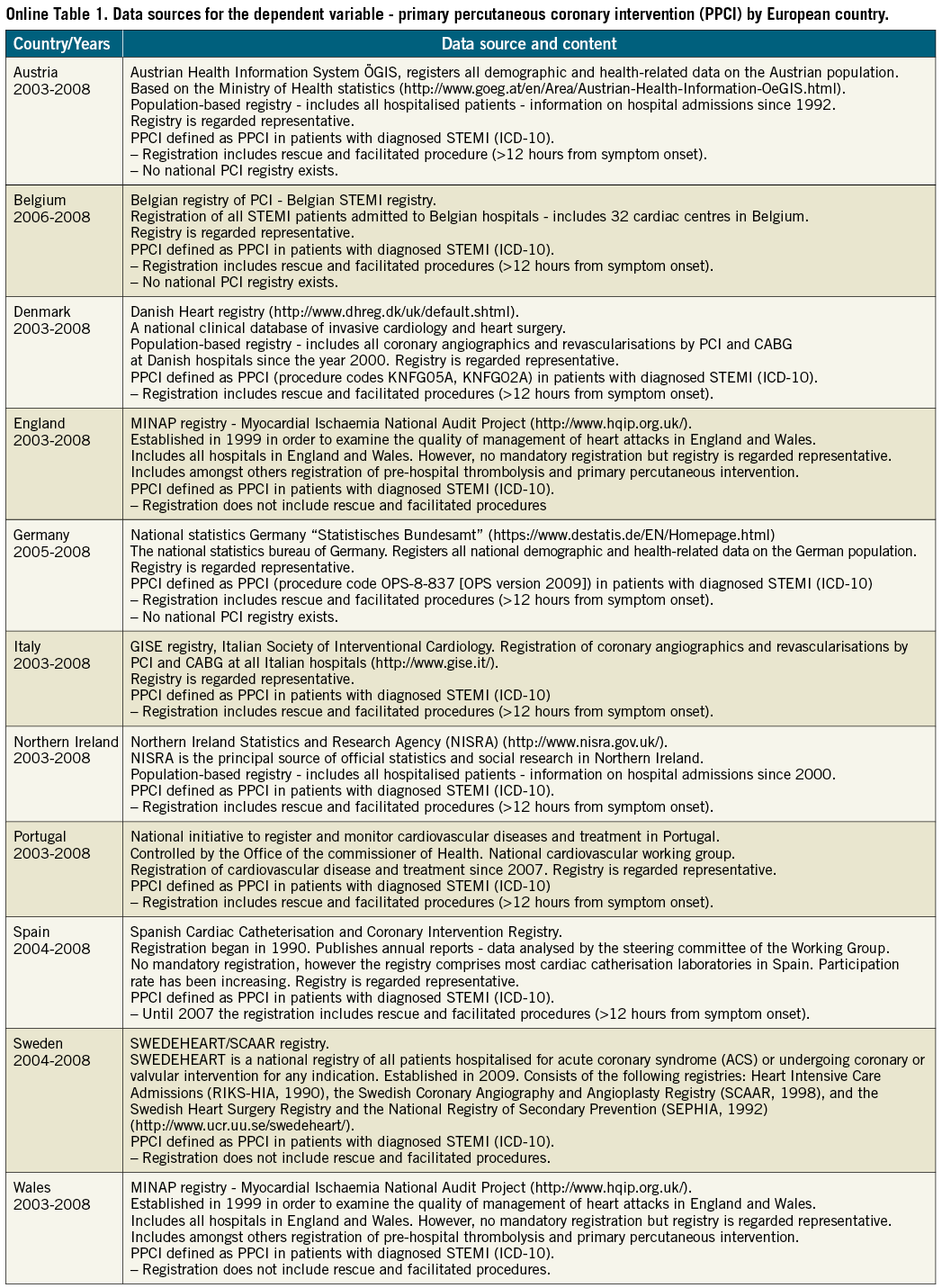
We obtained regional data on numbers of PPCI procedures per one million inhabitants per year from 2003 to 2008 from national and international registries (Online Table 1). PPCI was defined as the annual number of patients with a diagnosis of STEMI who underwent acute percutaneous coronary intervention (PCI). To be eligible for PPCI, patients had to meet the following criteria: symptom duration of ≤12 hours and ST-segment elevation ≥0.1 mV in at least two continuous leads (≥0.2 mV in V1-V3) or presumed new-onset left bundle branch block6. In countries where no national PCI registry existed (such as Germany), we received information on ICD-10 STEMI codes combined with the specific procedure codes for PCI. Data specifications and code numbers of STEMI according to the tenth revision of the International Classification of Diseases (ICD-10) are given in Online Table 2. Some countries were unable to provide data concerning PPCI frequency according to symptom duration (and therefore included patients undergoing PCI with a symptom duration of >12 hours) (Online Table 1).
The regional analyses were based on the original country-specific area divisions. Regional data were available for 10 countries, comprising a total of 120 regions. England and Wales were grouped as a “country”, although there are differences in care7.
We selected 2003 as baseline because this was the year in which the European guidelines recommending PPCI as the first choice treatment for STEMI was introduced8. The guideline was revised for the first time in 2008, when we therefore censored our analyses2,9. Moreover, it was not possible to obtain valid data from the majority of regions after 2008.
