Abstract
Aims: Current recommendations on the use of bivalirudin in patients treated with percutaneous coronary intervention (PCI) are mostly based on trials comparing bivalirudin versus heparin plus planned glycoprotein IIb/IIIa inhibitor (GPI). Whether bivalirudin is also superior to heparin alone is still not well established. This meta-analysis investigates the efficacy and safety of bivalirudin versus heparin in patients treated with PCI without planned use of GPI.
Methods and results: Scientific databases and websites were searched for randomised controlled trials. The primary efficacy and safety outcomes were the 30-day incidence of death and major bleeding, respectively. The secondary outcomes were the 30-day incidence of myocardial infarction (MI), definite stent thrombosis (ST), urgent target vessel revascularisation (TVR), and overall death at the longest available follow-up. Odds ratio (OR) and 95% confidence interval (95% CI) served as summary statistics. Ten trials were identified including a total of 18,065 PCI patients randomised to bivalirudin (n=9,033) versus heparin (n=9,032). At 30 days, bivalirudin versus heparin showed a comparable risk of death (1.09 [0.83-1.41], p=0.54), and MI (1.10 [0.83-1.46], p=0.50) with a trend towards a higher risk of urgent TVR (1.37 [0.96–1.96], p=0.08). The risk of major bleeding was lower with bivalirudin (0.57 [0.40-0.80], p=0.001) and the bleeding reduction was more evident when high doses of heparin were used as comparator (p for interaction <0.001). The risk of definite ST (2.09 [1.26-3.47], p=0.005) and, in particular, the risk of acute ST (3.48 [1.66-7.28], p<0.001) was increased by bivalirudin.
Conclusions: Patients undergoing PCI randomised to therapy with either bivalirudin or heparin display a similar mortality. Bivalirudin as compared to heparin appears to reduce the risk of major bleeding at the expense of a higher risk of acute ST.
Introduction
Antiplatelet and anticoagulant agents represent core adjuvant antithrombotic therapy in patients undergoing percutaneous coronary intervention (PCI). In this context, heparin has for a long time been the dominant anticoagulant available and was the therapy of choice in virtually all settings1.
Bivalirudin is a synthetic peptide derived from the natural drug hirudin with a direct and reversible thrombin-inhibitor activity. Bivalirudin inhibits both circulating and clot-bound thrombin, as well as thrombin-mediated platelet activation and aggregation, without binding plasma proteins2. In patients undergoing PCI, bivalirudin has become an attractive therapeutic option by combining antiplatelet and anticoagulant effects with a predictable antithrombotic response in comparison with heparin1.
In patients treated with PCI, bivalirudin reduced bleeding-associated complications3. In line with this evidence, guideline-writing authorities have assigned to bivalirudin a class I recommendation among anticoagulant agents available for PCI1,4. However, most of this supportive evidence has come from earlier comparisons of bivalirudin versus heparin plus planned glycoprotein IIb/IIIa inhibitor (GPI), a strategy which is no longer the standard of care1,4. In addition, recent data have pointed to an increased risk of ischaemic complications with bivalirudin3,5, questioning its role in patients treated with PCI.
This meta-analysis sought to investigate the clinical impact of bivalirudin versus heparin in patients treated with PCI without planned use of GPI.
Methods
SEARCH STRATEGY AND SELECTION CRITERIA
We searched Medline, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), scientific sessions’ abstracts and relevant websites (www.cardiosource.com, www.clinicaltrialresults.org, www.escardio.org, www.tctmd.com, www.theheart.org) without restricting language or publication status. Search terms included the keywords and the corresponding Medical Subject Headings for: “anticoagulation”, “bivalirudin”, “heparin”, “percutaneous coronary intervention”, “angioplasty”, “stent”, “trial”, and “randomised trial”. The references listed in all eligible publications, as well as in prior meta-analyses6-10 on the same topic, were checked to identify further citations. Inclusion criteria were: (1) randomised design; (2) bivalirudin or heparin administration for elective or urgent PCI; (3) >150 patients enrolled; (4) intention-to-treat analysis. Exclusion criteria were: (1) anticoagulation for elective or urgent PCI other than bivalirudin or heparin; (2) planned GPI use; (3) thrombolysis before randomisation; (4) duplicated data. The final search was performed on 5 July 2014.
