Abstract
Aims: To examine outcomes in patients with ST-segment elevation myocardial infarction (STEMI) treated with primary percutaneous coronary intervention (PCI) at US sites versus sites outside the US (OUS).
Methods and results: In the HORIZONS-AMI trial 3,602 STEMI patients in 11 countries were randomised to primary PCI with bivalirudin versus heparin + glycoprotein IIb/IIIa inhibitors. US patients (n=814) had more diabetes, prior infarction, prior bypass surgery, and renal insufficiency. OUS patients (n=2,788) had longer door-to-balloon times, more radial access, fewer bypass surgeries, and were discharged more often on beta-blockers and statins. At three years US patients had higher mortality (9.7% vs. 6.0%, p=0.0003), reinfarction (10.2% vs. 6.4%, p=0.001), major adverse cardiac events (MACE; 28.2% vs. 20.1%, p<0.0001), major bleeding (16.9% vs. 6.4%, p<0.0001) and net adverse clinical events (NACE; 36.6% vs. 23.8%, p<0.0001), which persisted after adjusting for baseline risk.
Conclusions: In the HORIZONS-AMI trial, STEMI patients undergoing primary PCI at US versus OUS sites had higher rates of adverse events, which persisted after adjusting for baseline risk. The reasons for these differences are not clear but may be due to unmeasured confounders, different thresholds for event reporting, or valid differences in systems of care and treatments. Registration: ClinicalTrials.gov number NCT00433966.
Introduction
Primary percutaneous coronary intervention (PCI) reduces mortality, reinfarction and stroke compared to fibrinolytic therapy, and has become the preferred reperfusion strategy for patients with ST-segment elevation myocardial infarction (STEMI)1-3. Despite similarities between evidence-based guidelines for the management of myocardial infarction in the US and other countries1,2, there are considerable variations in practice patterns and outcomes between different countries4-6. There are limited data examining outcomes in STEMI patients treated in the US compared to sites outside the United States (OUS), and most such data are from the thrombolytic era4-6.
The HORIZONS-AMI (Harmonizing Outcomes with RevascularIZatiON and Stents in Acute Myocardial Infarction) trial was a prospective, randomised, international trial that evaluated bivalirudin versus heparin plus glycoprotein IIb/IIIa platelet inhibitors (GPI) and paclitaxel-eluting stents (PES) versus bare metal stents (BMS) in patients undergoing primary PCI for STEMI7,8. As one of the largest international, randomised trials of primary PCI to date, HORIZONS-AMI provided a unique opportunity to compare patient outcomes according to geographic region.
The purpose of the present study is to compare outcomes between patients enrolled at US and OUS sites, and to evaluate potential reasons for differences in outcomes by comparing geographic-specific patient risk profiles and variations in clinical practice patterns.
Methods
PATIENT POPULATION AND STUDY PROTOCOL
HORIZONS-AMI was a prospective, randomised, multicentre trial comparing bivalirudin monotherapy to heparin plus GPI, and PES to BMS in patients with STEMI undergoing a primary PCI management strategy7. Consecutive patients ≥18 years old with symptom duration >20 minutes and <12 hours and ST-segment elevation of ≥1 mm in ≥2 contiguous leads, new left bundle branch block, or true posterior MI were considered for enrolment. The study complies with the Declaration of Helsinki, was approved by the institutional review board or ethics committee at each participating centre, and all patients signed informed consent.
RANDOMISATION
Patients were randomised to bivalirudin alone or unfractionated heparin plus GPI. Randomisation was stratified by: a) administration of pre-randomisation heparin; b) administration of clopidogrel 300 mg or 600 mg prior to catheterisation; c) administration of abciximab vs. eptifibatide in patients randomised to the control group; and d) US or OUS study site. Following coronary angiography, patients were treated by PCI, coronary artery bypass graft surgery (CABG) or medical management at the physician’s discretion7. Eligible patients undergoing PCI were randomised 3:1 to either TAXUSTM EXPRESS PES (Boston Scientific, Natick, MA, USA) or uncoated EXPRESS BMS (Boston Scientific).
