
Abstract
Aims: The aim of the study was to compare outcomes in unfractionated heparin (UFH) and bivalirudin-treated patients undergoing primary percutaneous coronary intervention (PPCI).
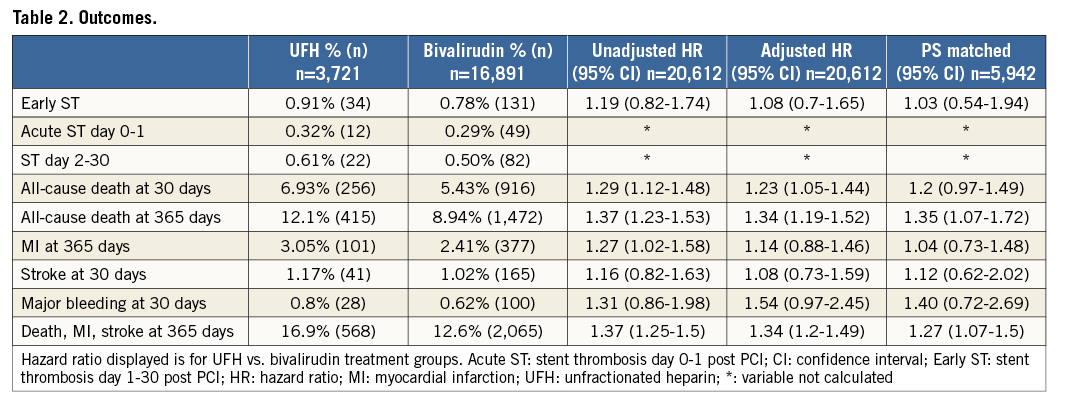
Methods and results: This observational study contained 20,612 PPCI patients treated with either UFH monotherapy or bivalirudin with or without concomitant UFH. Patients with oral anticoagulant or glycoprotein IIb/IIIa inhibitor (GPI) treatment were excluded. The primary outcome measure was definite early stent thrombosis (ST) that occurred at low and similar rates in UFH only and bivalirudin-treated patients: 0.9% vs. 0.8% (adjusted hazard ratio [HR] 1.08, 95% confidence interval [CI]: 0.7-1.65). All-cause death at 30 days occurred in 6.9% vs. 5.4% of patients (adjusted HR 1.23, 95% CI: 1.05-1.44) and within 365 days in 12.1% vs. 8.9% (adjusted HR 1.34, 95% CI: 1.19-1.52) in the two groups, respectively. The incidence of major bleeding within 30 days was 0.8% vs. 0.6% (adjusted HR 1.54, 95% CI: 0.97-2.45). The incidence of reinfarction within 365 days and stroke within 30 days was similar between groups.
Conclusions: In this large, nationwide observational study we found low and similar rates of early ST in UFH only and bivalirudin-treated patients undergoing primary PCI. Mortality was higher in UFH compared with bivalirudin-treated patients.
Abbreviations
ACS: acute coronary syndrome
CABG: coronary artery bypass graft
CI: confidence interval
GPI: glycoprotein IIb/IIIa inhibitor
HR: hazard ratio
MI: myocardial infarction
NPR: National Patient Register
PPCI: primary percutaneous coronary intervention
RCT: randomised clinical trial
SD: standard deviation
ST: stent thrombosis
STEMI: ST-elevation myocardial infarction
SWEDEHEART: Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies
UFH: unfractionated heparin
Introduction
Potent adjunctive antithrombotic agents are crucial for the treatment of ST-elevation myocardial infarction (STEMI) with primary PCI (PPCI). Periprocedural parenteral antithrombotic treatment choices include anticoagulation with bivalirudin, unfractionated heparin (UFH) or enoxaparin, and antiplatelet treatment with cangrelor and glycoprotein IIb/IIIa inhibitors (GPI). Bivalirudin, as compared to UFH with or without GPI, improved event-free survival at 30 days and reduced major bleeding, but also increased the risk of acute stent thrombosis (ST) in a pivotal PPCI STEMI trial1. Mainly based on these results, the American College of Cardiology/American Heart Association and European Society of Cardiology have included bivalirudin in STEMI treatment guidelines2-4. The majority of subsequent trials have shown that bivalirudin as compared with UFH with or without GPI results in lower bleeding risk but increased rates of ST5-7.