DATA COLLECTION AND ASSESSMENT OF RISK OF BIAS
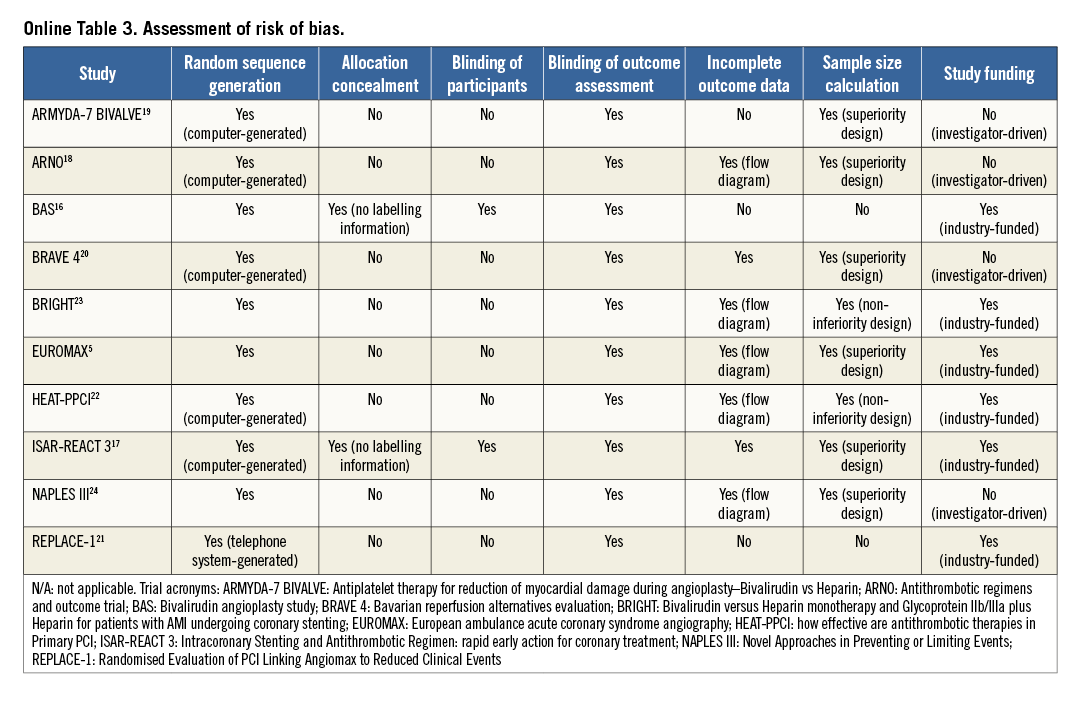
Two investigators (SC and RAB) independently assessed publications for eligibility at title and/or abstract level, with divergences resolved by a third investigator (AK). Studies that met the inclusion criteria were selected for further analysis. Freedom from bias was evaluated for each study by the same investigators, in accordance with The Cochrane Collaboration method11, based on the following methodological items: adequacy of random sequence generation and allocation concealment, blinding (at participant or outcome assessor level), completeness of reporting outcome data, completeness and adequacy of description of sample size calculation and appropriate disclosure of funding sources. We avoided formal quality score adjudication, which has previously been considered potentially misleading12.
OUTCOME VARIABLES
The primary efficacy and safety outcomes were the 30-day incidence of death and major bleeding, respectively. The secondary outcomes were the 30-day incidence of myocardial infarction (MI), definite stent thrombosis (ST), urgent target vessel revascularisation (TVR), and the cumulative incidence of death at the longest available follow-up. Endpoints of interest were prospectively defined and were evaluated as per protocol definitions. Where further details were required, we attempted to obtain them from the study investigators directly.
STATISTICAL ANALYSIS
Statistical analysis was performed using the RevMan (Review Manager [RevMan] Version 5.1; The Cochrane Collaboration, Copenhagen, Denmark), and Stata 11.2 (StataCorp, College Station, TX, USA) software packages. Distribution of patient characteristics was presented as median (interquartile range). Odds ratio (OR) and 95% confidence interval (95% CI) served as summary statistics and were calculated for comparison of bivalirudin versus heparin. The Mantel-Haenszel random effects model (DerSimonian and Laird) was used to obtain pooled OR. In case of statistical significance, the number needed to treat (NNT) or the number needed to harm (NNH) with relative (95%) CI was provided. Treatment effects could not be assessed in trials in which no event was reported within the groups. For trials in which only one of the treatment groups had no events of interest, the risk estimates were approximated from 2×2 contingency tables after adding 0.5 to each cell13. The Breslow-Day χ2 test and the I2 statistic were used to test heterogeneity across the trials. As a guide, I2 values <25% indicated low, 25-50% moderate, and >50% high heterogeneity11. To estimate the additive (between-study) component of variance, the restricted maximum likelihood method (Tau2) took into account the occurrence of residual heterogeneity. Visual estimation of funnel plots as well as statistical tests assessed possible publication bias for primary outcome, as previously published14. Similarly, an influence analysis, in which meta-analysis estimates are computed omitting one trial at a time, was performed for all outcomes. A random effects sensitivity analysis evaluated the extent to which several covariates might have influenced the risk estimates for endpoints showing significant difference. The following covariates were included: the size of the study (under/above median number of patients enrolled), the average of females enrolled (under/above median value), the average of PCI performed with drug-eluting stent (DES, under/above median value), the average of GPI use in the heparin arm (under/above median value), the dose of heparin (≤70 or >70 IU/kg), the inclusion of patients with ST-elevation myocardial infarction (STEMI), the routine measurement of activated clotting time (ACT) and the presence of industry funding. The same statistical method was used to address the time dependence of risk estimates for definite ST (acute, ≤24 hours; subacute, >24 hours and ≤30 days) associated with bivalirudin versus heparin.