MEDICATIONS
Bivalirudin was administered as an IV bolus of 0.75 mg/kg and infusion of 1.75 mg/kg/hr. Heparin was administered in the control group as an IV bolus of 60 IU/kg with subsequent boluses targeted to an activated clotting time of 200-250 seconds. Both antithrombin agents were discontinued as per protocol at the completion of angiography or PCI. Either abciximab or double-bolus eptifibatide was permitted as per the investigator’s discretion in patients assigned to heparin plus GPI, and was continued for 12 hours (abciximab) or 12-18 hours (eptifibatide). Aspirin 324 mg chewed or 500 mg IV was given in the emergency room, followed by 300-325 mg PO daily during the hospitalisation and minimum 75 mg daily indefinitely thereafter. Clopidogrel (300 or 600 mg as per the investigator’s discretion) was administered prior to catheterisation, followed by 75 mg PO daily for 6-12 months.
FOLLOW-UP AND ENDPOINTS
Clinical follow-up was performed at 30 days, six months, one year, two years and three years. Prespecified endpoints included death, reinfarction, ischaemia-driven target vessel revascularisation (TVR), stroke, protocol-defined major bleeding not related to CABG, definite or probable stent thrombosis (ARC criteria9), MACE (major adverse cardiac events comprising death, reinfarction, ischaemic TVR or stroke), and NACE (net adverse clinical events comprising MACE or non-CABG-related major bleeding). An independent clinical events committee blinded to treatment assignment adjudicated all adverse ischaemic and bleeding events using original source documents and procedural angiograms.
STATISTICAL METHODS
