Abstract
Background: Unfractionated heparin (UFH) is frequently administered before percutaneous coronary intervention (PCI) in patients with ST-segment elevation myocardial infarction (STEMI).
Aims: The aim of the study was to investigate if pretreatment with UFH prior to arrival at the catheterisation laboratory affects coronary artery occlusion, mortality, and in-hospital major bleeding in patients with STEMI undergoing PCI.
Methods: Patients with a first STEMI event undergoing PCI between 2008 and 2016 were extracted from the Swedish Coronary Angiography and Angioplasty Registry. Risk ratios for UFH pretreatment versus no pretreatment regarding coronary artery occlusion at presentation in the catheterisation laboratory, 30-day mortality, and bleeding were obtained using adjusted Poisson regression models with robust standard errors. Analyses of propensity score (PS)-matched groups were performed to obtain absolute risk differences.
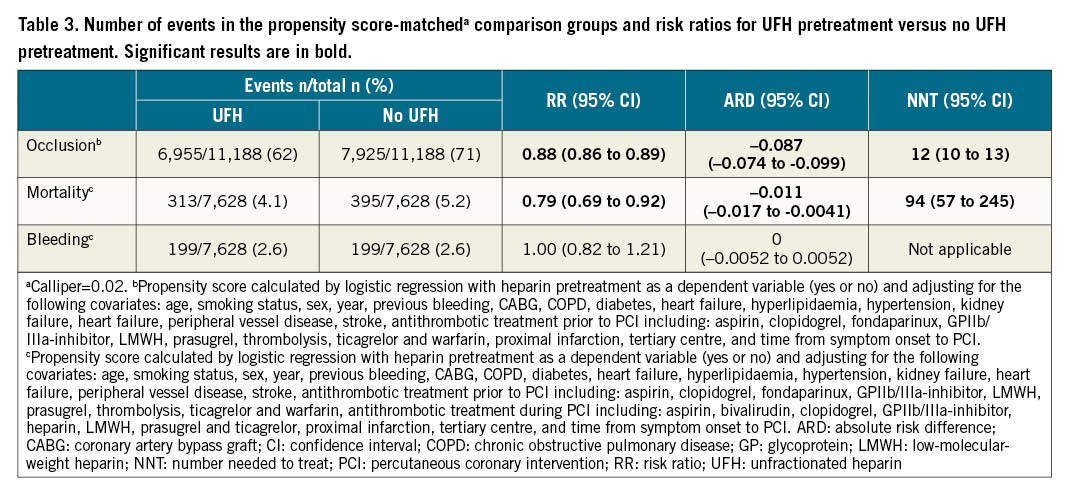
Results: In all, 41,631 patients were included, 16,026 (38%) with and 25,605 (62%) without UFH pretreatment. Adjusted risk ratios were 0.89 (95% confidence interval [CI]: 0.87 to 0.90) for coronary artery occlusion, 0.87 (0.77 to 0.99) for mortality, and 1.01 (0.86 to 1.18) for bleeding. In the PS-matched analyses, the absolute risk differences were −0.087 (−0.074 to −0.099) for coronary artery occlusion, −0.011 (−0.017 to −0.0041) for mortality, and 0 (−0.0052 to 0.0052) for bleeding.
Conclusions: Pretreatment with UFH was associated with a reduction in coronary artery occlusion among patients with STEMI, with a number needed to treat (NNT) of 12, without increasing the risk of major in-hospital bleeding. Regarding mortality, a reduction was found with UFH pretreatment, with an NNT of 94, but this effect was not robust over all sensitivity analyses and residual confounding cannot be excluded.
Introduction
Percutaneous coronary intervention (PCI) is the gold standard for reperfusion therapy in patients with ST-segment elevation myocardial infarction (STEMI). Pretreatment with unfractionated heparin (UFH) prior to arrival at the coronary catheterisation laboratory is often administered with the intention to improve spontaneous reperfusion rates and reduce clot burden1. Improved coronary blood flow prior to PCI has previously been shown to improve patient outcome2. After intravenous administration, the maximum effect of UFH is achieved within minutes. Furthermore, the half-life is short, between 1 and 2 hours. These characteristics, combined with the availability of an antidote, potentially makes UFH a good candidate for early administration in STEMI patients.
Scientific evidence regarding UFH pretreatment in patients with STEMI undergoing PCI is scarce. Only 1 small randomised controlled trial (RCT) dating back to the late 90s3 and 10 observational studies with varying results regarding patient-relevant outcomes exist1456789101112. Furthermore, evidence on absolute risk differences is sparse, restricted to 1 small non-randomised study4, and available evidence regarding mortality is inconclusive. This is reflected in current guidelines where UFH is endorsed for use during PCI, but there are no clear recommendations for UFH pretreatment prior to arrival at the coronary catheterisation laboratory1314. In Sweden, there are 30 centres performing PCI, each with their own local routines and traditions; no consensus has been reached regarding UFH pretreatment. Thus, the aim of the present study was to investigate relative risks and absolute risk differences for the clinical effects of UFH pretreatment, including coronary artery occlusion at presentation in the catheterisation laboratory, mortality at 30 days, and major in-hospital bleeding for patients with STEMI undergoing primary PCI.