One open-label, single-centre trial that evaluated UFH alone as compared with periprocedural bivalirudin alone showed a lower rate of major adverse ischaemic events, including ST at 30 days, with UFH8. Interestingly, bleeding rates were similar with UFH and bivalirudin. As a result of that trial, the advantages of bivalirudin vs. UFH in PPCI have been questioned. In this observational study, we compared ST, reinfarctions, stroke, all-cause mortality and major bleeding in a large contemporary Swedish PPCI STEMI population treated with either UFH only or bivalirudin (with or without concomitant UFH).
Methods
STUDY POPULATION AND OUTCOME MEASURES
Patients were identified using the Swedish Coronary Angiography and Angioplasty Register (SCAAR), part of the Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) register9. We included all STEMI patients undergoing PPCI, treated with bivalirudin and/or UFH in Sweden, from January 2007 until December 2014. Exclusion criteria were concomitant GPI or oral anticoagulant treatment. The study population (n=20,612) was divided into two groups, those treated with bivalirudin, with or without concomitant UFH (n=16,891), and those treated with UFH only (n=3,721) (Figure 1). The primary outcome measure was definite stent thrombosis within 30 days of PPCI (early ST). Secondary outcome measures included all-cause mortality within 30 and 365 days, reinfarction within 365 days, stroke within 30 days and major bleeding within 30 days. Drug dosage and duration are not registered in SCAAR and not reported in this study.
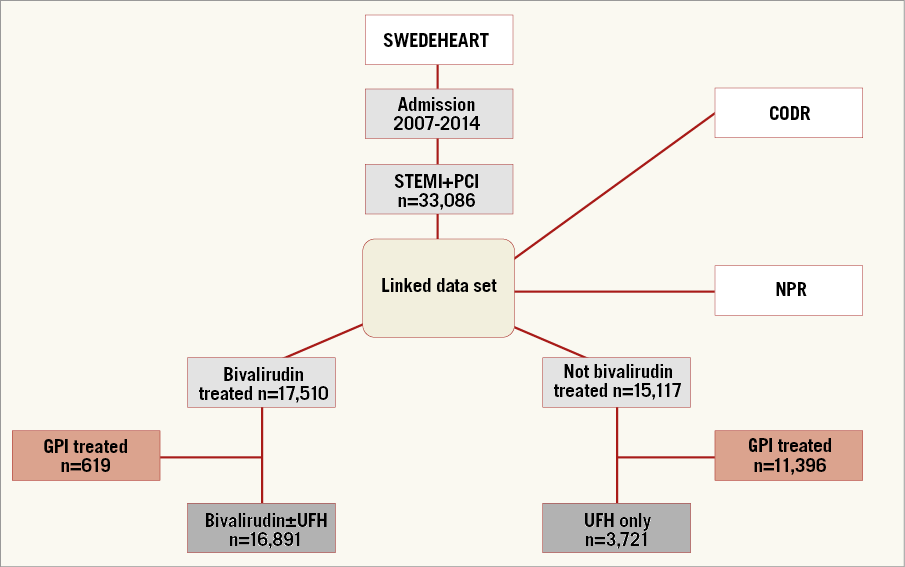
Figure 1. Patient population flow chart. CODR: National Board of Health and Welfare’s Cause of Death Register; GPI: glycoprotein IIb/IIIa inhibitor; NPR: National Patient Register; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction; UFH: unfractionated heparin
DATA COLLECTION
The SCAAR/SWEDEHEART registry includes baseline characteristics, angiographic findings, procedural data and index hospitalisation medications. The interventionist enters all information. Restenosis and ST are actively recorded by mandatory questions for each stent at any subsequent coronary angiography. Data from SWEDEHEART were merged with the National Board of Health and Welfare’s Cause of Death Register and the National Patient Register (NPR)10, providing mortality, stroke and major bleeding data. The study complies with the Declaration of Helsinki. Uppsala regional ethics committee approved the protocol (reference number 2011/333/2).