Categorical outcomes were compared by chi-square or Fisher’s exact test. Continuous variables were compared by the Wilcoxon rank-sum test. The 30-day and three-year event analyses were performed using Kaplan-Meier estimates, and were compared with the log-rank test. Multivariable analyses were performed with Cox regression. Baseline variables with p-values <0.1 on univariable analysis were entered into the models and retained with p-values <0.1. Treatment site (US versus OUS) was included in all models.
Results
PATIENTS AND BASELINE CHARACTERISTICS
Between March 2005 and May 2007, 3,602 patients with STEMI undergoing primary PCI were enrolled at 123 centres in 11 countries; 814 patients (22.6%) were from the US and 2,788 (77.4%) from OUS. Patients enrolled at US sites had more hypertension, hyperlipidaemia, diabetes and a higher body mass index (BMI) (Table 1). US patients were more likely to have prior infarction, prior coronary artery bypass surgery, were more often black or Hispanic, had lower left ventricular ejection fraction, and less TIMI 2-3 flow on initial angiography. The infarct artery for US patients was less often the left anterior descending and more often the right coronary artery or a saphenous vein graft. OUS patients had a higher incidence of smoking. US patients had higher baseline haemoglobin and platelet count but a lower baseline white blood cell count and creatinine clearance.
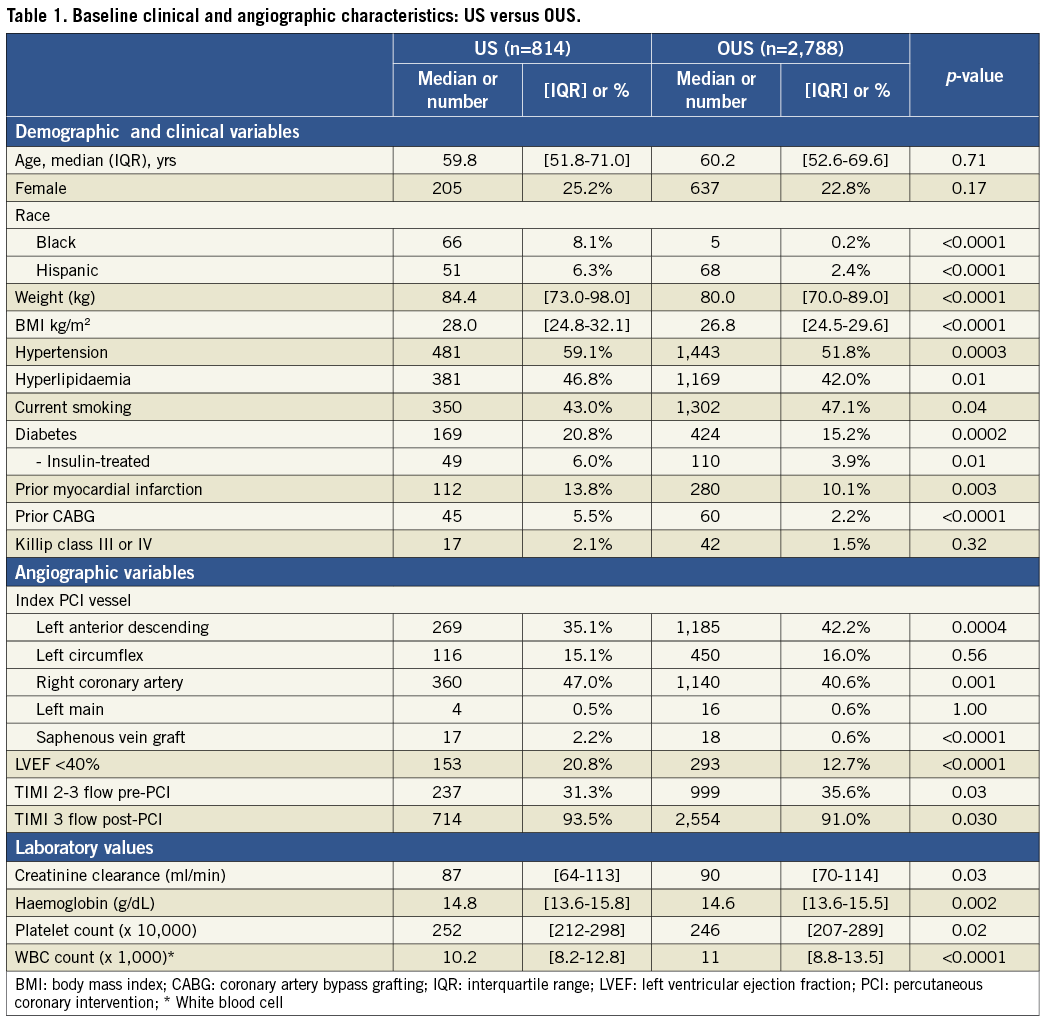
PATIENT MANAGEMENT
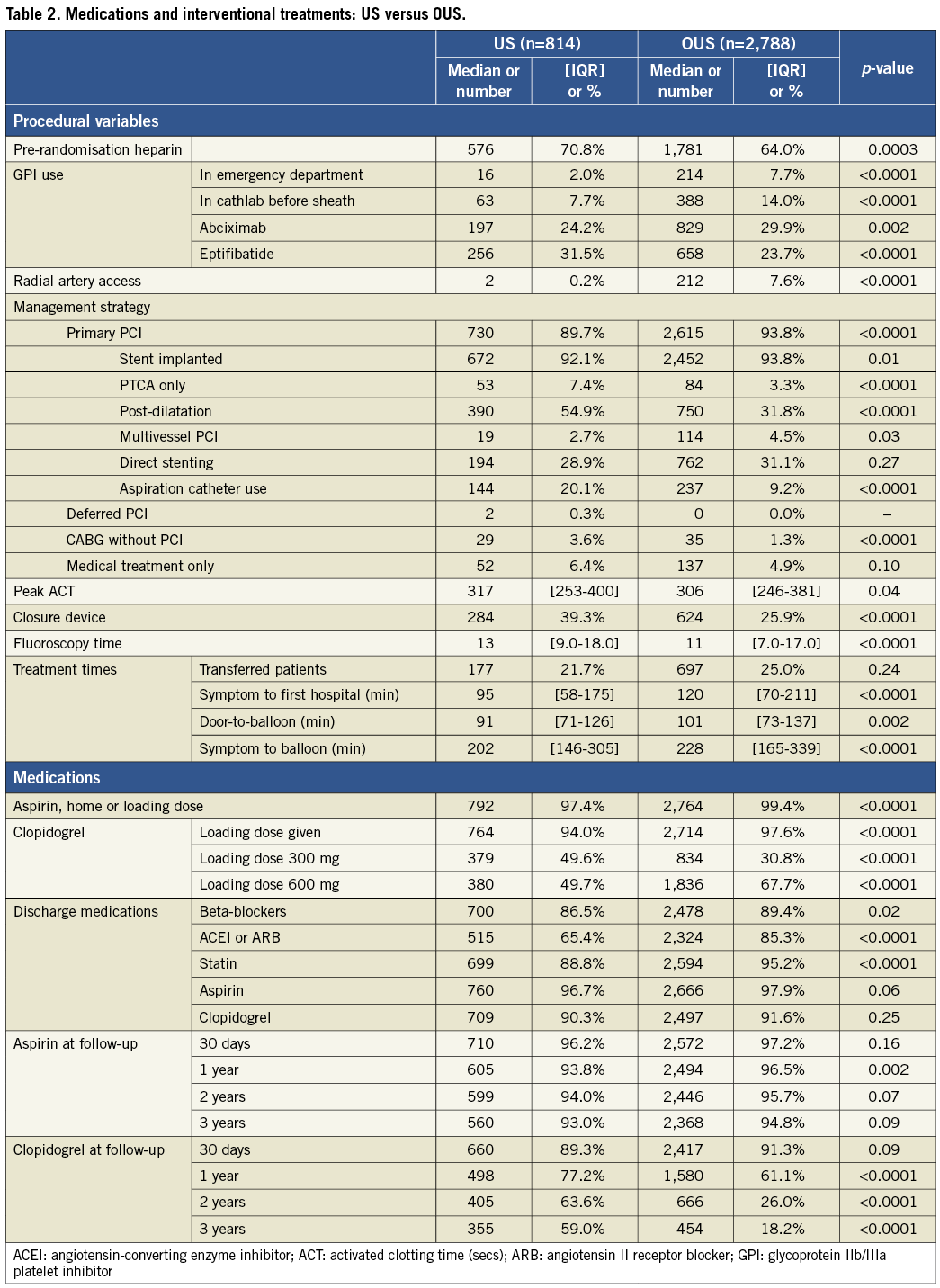
Medical and interventional treatments varied between US and OUS patients (Table 2). OUS patients had less use of pre-randomisation heparin, more upfront use of GPI in the emergency department and in the catheterisation laboratory before sheath insertion, more abciximab and less eptifibatide use, greater use of radial access, more primary PCI and stenting performed, and more multivessel PCI. OUS patients also had lower ACTs, shorter