EXPLANATORY VARIABLES
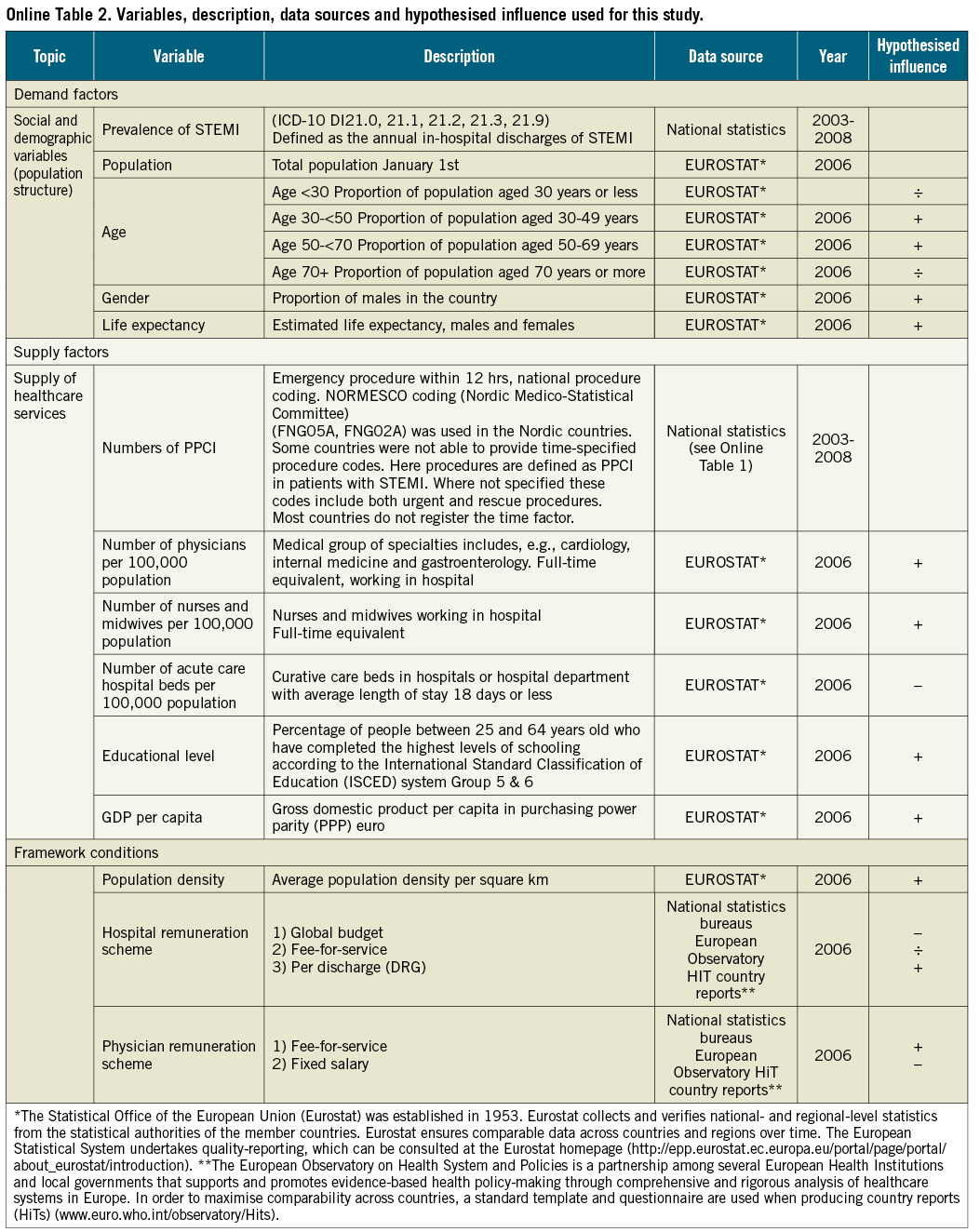
Data on the potential explanatory variables were obtained from Eurostat´s regional statistics (Online Table 2)10. Validated information on the explanatory variables from Eurostat was available for 200610. We collected data on each region’s demographics, supply factors, and healthcare system characteristics. Demographic data included age (proportion of the population in each age group), gender distribution and life expectancy of men and women. Supply factors included the number of physicians per 100,000 inhabitants, the number of nurses and midwives per 100,000 inhabitants, the percentage of the population with tertiary educational level according to the International Standard Classification of Education (ISCED) system group 5 & 611, gross domestic product (GDP) per capita (euros purchasing power parity), and the number of general hospital beds per 100,000 inhabitants. Information on the characteristics of each country´s healthcare system and remuneration schemes for hospitals and physicians in 2006 was available only at a national level, and therefore this information was excluded from the analysis. Regional data concerning the number of interventional or general cardiologists was not available; the number of medical physicians was used as a surrogate. In Northern Ireland the number of general hospital beds and educational level were available at a national level only: therefore, this single value was used for all the regions.
ANALYSIS
The annual change in utilisation of PPCI per region was modelled using linear regression. To investigate the effect of regional characteristics on regional differences in change in PPCI use, we performed the following analyses:
1. An overall analysis for all countries combined. We built an exploratory linear mixed-effects random slope and random intercept regression model (regions nested within countries, where covariates were included as grouped variables) using all available data (Online Table 1). Life expectancy, sex distribution and educational level were not included in any of the models due to collinearity. The model was fitted via maximum likelihood.
2. Within country analyses. To quantify the relationship between the explanatory variables and changes in PPCI utilisation in England, Italy, Germany, Spain and Sweden we fitted a stepwise linear mixed-effects regression model with random intercepts for each region and a random slope for each explanatory variable (Online Table 2). Inclusion of explanatory variables was informed by clinical and automated processes. Maximum likelihood estimation was used so that the null model could be calculated to compare statistically the addition of the predictor (p<0.05 suggested a better fitting model).
A natural log transformation was used to correct for right skewness of PPCI per one million inhabitants. All regression estimates are presented as antilogarithms along with their 95% confidence intervals (CI) and p-values, and therefore they represent the geometric mean change in PPCI use. All tests were two-sided, and a p-value <0.05 was used as a cut-off for statistical significance. All analyses were conducted using Stata Statistical Software: version 11.0 (Stata Corporation, College Station, TX, USA).