This study was performed in compliance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement15. The PRISMA research checklist has been provided as an Online Appendix.
Results
ELIGIBLE STUDIES
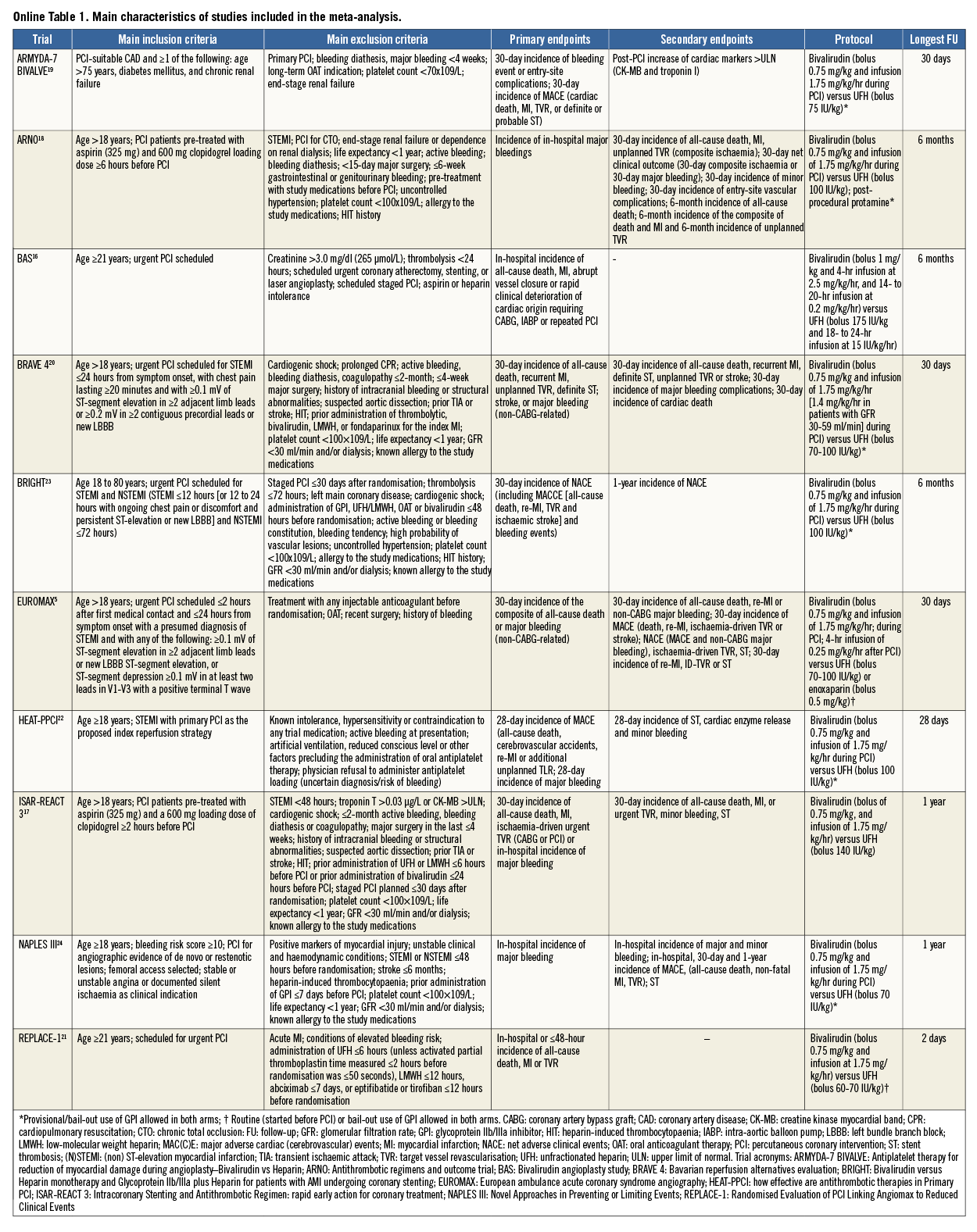
The process of study selection is summarised in Online Figure 1. Ten studies –eight full-length manuscripts5,16-22, two meeting presentations23,24– totalling 18,065 patients (bivalirudin, n=9,033, versus heparin, n=9,032) were selected for inclusion in the meta-analysis. Seven trials5,16,17,19-21,23 had a multicentre design. The main characteristics of the trials are described in detail in Online Table 1. Briefly, patients with significant stable or unstable coronary artery disease (CAD) scheduled for PCI were randomised to receive bivalirudin versus heparin. Four trials5,20,22,23 enrolled patients with acute STEMI.