fluoroscopy times, less use of aspiration thrombectomy, less post-dilatation, less use of access closure devices, and less frequent performance of bypass surgery. OUS patients had longer times from symptom onset to first hospital arrival, longer door-to-balloon times and longer reperfusion times. OUS patients were more frequently given loading doses of aspirin and clopidogrel and more often received the higher 600 mg loading dose of clopidogrel. At hospital discharge, OUS patients had more frequent use of beta-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and statins. OUS patients had higher rates of aspirin use at one and two years, but lower clopidogrel use at one, two, and three years.
CLINICAL OUTCOMES
Patients at US sites had higher rates of adverse events than those treated in other countries (Table 3). At three years, US patients had the highest incidence of NACE, MACE, reinfarction and major non-CABG bleeding. Patients from the US had the second highest incidence of death, stroke, and stent thrombosis, and the third highest incidence of ischaemic target vessel revascularisation.
At 30 days, US versus OUS patients had significantly higher unadjusted rates of death, non-CABG-related major bleeding, MACE and NACE (Table 4). After adjusting for differences in baseline risk, US site enrolment was independently associated with increased 30-day rates of non-CABG-related major bleeding, MACE and NACE.
At three years, US patients had significantly higher unadjusted rates of death, reinfarction, stroke, major non-CABG bleeding, stent thrombosis, MACE and NACE compared to patients enrolled at sites outside the US (Table 4, Figure 1- Figure 6). All of these differences, except stent thrombosis, persisted after adjusting for differences in baseline risk.