DEFINITIONS
SCAAR applies the universal definition of myocardial infarction and the Academic Research Consortium definition of definite ST11.
Major bleeding was defined as any major bleeding reported in the NPR as a hospitalisation discharge diagnosis of: intracerebral, subarachnoidal or subdural haematoma; gastrointestinal tract bleeds including oesophageal, gastric, duodenal, lower gastrointestinal tract and rectal bleeds; haematemesis or melena. Stroke was defined as any stroke reported in the NPR as cerebral infarction, intracerebral haematoma or subarachnoidal haemorrhage.
STATISTICAL ANALYSES
Continuous variables were presented as mean±SD or median (interquartile range) and categorical variables as numbers (percentages). Incidences were estimated by the proportion of patients with an outcome. Corresponding 95% confidence intervals were calculated using normal approximation. Adjusted analyses were performed using:
1) propensity scores as covariate in the Cox model with multiple imputation on missing data;
2) propensity score-matched cases with multiple imputed data sets using robust sandwich estimators to estimate the variance of the coefficients accounting for the matched pairs (sensitivity analyses).
When analysing reinfarction within 365 days, an initial 28-day blanking period was applied at discharge in order to avoid multiple inclusion of one event12. From 2010, the registry was modified to overcome the uncertainty whether a new hospitalisation for myocardial infarction was a true recurrent