Methods
In this cohort study, data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) and the National Patient Register were used. SCAAR is a Swedish nationwide register where data regarding myocardial infarctions (MI) from all hospitals with a cardiac intensive care unit as well as data from all coronary angiographies and PCI procedures in Sweden are recorded. Patient data are reported by the handling physician via a web interface. In 2020, the register had a coverage rate of 98.1%15, and data can be linked to other registers through patients’ unique Swedish personal identity number16. The Swedish National Patient Register contains International Classification of Diseases (ICD)-10 diagnoses from hospital admissions as well as from specialist outpatient care, with a 99% coverage of hospital discharges17.
The study population consisted of unique patients with a first STEMI event undergoing primary PCI during the study period from January 2008 to December 2016. The date of PCI was defined as the index date. Patients with chronic total occlusions, missing information regarding UFH pretreatment, and with rescue PCI following thrombolysis were excluded.
From SCAAR, data regarding age, sex, body mass index (BMI), smoking status, year of PCI, previous medical history (coronary artery bypass graft [CABG], diabetes, hyperlipidaemia, hypertension, MI), antithrombotic treatment before and during PCI, and the time from symptom onset to PCI were extracted. In Sweden, the dose of UFH pretreatment is often 5,000 U. However, the dose and the exact timing of the treatment is not recorded in the register. To reflect the severity of the STEMI as well as differences in procedure, the location of the infarction (proximal versus not proximal) and access (radial versus non-radial) were recorded. We also recorded whether the PCI was performed at a tertiary hospital, defined as a centre with cardiac surgery available 24/7. From the patient register, we extracted diagnoses before the index date: bleeding, chronic obstructive pulmonary disease (COPD), heart failure, kidney failure, peripheral vessel disease, and stroke.
Outcome definitions
The outcomes were coronary artery occlusion at presentation in the catheterisation laboratory, 30-day mortality, and major in-hospital bleeding. Coronary artery occlusion was determined by the PCI physician prior to the PCI. In-hospital major bleeding included the following events during the index hospitalisation: intracranial bleeding, a haemoglobin decrease >30 g/L caused by bleeding, bleeding leading to prolonged hospitalisation, or bleeding which required treatment other than compression.
Statistical analyses
We performed 2 adjusted statistical models using (i) a Poisson regression model (our main model) and (ii) a propensity score (PS)-matched model. For sensitivity analyses, we used multiple imputation followed by a PS-matched analysis, as well as inverse probability treatment weighting (IPTW).
Main analysis
Poisson regression with robust error margins (HC3) was used to obtain risk ratios with 95% confidence intervals (CI)18. Goodness-of-fit was investigated with the chi-squared test. In the adjusted models, we a priori identified age, smoking status, sex, hyperlipidaemia, hypertension, type of centre (tertiary versus not tertiary), and time from symptom onset to PCI as covariates. In addition, we adjusted for factors differing significantly (p-value<0.05) between patients with and without UFH pretreatment and with a prevalence of at least 2%. BMI was not included in the models as information was missing for 26% of the patients. For each outcome, we applied 1 crude and 3 adjusted models, with an increasing number of adjusting variables for each model. The first model was adjusted for demographics and previous medical history and the second model additionally included other antithrombotic treatment. In the third model, we adjusted for age, smoking status, sex, year, hyperlipidaemia, hypertension, previous bleeding, CABG, COPD, diabetes, heart failure, kidney failure, peripheral vessel disease, stroke, antithrombotic treatment prior to PCI (aspirin, clopidogrel, fondaparinux, glycoprotein [GP] IIb/IIIa-inhibitor, low-molecular-weight heparin [LMWH], prasugrel, thrombolysis, ticagrelor, and warfarin) and antithrombotic treatment during PCI (aspirin, bivalirudin, clopidogrel, GPIIb/IIIa-inhibitor, UFH, LMWH, prasugrel, and ticagrelor), proximal infarction, type of centre, and time from symptom onset to PCI. Antithrombotic treatment during PCI was included for the outcomes concerning mortality and bleeding, but not for coronary artery occlusion; treatment during PCI occurs after coronary artery occlusion has been determined. Furthermore, radial access was not adjusted for as this variable could be affected by the UFH treatment. However, a subgroup analysis on radial access was performed (see below). Tolerance tests were performed to investigate multicollinearity.