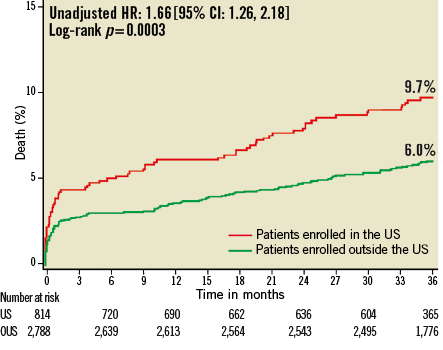
Figure 1. Kaplan-Meier estimates of cumulative frequency of mortality up to 3 years following primary PCI for STEMI comparing events at US versus OUS sites. P-values are univariate log-rank p-values, and hazard ratios and 95% confidence intervals are unadjusted values using univariate Cox regression.
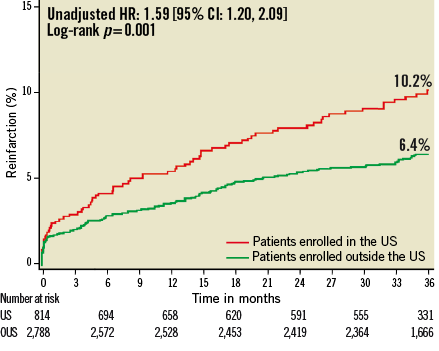
Figure 2. Kaplan-Meier estimates of cumulative frequency of reinfarction up to 3 years following primary PCI for STEMI comparing events at US versus OUS sites.
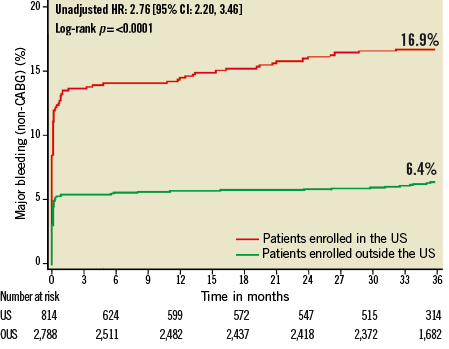
Figure 3. Kaplan-Meier estimates of cumulative frequency of major bleeding (non-CABG) up to 3 years following primary PCI for STEMI comparing events at US versus OUS sites.
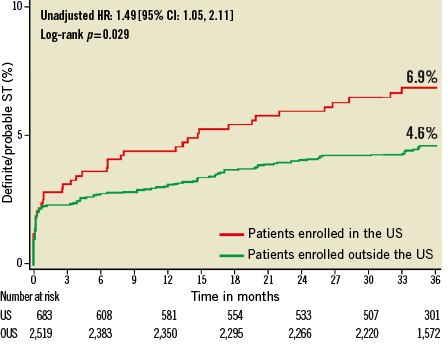
Figure 4. Kaplan-Meier estimates of cumulative frequency of stent thrombosis (ARC definite or probable) up to 3 years following primary PCI for STEMI comparing events at US versus OUS sites.
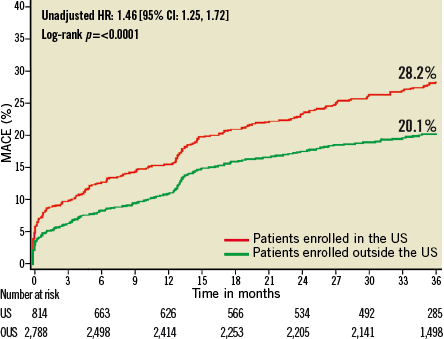
Figure 5. Kaplan-Meier estimates of cumulative frequency of MACE (major adverse cardiac events) up to 3 years following primary PCI for STEMI comparing events at US versus OUS sites.
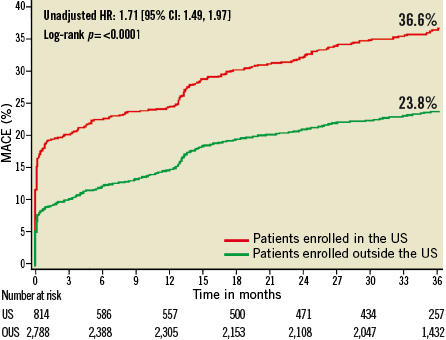
Figure 6. Kaplan-Meier estimates of cumulative frequency of NACE (net adverse clinical events) up to 3 years following primary PCI for STEMI comparing events at US versus OUS sites.