Results
TRENDS IN REGIONAL PPCI UTILISATION
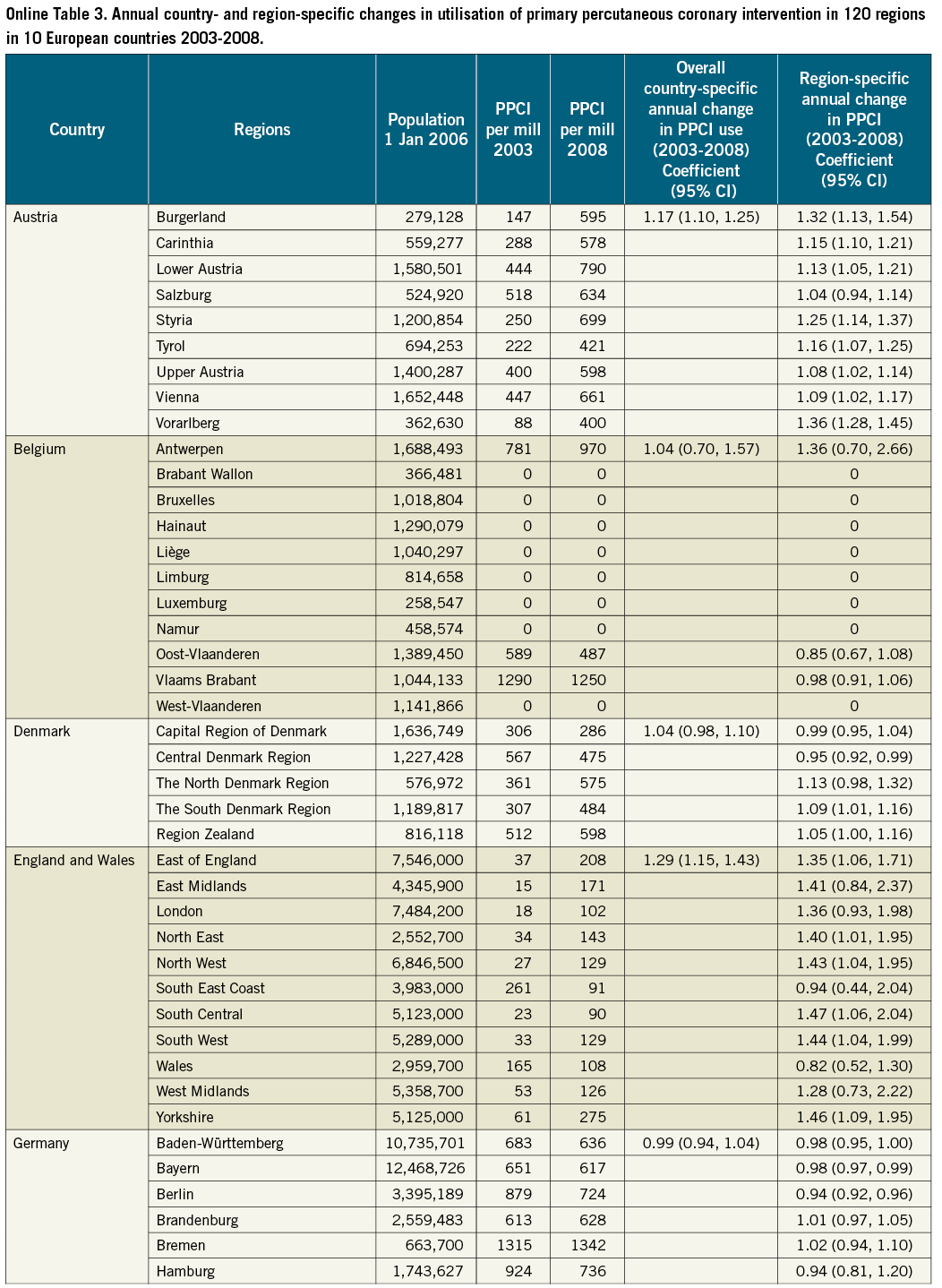
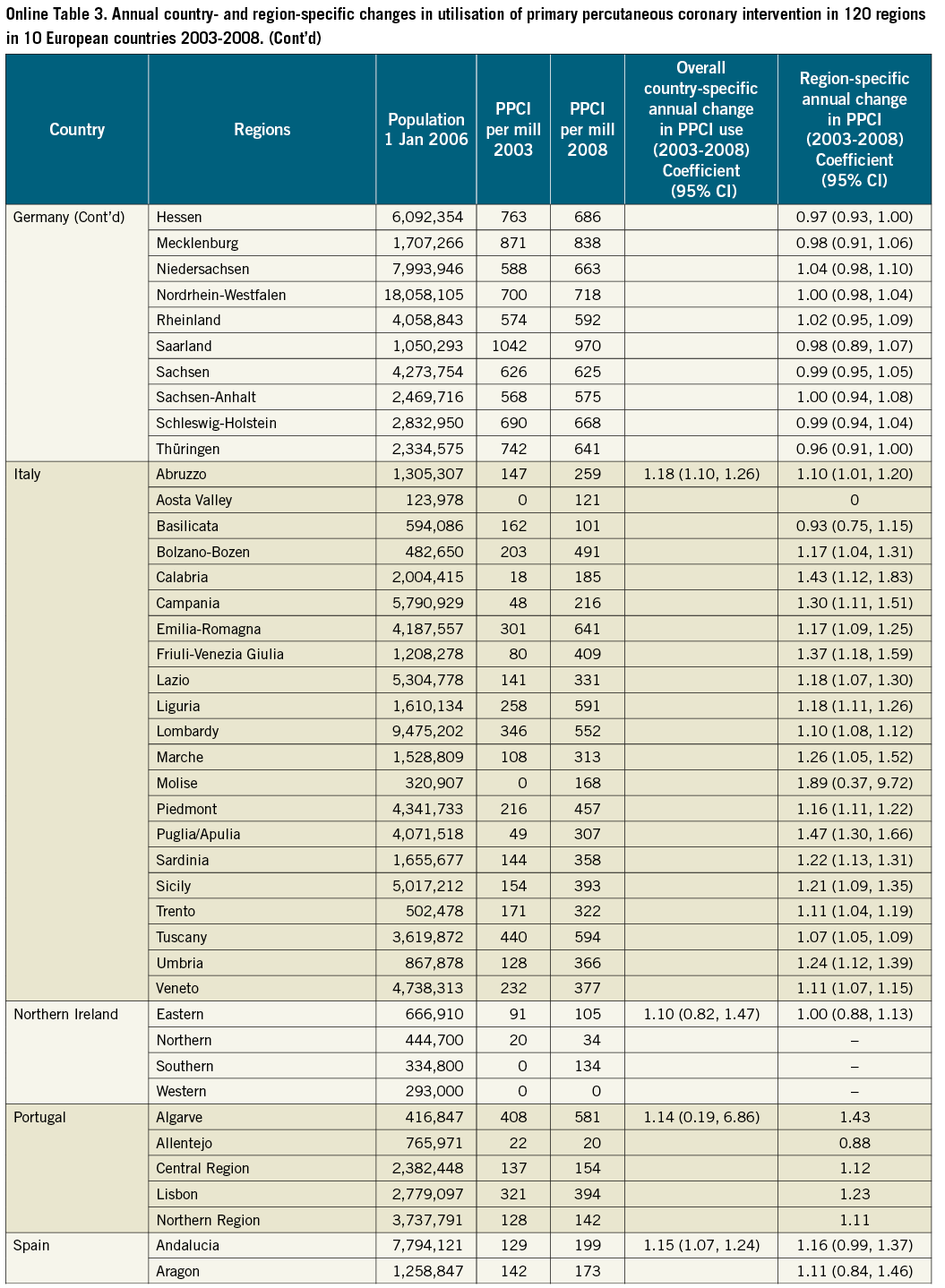
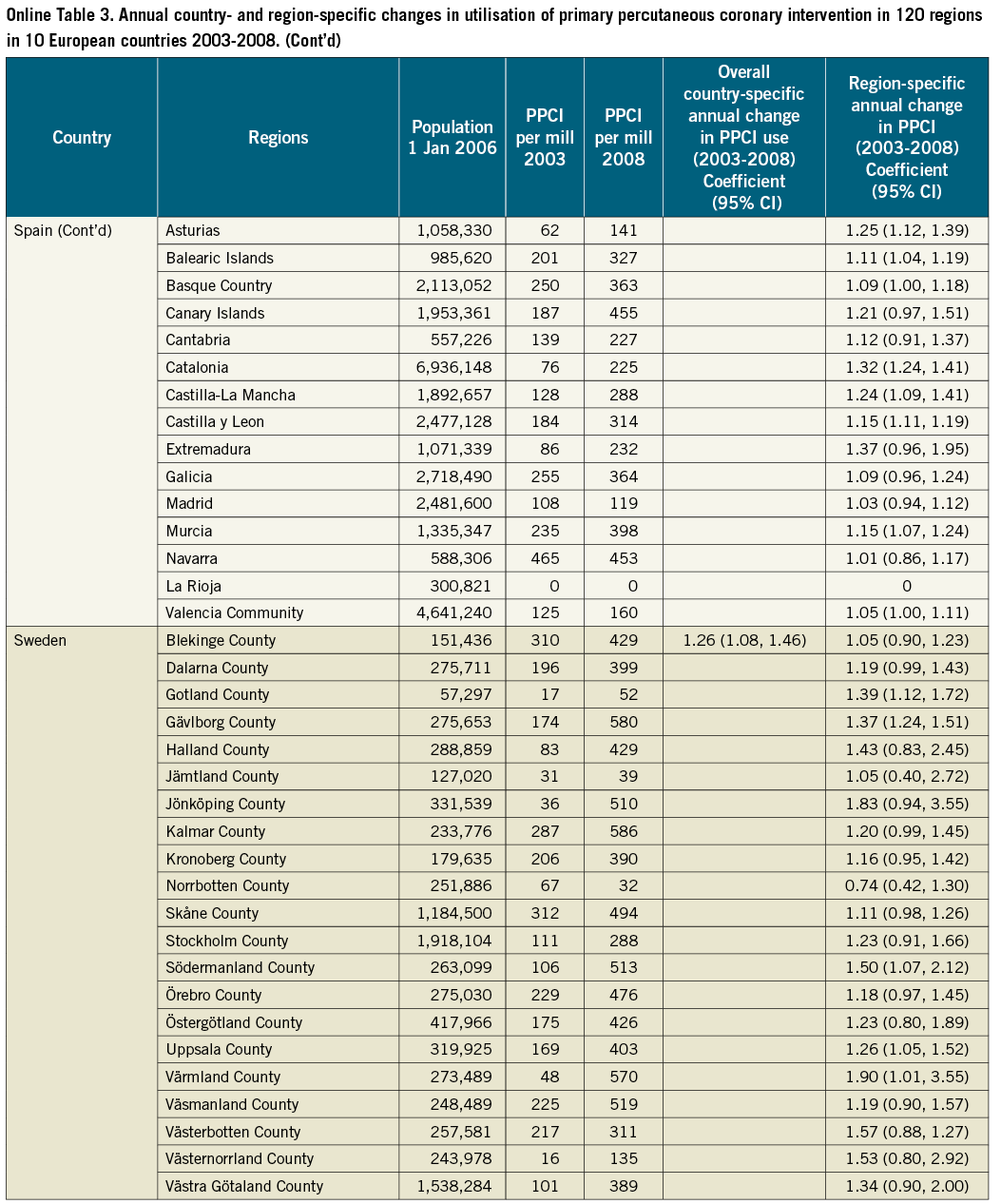
Figure 1 depicts the regional rates of PPCI for STEMI per one million inhabitants for each country from 2003 to 2008. The rates varied substantially both by country and by region. Overall, rates of utilisation levels were highest in Germany and Austria and lowest in England, Northern Ireland and Spain (Figure 1). The mean yearly change in PPCI utilisation was, however, largest in England and Wales with a growth of 1.29 (95% CI: 1.15, 1.43) per one million inhabitants per year from 2003 to 2008 whereas Germany had a stable level of utilisation (0.99 [95% CI: 0.94, 1.04]) (Online Table 3).
