
Introduction
The benefits of bivalirudin or unfractionated heparin (UFH) during primary percutaneous coronary intervention (PCI) remain controversial. Bivalirudin rather than UFH plus a routine glycoprotein IIb/IIIa receptor inhibitor (GPI) has been shown to improve overall and cardiac survival and reduce bleeding complications in patients undergoing primary PCI. Several trials comparing bivalirudin and UFH±GPI have since questioned this survival advantage and created controversy related to bivalirudin-associated improved bleeding outcome and the risk of acute stent thrombosis. Recently, the VALIDATE-SWEDEHEART bivalirudin versus heparin monotherapy in myocardial infarction during PCI trial reported no differences in myocardial infarction, major bleeding, definite stent thrombosis and death1. Clinically meaningful reduced efficacy of either regimen in antithrombotic protection during primary PCI is likely to lead to increased myocardial damage. Therefore, the present analysis evaluated myocardial salvage, infarct size, microvascular obstruction (MVO) and left ventricular ejection fraction (LVEF) by cardiac magnetic resonance (CMR), according to the antithrombotic strategy.
Methods
This is a non-randomised post hoc analysis of the DANAMI-3 trial programme in patients with available CMR2. Patients (n=730) were evaluated with an acute CMR after primary PCI during the index admission and with a second examination at 90-day follow-up. We first stratified patients by the administration of GPI. In the DANAMI-3 trial, the choice of antithrombotic regimen was left to the operator. The decision to administer bail-out GPI in bivalirudin-treated patients was associated with the event of a complicated procedure, illustrated by prolonged procedure duration and a higher frequency of distal embolisation and no or slow reflow in the culprit artery. The decision to administer provisional GPI was associated with randomisation to the DEFER study in the DANAMI-3 trial programme (Supplementary Table 1, Supplementary Table 2). Patients with provisional or bail-out GPI treatment (n=166, 26.7%) were therefore excluded from the present analysis.
Results
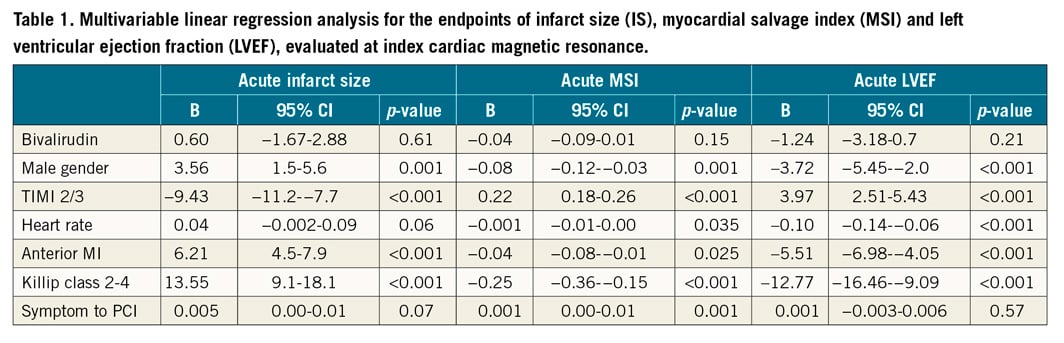
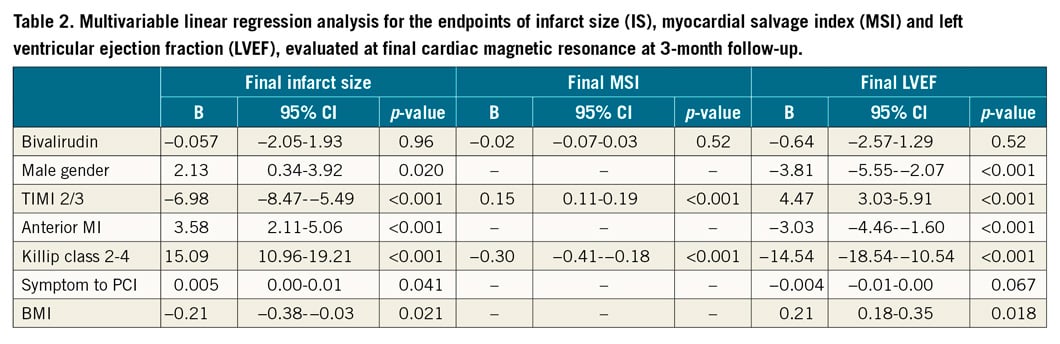
In patients without GPI treatment (n=564, 77.3%) and with very similar area at risk (% of LV) at index CMR (32.5 [±11.8] vs 33 [±11.3] [p=0.72]), we did not find significant differences in acute or final infarct size, myocardial salvage index (MSI), LVEF (Figure 1) or MVO (% of LV) (1.62 [±3.6] vs 2.04 [±3.4] [p=0.30]) according to the mono-comparison of UFH versus bivalirudin. Multivariable analysis adjusted for differences (p<0.1) in baseline and procedural characteristics and bivalirudin was forced into the models. Male gender, heart rate, anterior myocardial infarction, Killip class ≥2, preprocedural TIMI flow 0/1, and symptom onset to PCI were associated with acute and final infarct size (IS), MSI and LVEF, whereas bivalirudin versus UFH treatment was not (Table 1, Table 2). A sensitivity analysis in patients (bivalirudin n=234, UFH n=40) randomised to the conventional treatment groups in the DANAMI-3 trial programme without GPI treatment confirmed the above results.