PHARMACOLOGIC RANDOMISATION OUTCOMES AT US VERSUS OUS SITES
As previously reported, at 30 days in the entire cohort, randomisation to bivalirudin rather than heparin plus GPI resulted in significantly lower mortality, major bleeding and NACE7. Also as previously reported, at three years in the entire cohort, treatment with bivalirudin resulted in significantly lower mortality, reinfarction and major bleeding, and a trend for lower NACE8. There were no significant interactions between the treatment effects with bivalirudin compared to heparin plus GPI and enrolment at US versus OUS sites for any of the 30-day or three-year outcomes (Table 5).
Discussion
The major findings of our study are that among patients with STEMI undergoing primary PCI in the HORIZONS-AMI trial: 1) US compared to OUS patients had higher early and late rates of adverse events, including death, reinfarction, stroke, major bleeding, MACE and NACE, differences which persisted after adjustment for differences in baseline risk profile and baseline treatment; and 2) the beneficial treatment effects of bivalirudin compared with UFH and GPI were consistent at US and OUS sites.
There are surprisingly little data from previous studies comparing outcomes in the US with outcomes outside the US in patients with STEMI, and the data which exist have been reported mostly from the thrombolytic era with short-term follow-up4-6. Van de Werf and colleagues reported from the GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) trial that adjusted 30-day mortality was slightly lower at US versus OUS sites4. There were no significant differences in treatment effects between tissue plasminogen activator and streptokinase at US versus OUS sites. Giugliano and colleagues reported from the In-TIME-II (Intravenous nPA for Treatment of Infarcting Myocardium Early II) trial that US sites had lower unadjusted 30-day mortality compared with Western Europe, Eastern Europe and Latin America5. After adjustment for differences in baseline variables, US sites had significantly lower mortality than Latin America but there was no significant difference in mortality compared with Eastern and Western Europe. These authors documented significant regional variations in the use of medications and revascularisation procedures. Barbash and colleagues reported from the International Tissue Plasminogen Activator/Streptokinase trial that there were wide variations in outcomes between different countries in STEMI patients, but the authors did not identify the countries6.
In contrast to these previous studies that were performed in the pharmacologic reperfusion era, the present study, which enrolled STEMI patients targeted for primary PCI, found that patients treated at US sites compared to OUS sites had significantly higher early and late adverse event rates. Understanding the possible reasons for these differences is essential to improving clinical outcomes. There are several possible explanations for these differences. First, there are considerable differences in the baseline patient risk profile and management strategies between US and OUS sites. Patients enrolled at US sites had more frequent comorbid conditions, including hypertension, diabetes and obesity, were more likely to have had prior MI and prior coronary artery bypass surgery, had lower left ventricular ejection fraction, and less TIMI 2-3 flow on initial angiography. Differences in infarct artery distribution and haematologic parameters were also present. Following coronary arteriography, US sites performed less primary PCI and more CABG than OUS sites, and radial access was used less often. OUS patients were more often transferred from non-PCI hospitals and had longer door-to-balloon times and longer reperfusion times. US patients were more likely to receive a 300 mg rather than a 600 mg loading dose of clopidogrel. Frequency of radial access use and aspiration also varied according to geography. US patients were less likely to receive beta-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and statins at discharge but were more likely to be maintained on clopidogrel for three years. Other differences in baseline risk and treatments were also apparent. After adjusting for these differences in baseline and treatment variables, outcomes of US patients were still significantly worse. Although the HORIZONS-AMI trial collected extremely detailed data on clinical, angiographic and treatment variables, unmeasured confounders may exist which could impact on outcomes. For example, the extent to which genetic differences across geographies were present, impacting on drug metabolism or future plaque rupture, is unknown.
Second, although all events were monitored on-site by independent study personnel, and source documents were reviewed for event adjudication, it is possible that differences in thresholds for event reporting and documentation could explain some of the differences between US and OUS sites. While such an occurrence is unlikely to explain differences in mortality, varying thresholds for event documentation or biomarker assessment could in part explain differences in bleeding and reinfarction, as suggested in prior studies10.