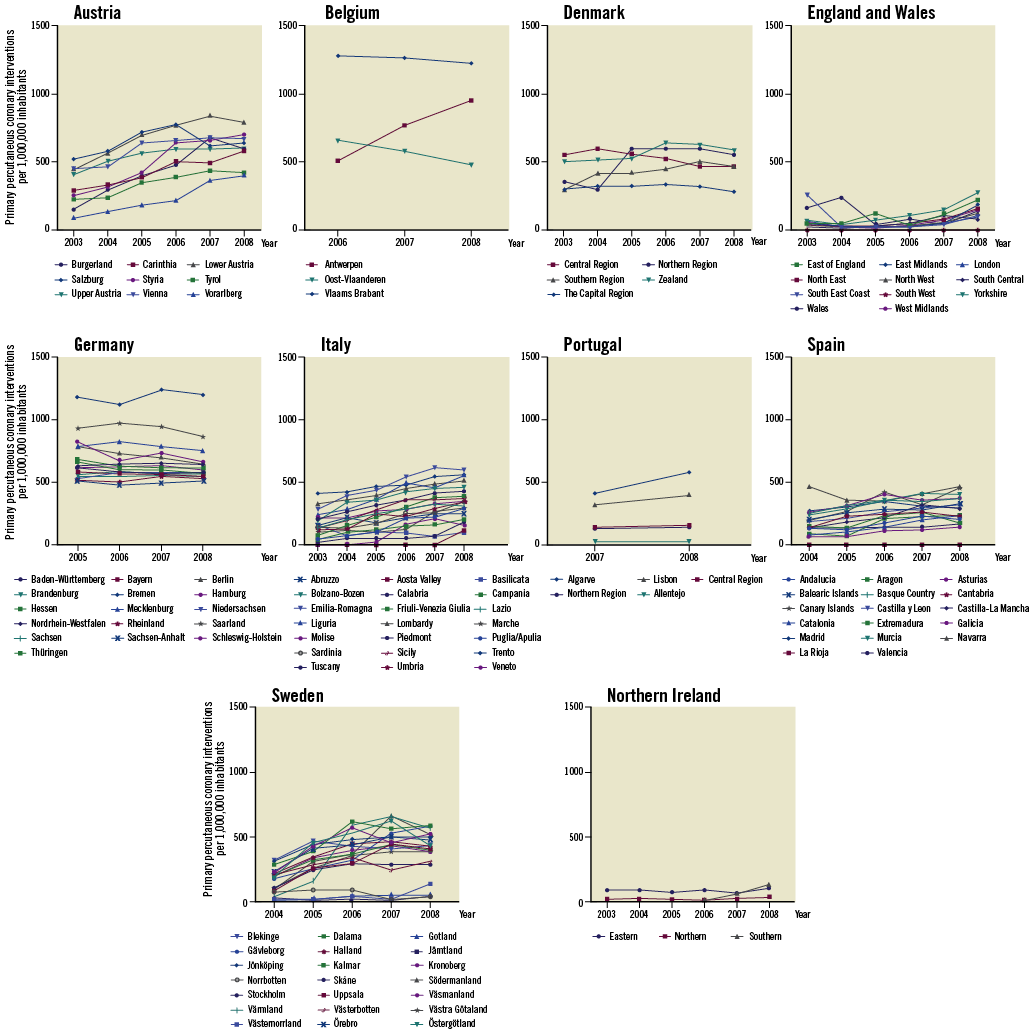
Figure 1. Frequency of primary percutaneous interventions per one million inhabitants, by place of residence and year of treatment in 10 European countries, 2003-2008.
The three countries with the largest regional inequalities were Italy, Spain and Sweden (Figure 1). The annual regional change in PPCI utilisation per one million inhabitants varied in Sweden between a mean decline in utilisation of 0.74 (95% CI: 0.42, 1.30) per one million inhabitants (Norrbotten County) and a mean growth of 1.90 (95% CI: 1.01, 3.55) per one million inhabitants (Värmland County). In Germany and Denmark utilisation was stable over the years and the regions (Figure 1, Online Table 3).
OVERALL FACTORS ASSOCIATED WITH CHANGES IN REGIONAL PPCI USE
Table 1 presents the multivariable analysis of the overall impact of the covariates on the change in regional use of PPCI over time. This revealed a significant positive association between PPCI utilisation and year of acute percutaneous revascularisation, the number of physicians per 100,000 inhabitants, the number of nurses and midwives per 100,000 inhabitants, and age distribution.
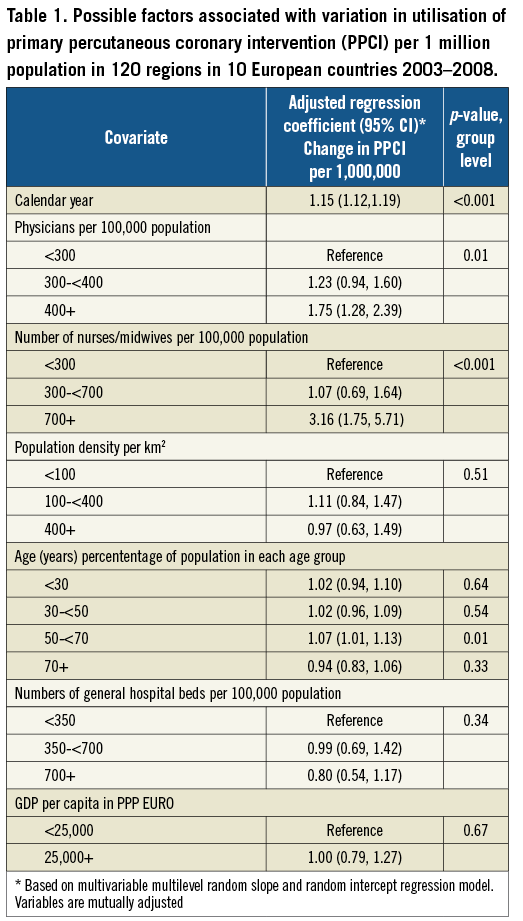
We observed a yearly growth in PPCI utilisation across all regions, with an overall mean growth of 1.15 per one million inhabitants per year (95% CI: 1.12, 1.19). Regions with >400 physicians per 100,000 inhabitants experienced a change in growth in PPCI utilisation of 1.75 (95% CI: 1.28, 2.39) per one million inhabitants compared with regions with <300 physicians per 100,000 inhabitants. The growth increased significantly with a higher underlying basis of physicians (p=0.01) (Table 1). Regions with a number of nurses and midwives employed of >700 per 100,000 inhabitants experienced a growth in PPCI utilisation of 3.16 (95% CI: 1.75, 5.71) per one million inhabitants compared with regions with <300 nurses and midwives per 100,000 inhabitants. Likewise the growth increased significantly with a higher underlying basis of nurses and midwives (p<0.001) (Table 1). Regions with a large proportion of people aged between 50 and <70 years experienced a faster growth in utilisation of PPCI (1.07 per one million inhabitants [95% CI: 1.01, 1.13]) (Table 1).