event or merely a registration related to inter-hospital transfer. A separate sensitivity analysis for reinfarction within 365 days was added for the period 2010-2014. Statistical analyses were performed using R statistical programme version 3.1.3 (The R Foundation, Vienna, Austria).
Results
BACKGROUND CHARACTERISTICS
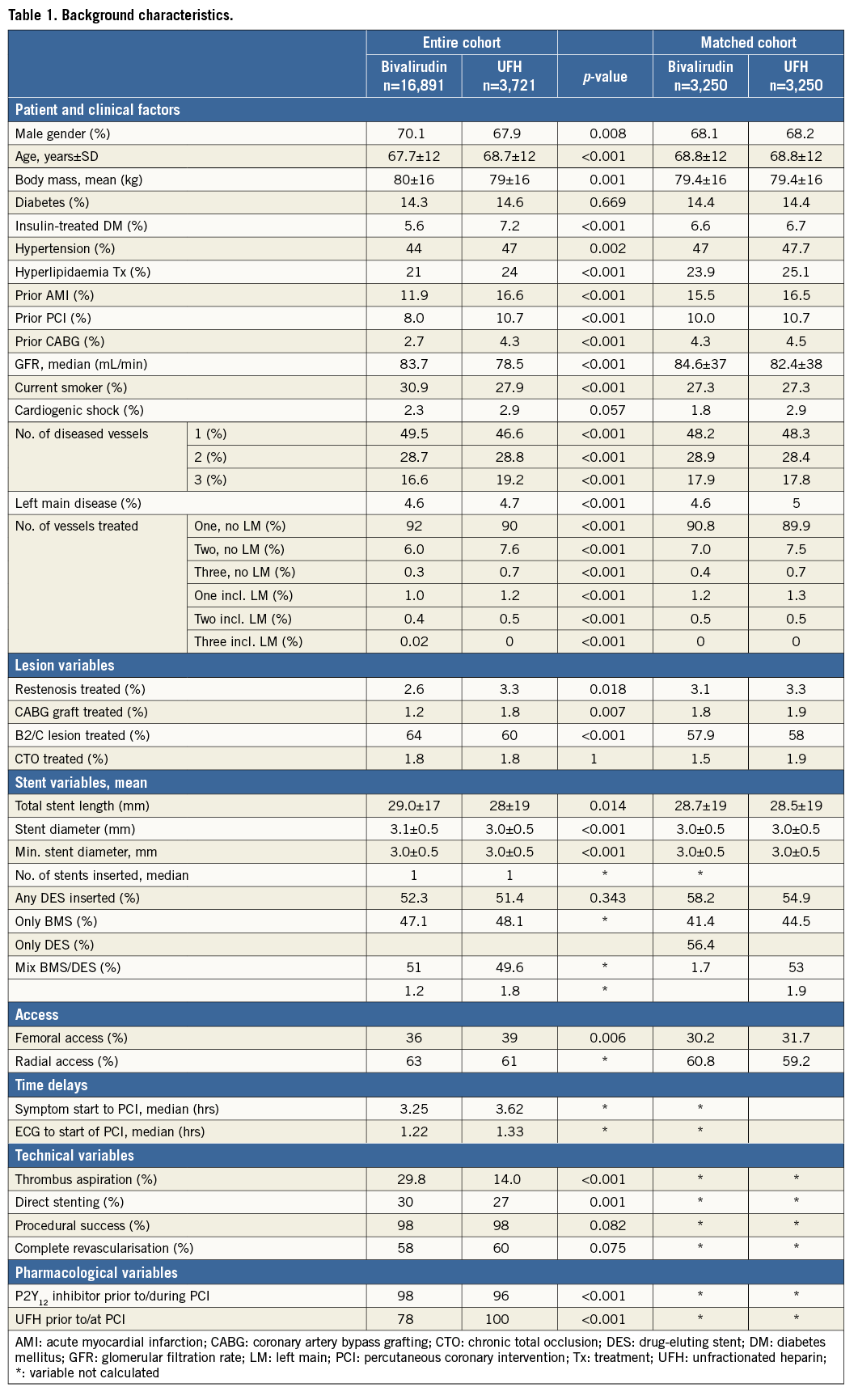
The treatment groups differed in baseline and procedural variables. UFH only treated patients were older, less likely to be male and generally at higher risk with more prior MI, PCI, CABG and longer time delays to PPCI. Thrombus aspiration and direct stenting were less frequently used. On the other hand, they were less often smokers and tended to have less severe lesions. Propensity score matching with imputed data sets generated very similar data groups (Table 1).
EARLY STENT THROMBOSIS
The crude incidence of early ST was low and similar in UFH only (0.91%, n=34) and bivalirudin (0.78%, n=131) treated patients (Figure 2, Table 2). Acute ST (day 0-1 post PCI) (0.32%, n=12 vs. 0.29%, n=49) and subacute ST (0.61%, n=22, vs. 0.50%, n=82) also occurred at similar rates. After multivariable adjustment using propensity score matching with an imputed data set, there was no difference in early ST: HR 1.08 (95% CI: 0.7-1.65). Also, using propensity score-matched groups with an imputed data set, there was no difference between the groups: HR 1.03 (95% CI: 0.54-1.94). Event numbers were too low to perform adjusted analyses for acute and subacute ST separately.