Finally, valid differences in systems of care and treatments between US and OUS sites may affect patient outcomes. OUS sites had a higher percentage of patients transferred from non-PCI hospitals and had longer door-to-balloon times, suggesting more centralised systems of care for primary PCI. Although longer door-to-balloon times may compromise outcomes, centralised systems of care may contribute to improved outcomes at OUS sites. OUS patients were more likely to receive up-front and loading doses of medications including GPI, aspirin and clopidogrel. OUS patients were more often treated with radial access and had more PCI and more stents used and less bypass surgery. OUS patients had lower ACTs which may have contributed to less bleeding. OUS patients more often received recommended discharge medications including beta-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and statins. US patients had a shorter length of stay compared with OUS patients, and there is recent evidence that a shorter length of stay may be correlated with an increased incidence of hospital readmissions11. Shorter length of stay may potentially affect post-hospital outcomes, although we have no data to support this. Patient compliance following hospital discharge varies from country to country and may affect outcomes, although we have little data to evaluate this.
In contrast to the overall difference in outcomes of patients enrolled at US vs. OUS sites, we did not find any significant differences in the relative treatment effects of bivalirudin versus heparin plus GPI between the US and OUS sites. This is in contradistinction to the PLATO (Platelet Inhibition and Patient Outcomes) trial which evaluated ticagrelor in patients with acute coronary syndromes and found a beneficial effect on outcomes at OUS sites but a trend towards worse outcomes at US sites, possibly related to geographic differences in chronic aspirin dosing12.
Limitations
Although comparison of outcomes at US versus OUS sites was prespecified, the present study is a non-powered subgroup analysis from a randomised trial and as such has all the limitations of subgroup analyses including unmeasured confounders that may differentially affect outcomes in US and OUS patients. The extent to which consecutive patients were enrolled in the different geographies is also uncertain, potentially contributing to selection bias. As such, these results should be considered hypothesis-generating, and require confirmation from other large databases.
Clinical implications
Our study has several important research and patient care-related implications. Considerable geographical variation exists regarding patient demographic, clinical and angiographic characteristics, management strategies and outcomes which may be important in the design and site selection for future randomised STEMI trials. Ethnic minorities were more represented at US than OUS sites but were under-represented at all sites. Attempts should be made in future trials to select sites with greater ethnic minority representation. Consideration should be given to standardising non-study treatments in order to isolate the effect of a randomised therapy. Although our study did not show any major regional interactions in the beneficial effects of treatment with bivalirudin versus heparin plus GPI, the variation in baseline risk profile and practice patterns in the US versus OUS countries could impact on the effect of other therapies.
Our study does not permit firm conclusions as to whether the higher event rates among US patients are related to unmeasured confounders between US and OUS patients or valid differences in systems of care and treatment which may meaningfully impact on outcomes. Since the latter is a real possibility, this study should provide a strong stimulus for further research regarding how our systems of STEMI care, both acute and long-term, may be improved.
Funding
This study is funded by Boston Scientific, Natick, MA, USA, and The Medicines Company, Parsippany, NJ, USA.
Conflict of interest statement
B. Witzenbichler has received lecture honoraria from The Medicines Company and Boston Scientific. C. Metzger is a consultant to Abbott, Cordis, IDEV, Medtronic, and Pathway and has received a speaker’s honorarium from The Medicines Company. G. Guagliumi is a consultant for Boston Scientific, Cordis and Volcano and has received research grants from Medtronic, Boston Scientific, Abbott Vascular and LightLab Imaging. M. Kellett has received research support from Abbott and Boston Scientific and has a small equity position with CSI. T. Stuckey is on an advisory board for Boston Scientific Medical, and has received speaker honoraria from Boston Scientific. R. Mehran has served as a consultant for Abbott, AstraZeneca, Ortho-McNeil, Regado, and has research grants from Bristol-Meyers-Squibb and Sanofi. G. Stone is a consultant to Boston Scientific and The Medicines Company. B. Brodie, P. Tobbia, J. Yu and M. Fahy have no conflicts of interest to declare.