COUNTRY-SPECIFIC FACTORS ASSOCIATED WITH REGIONAL PPCI UTILISATION
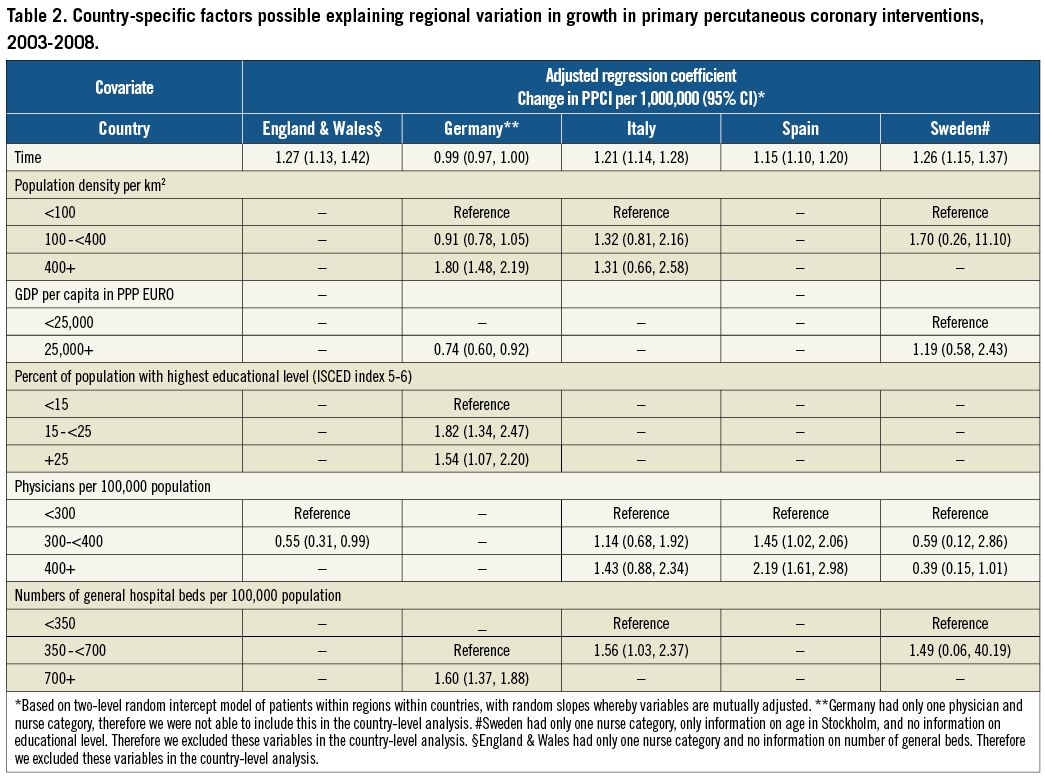
Table 2 presents the country-specific multivariate analyses.
ENGLAND AND WALES
In England and Wales, PPCI utilisation was positively associated with the year of acute revascularisation (Table 2). Overall, the mean annual growth in PPCI utilisation was 1.27 per one million inhabitants (95% CI: 1.13, 1.42) per year. Six regions (75.0%) demonstrated a significant increase in PPCI utilisation, with a highest mean change of 1.47 (95% CI: 1.06, 2.04) (Online Table 3) per one million inhabitants. There was a negative association between change in PPCI utilisation and the number of physicians (0.55 per 100,000 [95% CI: 0.31, 0.99]).
GERMANY
In Germany there was no mean change in PPCI utilisation (0.99 per one million inhabitants [95% CI: 0.97, 1.00]). The highest mean regional PPCI utilisation was 1.02 (95% CI: 0.95, 1.09) (Online Table 3).
Regions in Germany with >400 population density per km2 experienced a yearly growth in PPCI utilisation per one million inhabitants of 1.80 per 100,000 (95% CI: 1.48, 2.19) compared with regions with <100 population per km2. Regions with a GDP per capita of 25,000+ euros experienced smaller growth compared with regions with GDP per capita of <25,000 euros (0.74 per 100,000 [95% CI: 0.60, 0.92]) (Table 2).
ITALY
In Italy we found a positive association between the year of acute revascularisation and PPCI utilisation per one million inhabitants (1.21 [95% CI: 1.14, 1.28]) (Table 2). Seventeen regions (81%) demonstrated a significant increase in PPCI utilisation with a highest mean regional utilisation of 1.89 (95% CI: 0.37, 9.72) (Online Table 3). Regions with 350 to <700 hospital beds per 100,000 inhabitants (1.56 per 100,000 [95% CI: 1.03, 2.37]) experienced a greater yearly growth compared with regions with <350 beds per 100,000 inhabitants (Table 2).
SPAIN
In Spain we found a significant association between the year of acute revascularisation and the utilisation of PPCI per one million inhabitants (1.15 per 100,000 [95% CI: 1.10, 1.20]). Seven regions (41%) demonstrated a significant increase in PPCI utilisation with a highest mean regional PPCI utilisation of 1.32 (95% CI: 1.24, 1.41) (Online Table 3). Regions with 300 to <400 physicians per 100,000 inhabitants had an increase in PPCI utilisation of 1.45 per 100,000 (95% CI: 1.02, 2.06) procedures per one million inhabitants compared with regions with <300 physicians per 100,000 inhabitants. The growth was associated with a higher underlying number of physicians (p>0.01) (Table 2).
SWEDEN
In Sweden, the year of acute revascularisation was associated with growth in PPCI utilisation (1.26 per 100,000 [95% CI: 1.15, 1.37]) (Table 2). Four regions (19%) demonstrated a significant increase in PPCI utilisation with a highest mean regional utilisation of 1.90 (95% CI: 1.01, 3.55) (Online Table 3).