In the exploratory subgroup analyses, the outcomes were analysed according to access (radial or non-radial), sex (male or female), weight (<60 kg or ≥60 kg), diabetes (yes or no), age (<75 or ≥75 years), previous MI (yes or no), extent of the disease (one-vessel or multiple-vessel disease), and vessel affected (left anterior descending artery, left circumflex artery, right coronary artery, and other vessels). The fully adjusted model was applied. P-values for interaction were calculated in the subgroup analyses.
PS-matched analysis
To calculate the propensity score (PS), logistic regression, with UFH pretreatment as the dependent variable, was performed using all variables from the most adjusted Poisson model. We calculated 2 PS, one for analysing coronary artery occlusion and one for mortality and bleeding. For the outcome coronary artery occlusion, as opposed to mortality and bleeding, antithrombotic treatment during PCI was not included, since treatments during PCI occur after the occurrence of coronary artery occlusion has been established. Thereafter, 2 PS-matched datasets were generated, one for analysing coronary artery occlusion and one for mortality and bleeding. Calliper PS matching with nearest match was used. A calliper value of 0.02 was applied after testing values between 0.01 and 0.5 and looking at the distribution of PS between the groups and balance of covariates (data not shown). After matching, the groups were balanced for all variables and PS (standardised mean difference <0.1) (Supplementary Figure 1). PS distribution was also similar between the groups (Supplementary Figure 2).
Sensitivity analysis
As sensitivity analyses, we used multiple imputation, followed by PS matching, with 10 imputations and 10 iterations per imputation. We imputed variables with >5% unknown, including BMI (26% unknown), smoking status (10% unknown), and time from symptom onset to PCI (7.9% unknown). Variables used for imputing unknown data were those included in the most adjusted