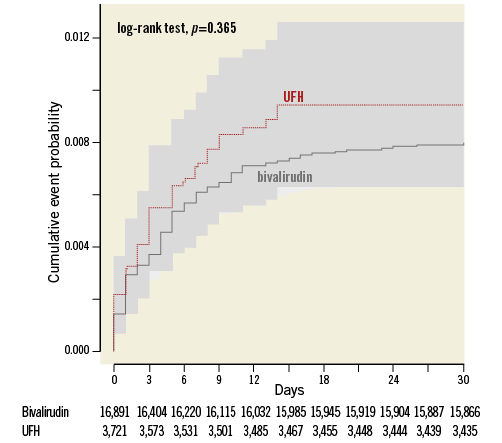
Figure 2. Stent thrombosis within 30 days of PPCI. Kaplan-Meier curve of early stent thrombosis. UFH: unfractionated heparin; x-axis: number of days post PPCI; y-axis: proportion of individuals with stent thrombosis
MORTALITY, REINFARCTION, STROKE AND MAJOR BLEEDING
The crude incidence of all-cause death at 30 days was higher in the UFH only as compared with bivalirudin-treated patients (6.93%, n=256 vs. 5.43%, n=916) (Figure 2-Figure 4, Table 2). The difference remained after adjustment: HR 1.23 (95% CI: 1.05-1.44) and with matched groups: HR 1.2 (95% CI: 0.97-1.49). Similarly, the crude incidence of all-cause death at 365 days was higher in the UFH only as compared with bivalirudin-treated patients (12.1%, n=256 vs. 8.94%, n=1,472). The difference remained with adjustment: HR 1.34 (95% CI: 1.19-1.52) and with matched groups: HR 1.35 (95% CI: 1.07-1.72) (Table 2).
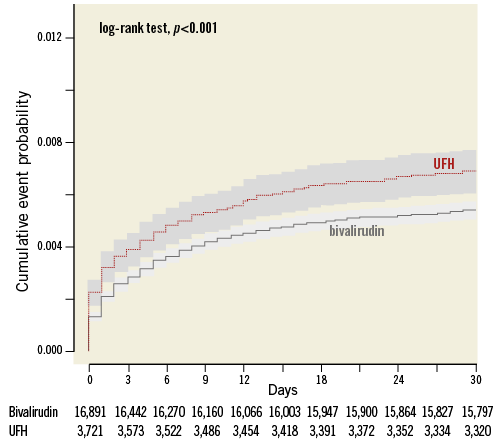
Figure 3. All-cause death within 30 days of PPCI. Kaplan-Meier curve of all-cause mortality within 30 days of PPCI. UFH: unfractionated heparin; x-axis: days post PPCI; y-axis: proportion of individuals diseased
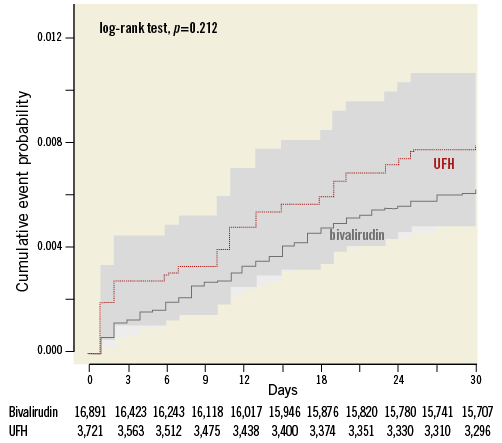
Figure 4. Major bleeding within 30 days of PPCI. Kaplan-Meier curve of major bleeding within 30 days of PPCI. UFH: unfractionated heparin; x-axis: days post PPCI; y-axis: proportion of individuals with major bleeding
The crude incidence of reinfarction within 365 days was higher in UFH only (3.05%, n=101) than bivalirudin-treated patients (2.41%, n=377) but with adjustment (HR 1.14 [95% CI: 0.88-1.46]) and with matched groups (HR 1.04 [95% CI: 0.73-1.48]) no significant difference remained (Table 2). A separate sensitivity analysis for reinfarction within 365 days was added for the period 2010-2014, with no difference between treatment groups: adjusted HR 1.13 (95% CI: 0.86-1.47).
The crude incidence of stroke within 30 days of PCI was similar with UFH (1.17%, n=41) and bivalirudin (1.02%, n=165), with no difference after adjustment (HR 1.08 [95% CI: 0.73-1.59]) or with matched groups (HR 1.12 [95% CI: 0.62-2.02]) (Table 2).
The crude incidence of major bleeding within 30 days was higher in the UFH only (0.80%, n=28) as compared with the bivalirudin-treated patients (0.62%, n=100), but the difference did not remain after adjustment (HR 1.54 [95% CI: 0.97-2.45]) or with matched groups (HR 1.40 [95% CI: 0.72-2.69]) (Table 2).