Discussion
In this study, based on regional-level data from 10 European countries, we found that PPCI for STEMI became more frequent in all countries between 2003 and 2008; however, the same was not evident within all regions of these countries. From the time when PPCI was recommended in European guidelines, we found that countries and regions differed substantially in the time and rates of utilisation of PPCI. Notably, regional variation among countries remained. Three patterns of change in PPCI uptake over time were evident. One pattern, evident in Germany and Denmark, involved an early start and a steady level. Austria, Italy and Sweden illustrated a second pattern: later start, relatively fast growth. The third pattern is illustrated by England and Northern Ireland with a late start and a rapid growth. Later initial adoption appears to be the primary reason why PPCI rates in England and Northern Ireland differed from the other countries.
Previous studies of international differences in implementation of intensive treatment show similar patterns, but studies on inter-regional differences in implementation are uncommon11-13.
These variations may have been influenced by the underlying demand for PPCI, expressed as the incidence of STEMI. The incidence and mortality from STEMI are not continuously monitored by surveillance registries, and few countries have valid regional data5,14. However, in recent years decline in the incidence of STEMI as well as decline in mortality in some countries has been evident. It is estimated that this decline is attributable to a reduction in risk factors and better preventive treatment, which may explain a reduction of PPCI utilisation in some countries (e.g., Denmark and Germany) towards the end of the study period1. However, comparing trends over time, as in this study, rather than point-in-time differences allows us to “factor out” some of the genetic, demographic and other influences on treatments of diseases across regions. These underlying factors are unlikely to change nearly as rapidly as medical practices. We do not believe, therefore, that differences in disease burden in western European countries fully explain our findings12.
Providing access to PPCI has prompted many European countries to establish regional networks involving pre-hospital services, community hospitals and PCI centres15,16. Our study was not designed to capture the existence of such networks or any cross-border trafficking due to them, which might explain the low PPCI activity in some regions.
COMMON FACTORS ASSOCIATED WITH REGIONAL VARIATION IN PPCI UTILISATION
From our combined analysis it appears that supply factors (healthcare professional workforce) are an important determinant of PPCI utilisation. Also, previous studies suggest that supply-side characteristics have a strong influence on treatment patterns. These studies found that, in particular, the regulation of specialist capacity to perform medical procedures, as well as the availability of catheterisation laboratories (“supplier-induced demand”), is a strong determinant of technology use11,13,17. However, disagreement exists as to whether this association is based on the fact that physicians relocate to regions where supply is high or based on an already existing high demand18-20. Our study did not have access to information on the number of existing catheterisation laboratories, which might explain part of the detected regional variation17.
Age and gender distribution may also be an important factor in healthcare utilisation. We found a significant association between the proportion of the population aged 50 to 70 years and growth in PPCI utilisation. This may be an expression of the regional demand for STEMI treatment in the region.
CONTEXT-SPECIFIC FACTORS
Factors explaining regional variation in PPCI use varied among countries, suggesting that implementation is complex. Our results support the literature in favour of context-specific implementation strategies that match the structures and needs of the society (region) in which they are to be implemented11,21. The need for context-specific implementation programmes within the field of PPCI were acknowledged in 2009 with the launch of the “Stent for Life Initiative”22. Preliminary reports from this initiative suggest a substantial increase in the number of PPCI cases performed23.
Apart from Germany, we found a positive correlation between the year of STEMI treatment and regional growth in PPCI utilisation. This meets our expectations that some natural increase in utilisation will happen as the technology matures and healthcare workers are trained. Not all of the other parameters estimated in the country-specific analyses are intuitive, and might be partly explained by the fragility of the models. For example, in Germany GDP was negatively associated with PPCI use, conflicting with earlier research on factors associated with technology implementation11,13,24. An unmeasured factor, such as the construction of new central government supported hospitals in poorer regions, may explain this25. Notably, collinearity between GDP and the number of physicians cannot be excluded and the determinants of the association should be considered in future studies.
In England and Sweden we found negative associations between the number of physicians and the change in PPCI utilisation. In England specialist care provided by a consultant cardiologist is associated with improved outcomes26, and it is likely therefore that the use of physicians (combined) as a surrogate marker for cardiology care was not appropriate in this instance. Some studies report an uneven distribution of physicians between rural and urban areas. Our aggregate figures might conceal large regional differences in physician distribution that could help explain these conflicting results. Future work should consider models in which implementation of PPCI is evaluated based on information at the patient level18,20,27,28.
STRENGTHS AND LIMITATIONS OF THE STUDY
A unique advantage of our study was that it incorporated an international gathering of regional-level data concerning PPCI utilisation. Many variables are important in the diffusion of technology: here we uncovered both overall (inter-regional) and context-specific (intra-regional) factors associated with geographical variations in PPCI utilisation in Europe. STEMI is a common and well-defined clinical condition, allowing a good platform for international comparisons of diagnoses and treatment. Uniform definitions of the explanatory variables were available through Eurostat, a reliable source of aggregated population-based data on demographics, social conditions, and economy. Moreover, macro-level studies allowed both demand and supply factors to be explored, which in earlier studies have been shown to have a significant impact on technology implementation.
However, our study has limitations. Our data are on an aggregate level, which prevented the inclusion of patient-level characteristics, and therefore our results might not apply to all individuals in the underlying population. There were differences among countries in aspects of the data collection of PPCI utilisation in STEMI patients, which might affect the size of the reported inequality in PPCI use. Incomplete or non-compulsory reporting from hospitals may bias the factual size of inequality, but the size and direction of bias is unknown. Moreover, inclusion of patients receiving PCI >12 hours from symptom debut in some countries may have led to an overestimation of PPCI use (Online Table 1). According to the available registries, these procedures comprise over 20% of the PPCI procedures per year (Italy and Berlin, Germany). Moreover, not all countries were able to provide data for five years. We used the ICD-10 coding system to obtain a comparable data registration of the STEMI diagnosis, which is well known worldwide. While some misclassification of the STEMI diagnosis cannot be ruled out, we do not expect the misclassification rates to vary over the time period of the study. Thus, potential misclassification does not explain the observed trends or the predictive variables of these trends. The study is based on registry data, which makes access to up-to-date data difficult due to the time for hospital reporting and validation of data. When initiating the study, we were not able to obtain valid data on PPCI utilisation from the majority of the countries after 2008. This will most likely underestimate the current level of use in all participating countries and regions. Our regional-level data models were based on an incomplete number of available variables, e.g., we had no access to data on the number and location of catheterisation laboratories. Finally, explanatory variables were registered for the year 2006, yet we modelled PPCI use from 2003 to 2008. Although interactions between time and the explanatory variables have been reported25, we have no reason to believe that the underlying explanatory factors have changed substantially during the relatively short observational period of our study.