model. Predictive mean matching was performed for imputing BMI and time from symptom onset to PCI, while logistic regression was performed for imputing smoking status. The distribution of the imputed data was similar for each imputation and available data (Supplementary Figure 3). Thereafter, PS were calculated using all the variables in the most adjusted Poisson model, including BMI, smoking status, and time from symptom onset to PCI. As an additional sensitivity analysis, we performed IPTW, adjusting for the same variables as in the main analysis. We used stabilised weights, and when extreme weights were obtained, we provided analyses with no weight truncation, truncation at the 99th percentile, and truncation at the 95th percentile.
Other statistical methods
To investigate potential differences between patients with and without UFH pretreatment, the Wilcoxon rank-sum test and Pearson’s chi-squared or Fisher’s exact test were used for continuous and categorical variables, respectively. Statistical significance was defined as a 2-sided p-value <0.05. The statistical analyses were performed using R version 4.1.0 (R Foundation for Statistical Computing).
Results
Main analysis
A total of 41,631 patients were included in the study population (Figure 1), 16,026 (38%) receiving and 25,605 (62%) not receiving UFH pretreatment. The mean age was 67, and 71% were male. In 2008 and 2016, a total of 26% and 49%, respectively, of patients were pretreated with UFH and, overall, the proportion of pretreatment varied between 1% and 85% across the PCI centres (Supplementary Figure 4). Baseline characteristics are presented in Table 1. Goodness-of-fit showed a good fit for the Poisson models. The overall results are summarised in the Central illustration.
Figure 1. Flowchart of the study population. PCI: percutaneous coronary intervention; SCAAR: Swedish Coronary Angiography and Angioplasty Registry; STEMI: ST-segment elevation myocardial infarction
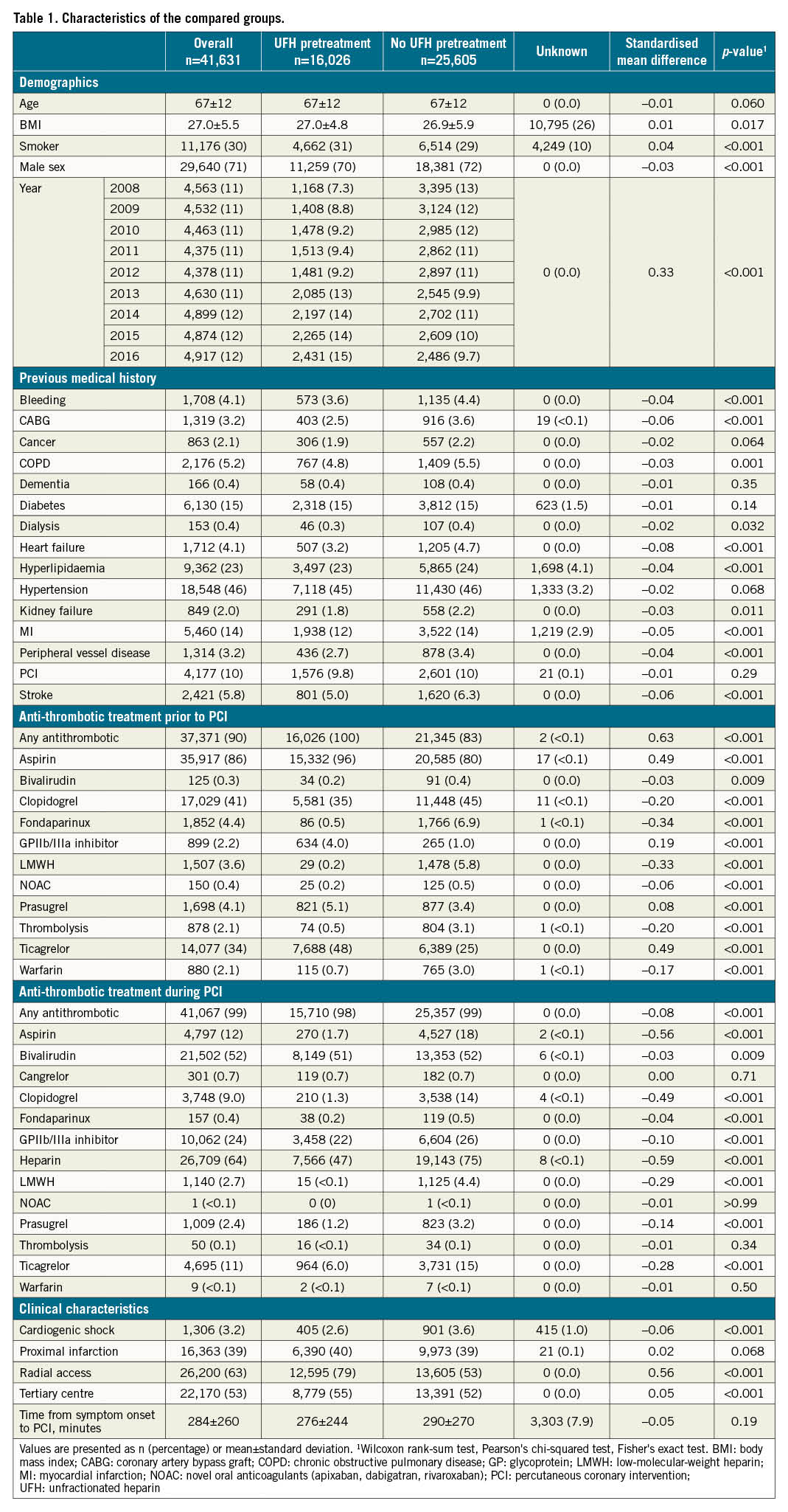