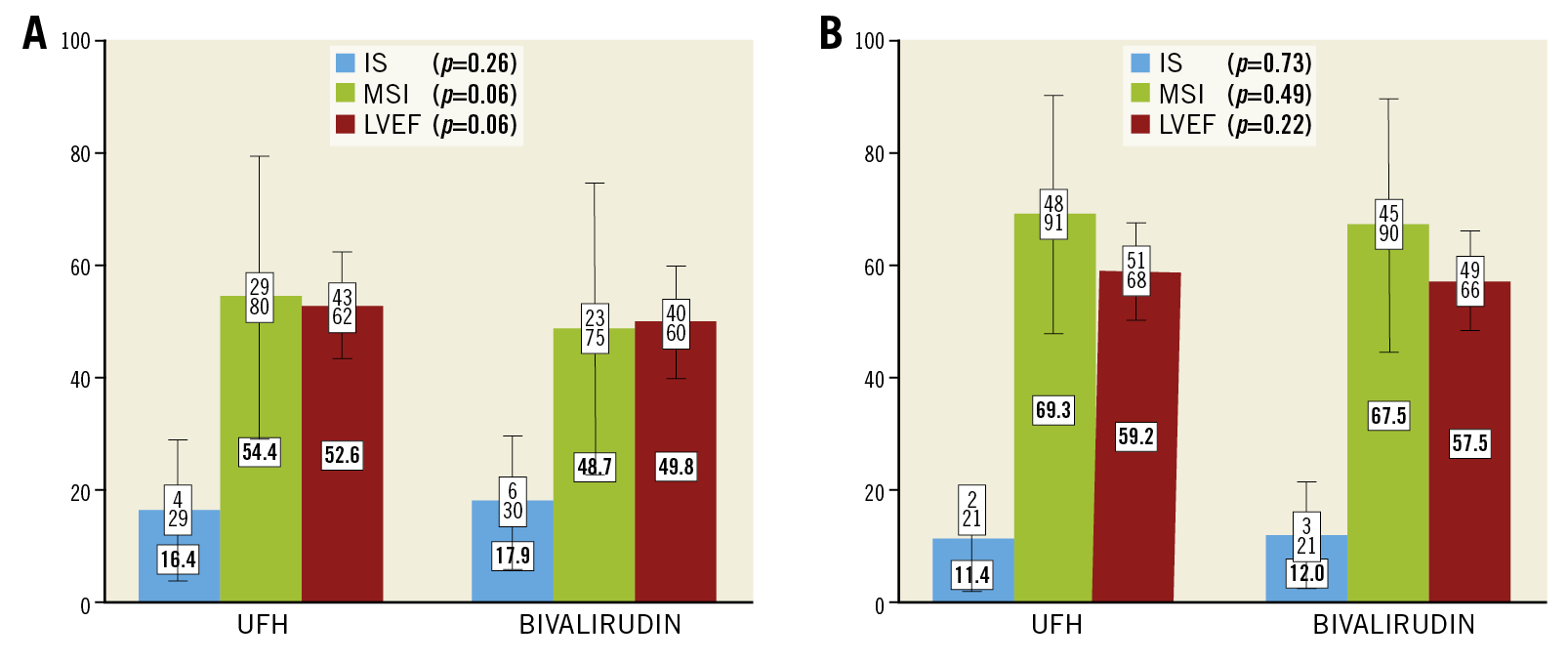
Figure 1. Infarct size (IS) (%±1 SD), myocardial salvage index (MSI) (±1 SD) and left ventricular ejection fraction (LVEF) (%±1 SD) at index (A) and follow-up (B) according to bivalirudin and unfractionated heparin (UFH). P-values are shown for the comparison of IS, MSI and LVEF according to bivalirudin and UFH.
Discussion
The present study is the first to compare bivalirudin and UFH according to indices of myocardial damage in a large CMR population of patients undergoing primary PCI. The use of bivalirudin alone compared to UFH alone is not associated with IS, MSI, LVEF or MVO. Our results indicate that bivalirudin and heparin have comparable outcome regarding indices of myocardial injury, when used as adjunctive pharmacotherapy during primary PCI. Our study therefore does not provide pathophysiological explanatory factors of myocardial damage, which could substantiate previously observed mortality benefits in patients treated with bivalirudin compared to UFH.
Limitations
The operators’ choice of antithrombotic strategy during primary PCI holds treatment attribution biases, which we attenuated by excluding GPI-treated patients and performing multivariate and sensitivity analyses. Potential selection biases affect the extrapolation of the present results to a general ST-segment elevation myocardial infarction (STEMI) population. Compared to CMR participants, randomised patients who dropped out before acute CMR assessment had a worse clinical risk profile upon admission3, and study participants in the DANAMI-3 trial had lower mortality compared to contemporary non-participants with STEMI from unselected registries4.
Conclusion
In a non-randomised analysis of patients undergoing primary PCI, the use of bivalirudin compared to UFH was not associated with myocardial damage.
|
Impact on daily practice Bivalirudin and heparin during primary PCI have comparable outcomes regarding indices of myocardial injury. Our results do not provide pathophysiological explanatory factors of myocardial damage in terms of differences in infarct size, myocardial salvage, microvascular obstruction or LVEF that could substantiate previously observed survival advantages in patients treated with bivalirudin compared to UFH. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