Implications and conclusions
With these caveats in mind, our findings offer some important contributions for research and clinical care. Our study is the first to examine regional-level variation across a large sample of countries over five years, thus providing a unique investigation of the variation in utilisation of PPCI. The study should be interpreted as a preliminary step in mapping PPCI practice across Europe. Our findings highlight the complexity of the factors that have a role in ensuring access to care. The implementation of PPCI takes place on many different levels and seems to differ from country to country. For this reason, interventions focused at several levels would most likely hasten PPCI uptake in the communities. Further studies accessing micro-level European data are needed and thus the development of high-quality databases at both national and international level is a prerequisite.
In conclusion, we found that the rate and level of PPCI implementation varied substantially among regions and countries across Europe. In the case of overall regional variation, this could partly be explained by both regional supply factors, such as the number of physicians, and demand factors, such as age distribution.
Acknowledgements
The list of colleagues who have given support in data collection can be found in the Online Appendix.
Funding
This work was supported by the European Critical Care Foundation and the EAPCI. C. P. Gale was supported by the National Institute for Health Research (NIHR) [NIHR CS009004].
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix
We thank several colleagues for their support with data collection.
AUSTRIA. Dr Gerhard Fülöp, Gesundheit Österreich GmbH/Geschäftsbereich ÖBIG (Austrian Health Institute), Responsible for working area “health care planning”, Stubenring 6, 1010 Vienna, Austria. Maria Hofmarcher-Holzhacker, Director Health and Care, European Centre for Social Welfare Policy and Research, Vienna, Austria.
BELGIUM. Professor Victor Legrand, MD, PhD, FESC, FACC, Department of Cardiology, CHU de Liège, Belgium.
DENMARK. The Danish Heart Register, The National Institute for Public Health. Lene Mia Von Kappelgaard, cand.sient.san.publ., Research assistant, National Institute of Public Health, Denmark. The National Board of Health, Denmark.
ENGLAND. Dr John Birkhead, c/o The Myocardial Ischaemia National Audit Project (MINAP) Academic Group, National Institute for Cardiovascular Outcomes Research (NICOR), University College London. This study includes data collected on behalf of the British Cardiovascular Society under the auspices of the National Institute for Cardiovascular Outcomes Research (NICOR).
GERMANY. Birga Maier, MD, MPH, MSc, Managing Director of the Berlin Myocardial Infarction Registry, Berlin. Professor Dr. med Heinz Theres, Medical Park Berlin Humboldtmühle, Berlin, Charité Universitätsmedicin Berlin, Germany. Diana Dinslage, employee of the health statistics at Federal Statistical Office, Germany. Helmut Schühlen, MD, FESC, FACC, Director of the Department of Cardiology & Invasive Care Medicine at the Vivantes Auguste-Viktoria-Klinikum in Berlin, Germany.
ITALY. Raffaele De Catarina, MD, PhD, Professor of Cardiology, Director at Institute of Cardiology, Institute of Cardiology, “G. d’Annunzio” University - Chieti-Pescara, Chieti, Italy. Aldo Pietro Maggioni, MD, FESC, Director ANMCO Research Center, Firenze, Italy.
PORTUGAL. Lino Gonçalves, MD, PhD, Department of Cardiology, Coimbra University Hospital, Portugal. Rui Cruz Ferreira, MD, National Coordinator for Cardiovascular Diseases, Lisbon, Portugal. Rui César das Neves, Engineer, National Cardiovascular Diseases Registry, Lisbon, Portugal.
SWEDEN. SCAAR/SWEDEHEART registry for sharing data on Swedish angiography and coronary interventions. Kalle Spångberg, Uppsala Clinical Research Center for administrative assistance. Tomas Jernberg, MD, PhD, Chairman of SWEDEHEART, Dept of Cardiology, Karolinska University Hospital, Stockholm. Bo Lagerqvist, MD, PhD, Department of Medical Sciences Uppsala University and Akademiska Sjukhuset Uppsala, Sweden. Torbjörn Nyqvist, Information Officer at the Customer Service, Communication Department Statistics Sweden, Stockholm, Sweden. Anna Sellin, Head of The Unit of Healthcare and Social Welfare, The National Board of Health and Welfare, Stockholm, Sweden. Max Köster, Researcher at Unit of Epidemiology, National Board of Health and Welfare, Sweden. Carl Hampus Lyttkens, Professor of Economics, Department of Economics Lund University, Sweden.
SPAIN. Manel Sabaté, MD, PhD, Chief of Department of Cardiology, Hospital Clínic, Barcelona. Héctor Bueno, MD, PhD, FESC, FAHA, Associate Professor of Medicine, Universidad Complutense de Madrid, Head of Clinical Cardiology/CCU Department of Cardiology, Hospital General Universitario Gregorio Maranon, President-Elect WG on Acute Cardiac Care, European Society of Cardiology, Spain.
SCOTLAND. Mr Adam Redpath, NHS National Services Scotland. Professor Jill Pell, Henry Mechan, Professor of Public Health, Institute of Health and Wellbeing, University of Glasgow.
NORTHERN IRELAND. Northern Ireland Statistical Research Agency, website: http://www.nisra.gov.uk/demography/default.asp.htm.
Suzanna McVeigh, Assistant Statistician, Information and Analysis Directorate, Department of Health, Social Services and Public Safety Northern Ireland. Dr Sharon Jamison, Assistant Statistician Hospital Information Branch, Annexe 2 Castle Buildings, Stormont, Northern Ireland.