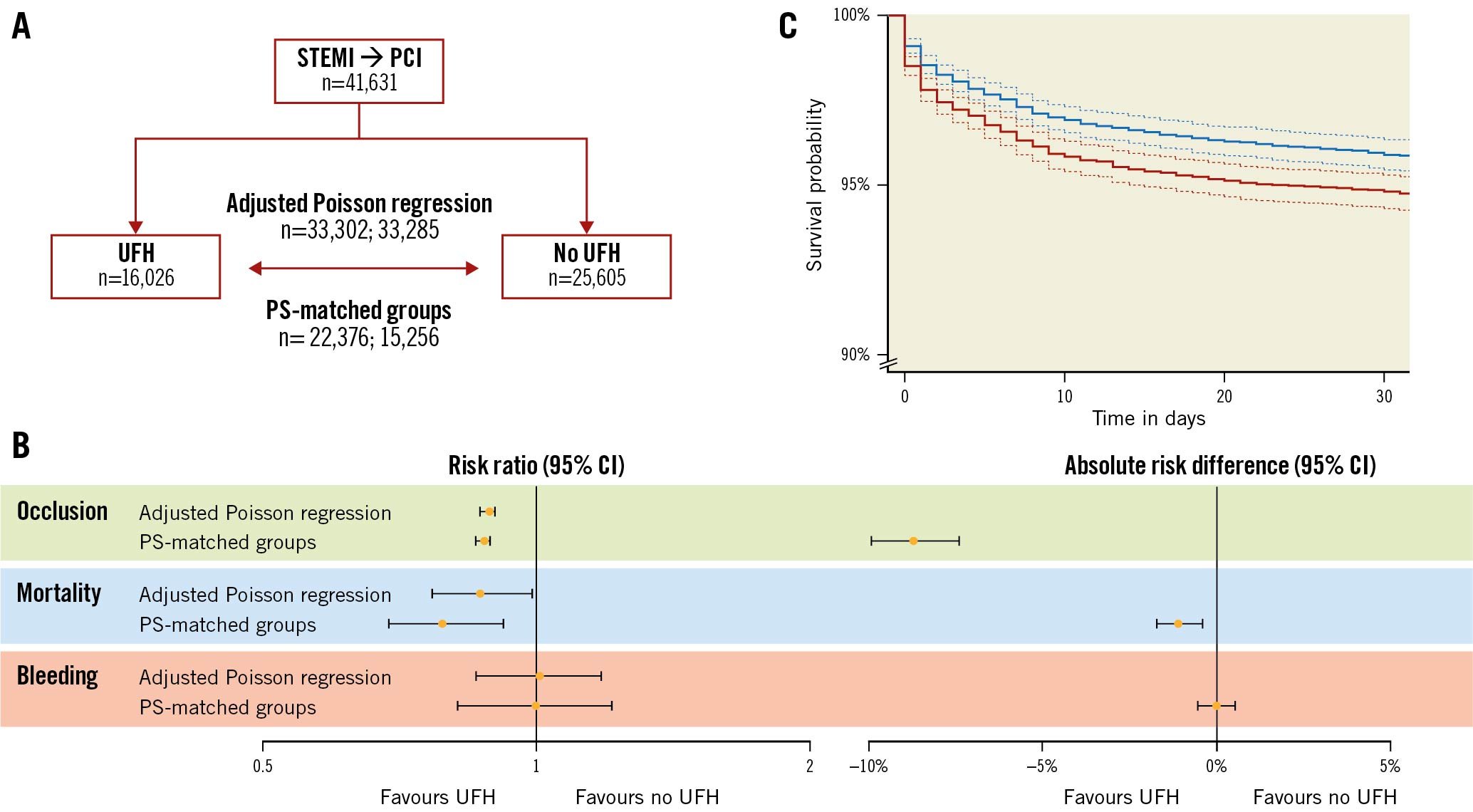
Central illustration. Patient outcomes with versus without heparin pretreatment in patients with ST-segment elevation myocardial infarction undergoing PCI. A) Flowchart of patients included for analysing occlusion or mortality and bleeding from the Swedish Coronary Angiography and Angioplasty Registry. B) Forest plots with risk ratios, calculated using adjusted Poisson regression as well as PS-matched groups, and absolute risk differences, calculated using PS-matched groups. C) Kaplan-Meier curves for overall survival with 95% confidence intervals (dashed lines) in PS-matched groups with (blue) and without (red) unfractionated heparin. CI: confidence intervals; PCI: percutaneous coronary intervention; PS: propensity score; STEMI: ST-segment elevation myocardial infarction; UFH: unfractionated heparin
A total of 27,478 (66%) patients presented with coronary artery occlusion at angiography, 2,712 (6.5%) died within 30 days, and 874 (2.1%) experienced in-hospital major bleeding (Table 2). In the most adjusted model, the risk ratios for UFH pretreatment versus no pretreatment were 0.89 (95% CI: 0.87 to 0.90) for coronary artery occlusion, 0.87 (95% CI: 0.77 to 0.99) for mortality, and 1.01 (95% CI: 0.86 to 1.18) for bleeding. Tolerance tests showed that all adjusted variables had a variance inflation factor <5, indicating that multicollinearity was not a problem. In the subgroup analyses, interaction tests revealed no effect modifiers for coronary artery occlusion (Figure 2). For mortality, access site and extent of vessels affected were effect modifiers. For bleeding, access site, weight, and age were found to be effect modifiers.
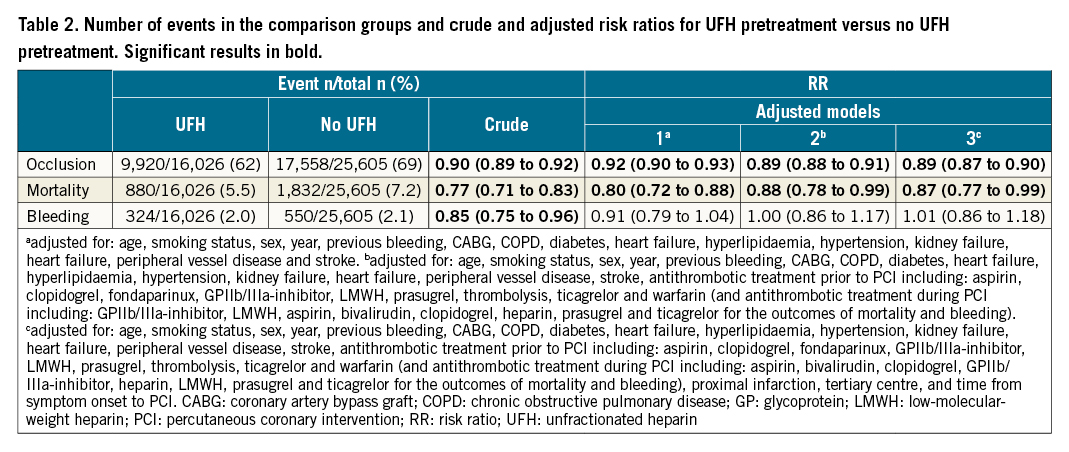
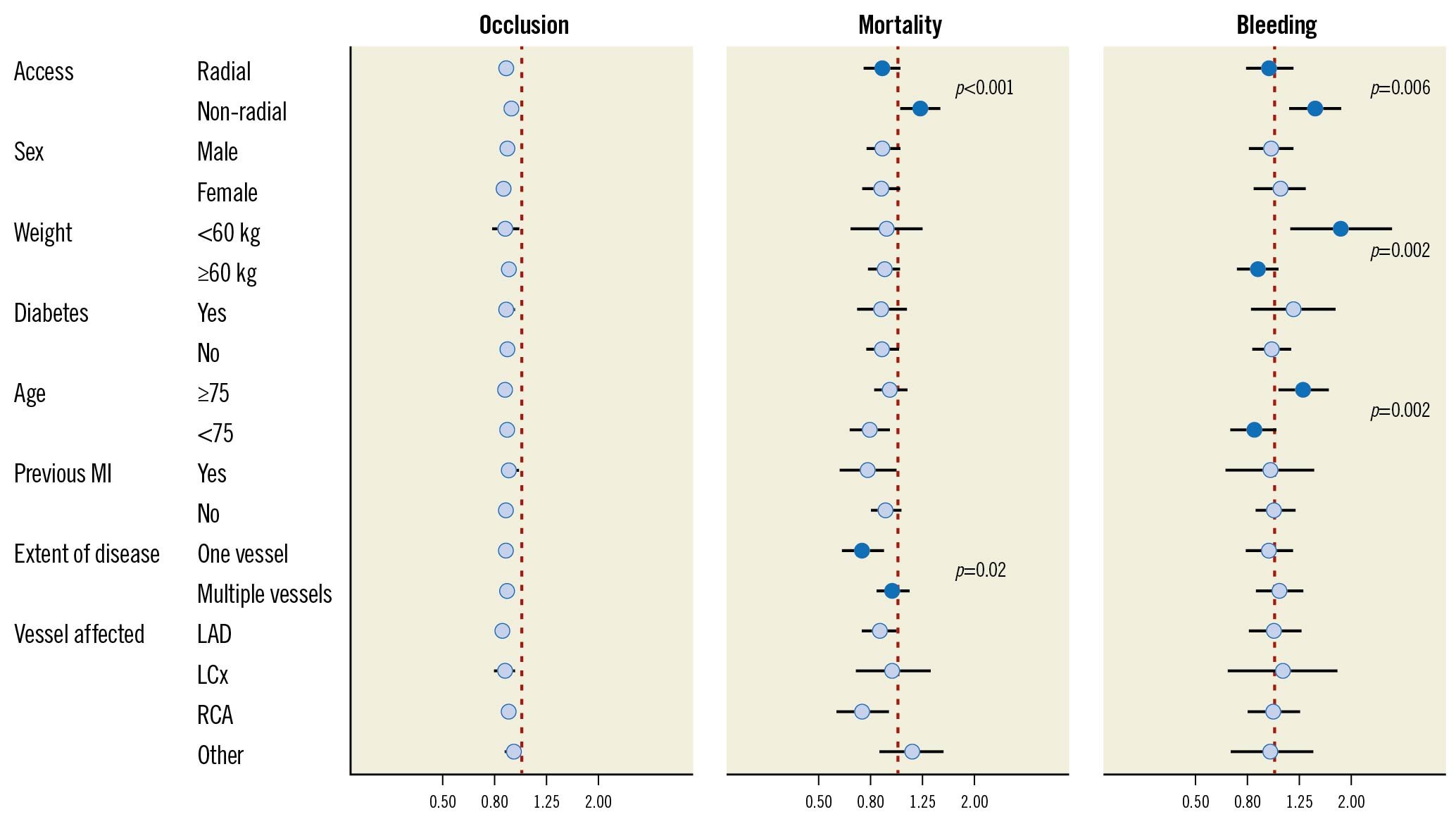