Discussion
In this large, nationwide observational study we found low and similar rates of early ST in UFH only and bivalirudin-treated patients undergoing primary PCI. Mortality was higher in UFH only compared with bivalirudin-treated patients. There were no differences in major bleeding, reinfarction or stroke.
Although randomised clinical trials are the gold standard for efficacy and safety, they have inherent limitations of selected enrolment and often limited sample size. Post-approval real-world registries may provide valuable supplementary data and important external validity to findings of randomised trials. SCAAR is a high-quality register, providing reliable data from a nationwide population, reflecting contemporary real-world practice in a high-risk, all-comer population. A mix of planned and bail-out GPI use in study arms limits many trials comparing UFH and bivalirudin.
MORTALITY
A recent meta-analysis of 14,000 randomised PPCI STEMI patients showed that bivalirudin, as compared to UFH with or without GPI, reduced the risk of all-cause mortality, cardiac mortality and major bleeding, but yielded comparable rates of major adverse cardiac events and net adverse clinical events at 30 days13. This meta-analysis contains several studies with marked differences in GPI use between study arms. Other meta-analyses with more balanced GPI exposure yield results with no mortality benefit for bivalirudin14-16. A recent UK registry report of more than 60,000 PPCI patients treated with either UFH/GPI or bivalirudin could not detect any mortality advantage with bivalirudin17. However, the study found a higher mortality in patients treated with UFH only. Our study results are consistent with the UK report, as we found higher all-cause mortality at 30 and 365 days associated with UFH only, compared with bivalirudin treatment.
STENT THROMBOSIS
A majority of randomised trials1,5,6,8 and several meta-analyses14-16 have shown increased ST rates with bivalirudin compared to UFH/GPI treatment. In our observational study, we found a very low incidence of early ST in both groups. Noted differences in ST rates between other trials and our study probably relate to pre- and post-randomisation patient characteristics, hospital treatment, downstream adverse event management, adherence to and continuation of key medications. Patient characteristics such as diabetes, smoking status, degree of coronary disease, previous AMI and PCI, parenteral antithrombotic treatment strategy, type or absence of P2Y12 inhibition, stent properties and appropriate stent deployment are all factors of documented importance.
Ticagrelor reduces ST rates as compared with clopidogrel across all ACS modalities18. Prehospital ticagrelor loading is common in Sweden19, and may partly explain the low ST rates20. The unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention trial (HEAT-PPCI) used only in-hospital ticagrelor loading and reported higher ST rates than other randomised clinical trials comparing UFH and bivalirudin8. Moreover, no concomitant UFH was used with bivalirudin, a practice that has been linked to increased ST rates in clinical trials and registries5,21,22. Bivalirudin was stopped at termination of PCI in HEAT-PPCI. Post-PCI continuation of bivalirudin, at PCI dose, is common in Sweden, but not reported in SCAAR. In the bivalirudin vs. heparin with or without tirofiban during primary percutaneous coronary intervention in acute myocardial infarction (BRIGHT) trial, prolonged bivalirudin infusion at PCI dose was postulated as an explanation for the similar ST rates observed with UFH and bivalirudin7. Risk of ST was not affected by prolonged bivalirudin infusion in the Minimizing Adverse Haemorrhagic Events by Transradial Access Site and Systemic Implementation of Angiox (MATRIX) trial6. An exploratory analysis of the MATRIX trial suggested a relationship between dose during prolonged infusion and ST risk. A post hoc analysis of the European Ambulance Acute Coronary Syndrome Angiography (EUROMAX) trial suggested that prolonging the bivalirudin infusion at PCI dose mitigates the risk of ST23. Optimal dose/duration to minimise ST remains to be ascertained.