Figure 2. Fully adjusted subgroup analyses regarding coronary artery occlusion, mortality, and bleeding (unfractionated heparin pretreatment versus no such treatment). Subgroups with statistically significant p-values for interaction are presented with filled circles with p-values provided. Subgroups without statistically significant interaction are presented with open circles. Occlusion adjusted for: age, smoking status, sex, year, previous bleeding, CABG, COPD, diabetes, heart failure, hyperlipidaemia, hypertension, kidney failure, heart failure, peripheral vessel disease, stroke, antithrombotic treatment prior to PCI (including: aspirin, clopidogrel, fondaparinux, GPIIb/IIIa-inhibitor, LMWH, prasugrel, thrombolysis, ticagrelor, and warfarin), proximal infarction, tertiary centre, and time from symptom onset to PCI. Mortality and bleeding adjusted for: age, smoking status, sex, year, previous bleeding, CABG, COPD, diabetes, heart failure, hyperlipidaemia, hypertension, kidney failure, heart failure, peripheral vessel disease, stroke, antithrombotic treatment prior to PCI (including: aspirin, clopidogrel, fondaparinux, GPIIb/IIIa-inhibitor, LMWH, prasugrel, thrombolysis, ticagrelor, and warfarin), antithrombotic treatment during PCI (including: aspirin, bivalirudin, clopidogrel, GPIIb/IIIa-inhibitor, heparin, LMWH, prasugrel, and ticagrelor), proximal infarction, tertiary centre, and time from symptom onset to PCI. CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; GP: glycoprotein; LAD: left anterior descending artery; LCx: left circumflex artery; LMWH: low-molecular-weight heparin; MI: myocardial infarction; PCI: percutaneous coronary intervention; RCA: right coronary artery
PS-matched analyses
As outlined in the methods section, 2 PS-matched datasets were created, one for coronary artery occlusion and a separate one for mortality and bleeding. In the PS-matched analysis regarding coronary artery occlusion, a total of 22,376 patients (54% of the primary population) were included (Supplementary Table 1). In the separate PS-matched dataset used for mortality and bleeding, 15,256 patients (37% of the primary population) were included. For both datasets, the mean age was 67 years and 71% were male.
A total of 14,880 (66%) patients had coronary artery occlusion, 708 (4.6%) died within 30 days, and 398 (2.6%) had major in-hospital bleeding (Table 3). Absolute risk differences were significant for coronary artery occlusion –0.087 (95% CI: –0.074 to –0.099), and for mortality –0.011 (95% CI: –0.017 to –0.0041), but not for major in-hospital bleeding 0 (95% CI: –0.0052 to 0.0052). A Kaplan-Meier curve is presented in the Central illustration.
Sensitivity analyses with multiple imputation before PS matching showed similar results to the main analysis (Supplementary Table 2). For coronary artery occlusion and bleeding, IPTW showed similar results to the main analysis, but for mortality the analyses were inconsistent.
Discussion
The current study is, to our knowledge, the largest study to date regarding the clinical effects of UFH pretreatment compared with no pretreatment in patients with STEMI undergoing primary PCI. Our results showed that UFH pretreatment was associated with an 11% relative risk reduction in STEMI patients presenting in the catheterisation laboratory with a coronary artery occlusion. The PS-matched analysis showed a corresponding 9% absolute risk reduction in coronary artery occlusion, corresponding to a number needed to treat (NNT) of 12. Regarding mortality, a 13% relative risk reduction was shown in the main analysis, and a 1% absolute risk reduction in the PS-matched analysis, corresponding to an NNT of 94. Regarding major in-hospital bleeding, our results suggest no harmful effects of UFH pretreatment.
Our positive findings regarding UFH pretreatment for coronary artery occlusion are in line with previous literature13456781011. Only 1 cohort study reported no difference in patency between the groups9. A potential explanation for the differing result in the latter study may be that a trained medical student instead of an experienced cardiologist was used for determining Thrombolysis in Myocardial Infarction (TIMI) flow. The previously largest cohort study, including 7,144 patients (who also were included in our study), reported a 36% reduction of total occlusion1, a figure substantially larger than ours. Possible explanations for our differing results could be different cut-offs for vessel occlusion. They also calculated odds ratios, in contrast to the relative risks reported in this study. These 2 estimates are not comparable when the outcome occurs in >10% of the sample19. A large difference between the groups regarding patency of the culprit coronary vessel was also reported in the only RCT on the topic; patency was 4 times more likely in the UFH-pretreated group3. However, this RCT was performed in the late 90s with a low sample size (n=48) and does not reflect the current care of STEMI patients. Furthermore, in the RCT, high-dose prehospital UFH was used, as opposed to the clinical routine in Sweden, where low-dose UFH (generally 5,000 U) is the choice for pretreatment prior to arrival in the coronary catheterisation laboratory.
As far as we are aware, PS-matched analyses have only been used in 1 previous study4. In that study, with 528 patients in each group, a statistically significant reduction in coronary artery occlusion with UFH pretreatment was found. The absolute risk reduction was 11 percentage points, which is in line with our results. In addition, a study examining the effects of pretreatment with different antithrombotic treatments in patients with STEMI including 10,064 patients and with 81% of pretreatment being UFH suggested an improved patency with pretreatment20. Overall, our results regarding coronary artery occlusion seem to be consistent with previous literature.