The HEAT-PPCI combination of in-hospital ticagrelor loading, bivalirudin without UFH and absence of prolongation may allow ST, as platelet inhibition may not have reached sufficient levels of inhibition at bivalirudin termination.
Frequent prehospital ticagrelor, concomitant UFH with bivalirudin (78%) and bivalirudin prolongation may reduce Swedish ST rates. Future use of the parenteral platelet inhibitor cangrelor with a predictable fast onset may further reduce acute ST24.
BLEEDING
Trials with major differences in GPI exposure to treatment arms have consistently shown decreased bleeding rates with bivalirudin, as compared with UFH/GPI1,5,6. Trials with balanced GPI exposure yield diverging results, with less clear bleeding advantages or even disadvantages for bivalirudin7,8. A recent real-world registry reported numerically higher bleeding rates in UFH monotherapy compared with bivalirudin-treated patients25. We excluded all GPI-treated patients and found no statistically significant differences in major bleeding. However, there was a trend towards more major bleeding in the UFH only treated patients. Although residual confounding may be the full explanation, we speculate that bleeding may be a contributing factor to our observed higher all-cause mortality in UFH only treated patients.
Limitations
The bivalirudin and UFH subgroups were different in clinical baseline characteristics. Despite propensity score adjustment and matching, residual confounding cannot be excluded. Inherent to all observational studies, there is a chance of under-reporting of events such as stent thrombosis and bleeding. Probable and possible ST events have not been included.
Exact drug dosage and duration are unknown in this study since they are not reported in SCAAR, although assumed to follow national and European guidelines. Unknown dosage is a major limitation, particularly for UFH with its narrow therapeutic range. Activated clotting time measurements are unknown. The exclusion of GPI-treated patients is a potential limitation, our primary reason being uncertainty as to whether GPI was a primary strategy or bail-out option. We acknowledge that bail-out GPI may have been used more frequently in either the UFH or bivalirudin-treated patients, which may have introduced a selection bias. In addition, bail-out GPI is used in high-risk cases with presumably large thrombus burden, possibly more exposed to stent thrombosis and other adverse events, leaving us with a lower-risk population. We recently published a descriptive report on ST rates in Sweden 2007-2014, reporting low early ST rates regardless of UFH, bivalirudin or GPI treatment26. Thus, excluding GPI in this study does not seem to have greatly affected rates of ST.
Conclusions
This large nationwide observational study shows overall low ST rates without any signals of increased ST rates associated with bivalirudin treatment. We found higher all-cause mortality in the UFH only as compared to bivalirudin-treated patients. Further randomised trials are needed to settle the uncertainty of optimal antithrombotic strategy in PPCI.
| Impact on daily practice This contemporary, large, nationwide observational study provides reassuring real-world data for the use of bivalirudin in STEMI patients treated with PPCI. We found that UFH compared with bivalirudin was associated with higher mortality at 30 and 365 days. The rates of early ST were low and similar with UFH and bivalirudin, and there were no differences in the rates of stroke, reinfarction and major bleeding. |
Funding
Uppsala Clinical Research Center has received institutional grants from The Medicines Company to support this work.
Conflict of interest statement
P. Grimfjärd reports institutional grants from The Medicines Company and lecture fees from AstraZeneca. D. Erlinge reports speaker fees from The Medicines Company and AstraZeneca. S. Koul reports lecture fees from AstraZeneca. B. Lagerqvist reports institutional grants from AstraZeneca. B. Svennblad reports institutional grants from AstraZeneca, The Medicines Company and Novartis. C. Varenhorst reports institutional grants from AstraZeneca and The Medicines Company, lecture and advisory board fees from AstraZeneca, The Medicines Company and Boehringer Ingelheim, lecture fees from Bayer, CSL Behring, Bristol Myers Squibb, Pfizer and is on the Clinical Endpoint Committee for Pfizer, Bristol Myers Squibb, Philips and AstraZeneca. S.K. James reports institutional grants and honoraria from AstraZeneca, The Medicines Company, Abbott and Boston Scientific.