Regarding mortality, the results from previous studies are mixed. No statistically significant difference in mortality between the groups was found in 5 studies145611, whereas a statistically significant difference in favour of UFH pretreatment was reported in 4 studies78912. The diverging results may be a result of power and/or bias issues. Indeed, a post hoc power analysis based on the mortality incidence in our PS-matched analysis revealed that 11,502 patients would be required to reject a 1.1% absolute risk difference with 80% certainty. In our study, a mortality difference between the groups in favour of UFH pretreatment was observed in both the main analysis and the PS-matched analysis. The interaction tests in our subgroup analyses revealed that the access site and extent of vessel disease were effect modifiers for mortality. About one-third of the study population had non-radial access and, for these patients, a higher risk of mortality with UFH pretreatment was found.
The sensitivity analyses using multiple imputation showed similar results. Interestingly, the IPTW analyses were not consistent, and the extreme weights obtained may be part of the explanation. For example, when investigating mortality and bleeding, the largest weight was 426, the mean 1.07, the median 0.68, and the third quartile 0.96. Consequently, few patients significantly affect the results, resulting in wide confidence intervals without truncation and biased results with arbitrary truncation. Indeed, one may speculate that if a patient receives UFH pretreatment, despite many underlying factors negatively associated with such treatment, they will be assigned an extreme weight in the IPTW analysis. These patients are likely to have an increased risk of poor outcomes and will contribute to biased results.
Regarding bleeding, our results are consistent with previous studies where no statistically significant differences were reported145678. However, our definition of bleeding is restricted to major bleeding only. Interestingly, the interaction tests in the subgroup analyses revealed that access site, age, and weight were effect modifiers. UFH pretreatment was associated with an increased risk of bleeding for patients that undergo non-radial access, are 75 years of age or older, or weigh below 60 kilograms. One may speculate that these three groups contain frail patients, i.e., patients particularly prone to bleeding complications. For patients with a low weight, one may also speculate that the increased risk of bleeding may be caused by a higher UFH dose relative to the patient’s body weight.
An important strength of our study is that we used data from SCAAR. This approach makes many relevant variables accessible for the analyses. In addition, our study sample is large with a high coverage; it includes nearly 6 times as many patients than the previously largest cohort study1 and enabled subgroup analyses. Another strength is that we calculated risk ratios instead of odds ratios, which have been used in several previous studies16789. Odds ratios may be more difficult to interpret. As opposed to risk ratios, they do not correspond to the relative risk for common outcomes, for example, occlusion. In addition, the PS-matched analyses allowed us to estimate the absolute risk differences with corresponding NNT. Furthermore, we used sensitivity analyses to investigate the robustness of the results.
Limitations
A limitation of the present study is that the UFH pretreated patients differed somewhat from the patients not receiving UFH pretreatment. We took this into account using 2 different statistical methods and multiple levels of statistical modelling. Nonetheless, residual confounding cannot be excluded. Another limitation is that the dose and exact timing of the pretreatments were unknown; these data are not recorded in SCAAR. An additional limitation is that several patients were excluded in the PS-matched analyses. This may affect the generalisability of the results. Furthermore, the IPTW sensitivity analyses regarding mortality were not robust. Finally, the National Patient Register does not include diagnoses recorded in primary care. Therefore, some background characteristics may be missing.
Conclusions
In summary, our results show that pretreatment with UFH prior to arrival at the catheterisation laboratory in patients with STEMI undergoing primary PCI is associated with a reduced risk of coronary artery occlusion at presentation, with an NNT of 12, without increasing the risk of major in-hospital bleeding. Regarding mortality, our analyses show a reduced risk with UFH pretreatment, with an NNT of 94, but this effect was not robust over all sensitivity analyses. Residual confounding cannot be ruled out; caution must be taken in interpretation, and a future RCT could add further insights.
Impact on daily practice
In this study, comparing unfractionated heparin pretreatment with no pretreatment in patients with STEMI undergoing primary PCI, we show a significant relative risk reduction in coronary artery occlusion at the time of angiography as well as an absolute risk reduction with an NNT of 12, without an increased risk of major bleeding. A reduced risk of mortality was found, with an NNT of 94, but this result was not robust over all sensitivity analyses. Providing risk ratios as well as absolute risk differences in a well-characterised cohort of 41,631 patients, which constitutes the largest published study so far on this topic, the study contributes further evidence that could be useful for future guidelines as the results suggest a clinical benefit of unfractionated heparin pretreatment.
Acknowledgements
This study was supported by grants from the Faculty of Medicine at Lund University and the Märta Winkler Foundation.
Conflict of interest statement
G.O. Olivecrona received consulting fees from Edwards Lifesciences, honoraria from Biotronik, EPS Vascular, and Abbott and participates on the Board of Biosensor CEC BioFreedom STEMI. M. Götberg received consulting fees from Boston Scientific and Medtronic and participates in the EAPCI Fellowship Grant Committee and the Case Review Committee of Boston Scientifc. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