Bivalirudin was administered as a bolus of 0.75 mg/kg, followed by a periprocedural infusion of 1.75 mg/kg/hr and an optional additional infusion of 0.25 mg/kg/hr after PCI in almost all trials. In one trial, a bolus of bivalirudin (1 mg/kg) followed by two-hour infusion at 2.5 mg/kg/hr and a 14- to 20-hour infusion at 0.2 mg/kg/hr was administered16.
In the control arm, anticoagulation was achieved predominantly with a bolus of unfractionated heparin (UFH) ranging between 60 and 175 IU/kg. In one trial16, the bolus of UFH was followed by eight to 24-hour infusion of 15 IU/kg. In another trial5 enrolling patients scheduled for primary PCI, a bolus of 50 IU (0.5 mg)/kg of enoxaparin could be used as an alternative to UFH. Six trials16,18,20-22,24 administered additional boluses of bivalirudin or heparin according to ACT values. One trial18 administered protamine sulphate at a dose of 0.5 mg/100 IU of UFH at the end of PCI.
In eight trials5,18-24, bail-out (in the presence of intracoronary abundant thrombotic material or sustained microvascular obstruction) or provisional (optional) use of GPI was allowed. One trial23 comprised a third treatment arm in which patients (n=730) were randomly allocated to UHF plus planned GPI administration: these patients were not included in the analysis.
All trials except two23,24 clearly reported that loading doses of thienopyridines (clopidogrel 300 or 600 mg, prasugrel 30 or 60 mg, ticagrelor 180 mg, oral) as well as aspirin (100 to 325 mg orally or 500 mg i.v.) were administered to all patients before PCI. In one trial20, enrolling exclusively STEMI patients, participants were randomised to a combination of bivalirudin plus prasugrel versus heparin plus clopidogrel. In all trials, aspirin was recommended indefinitely at a dose of 75 to 200 mg/d, whilst thienopyridines were prescribed for a period of time ranging from one to 12 months according to clinical indication or type of stent implanted during index PCI. In all but one trial16, the predominant revascularisation strategy consisted of PCI with stent implantation: in the remaining trial only balloon angioplasty was used.
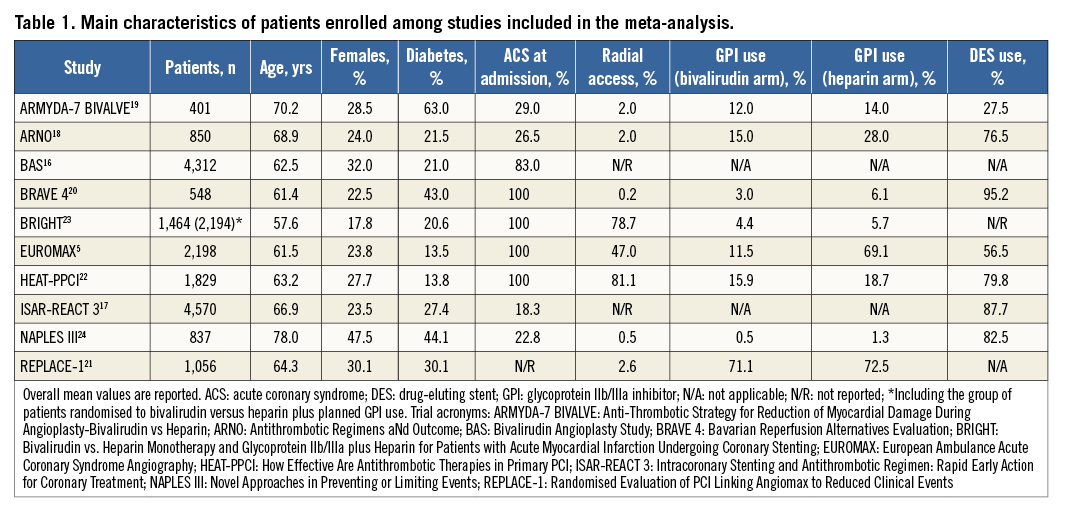
The median number of patients included in each trial was 1,260 (837-2,198). The principal clinical characteristics are shown in Table 1. The median age was 63.7 years (61.5-68.9), 25.8% (23.5-30.1) were females, and 24.4% (20.6-43.0) had a diagnosis of diabetes mellitus. More than half of the patients (56.0% [22.8-100]) presented with acute coronary syndrome. The radial artery served as access route for PCI in a small proportion of patients (2.3% [1.2-62.8]). The predominant type of stent used was DES (79.8% [56.5-87.7]).
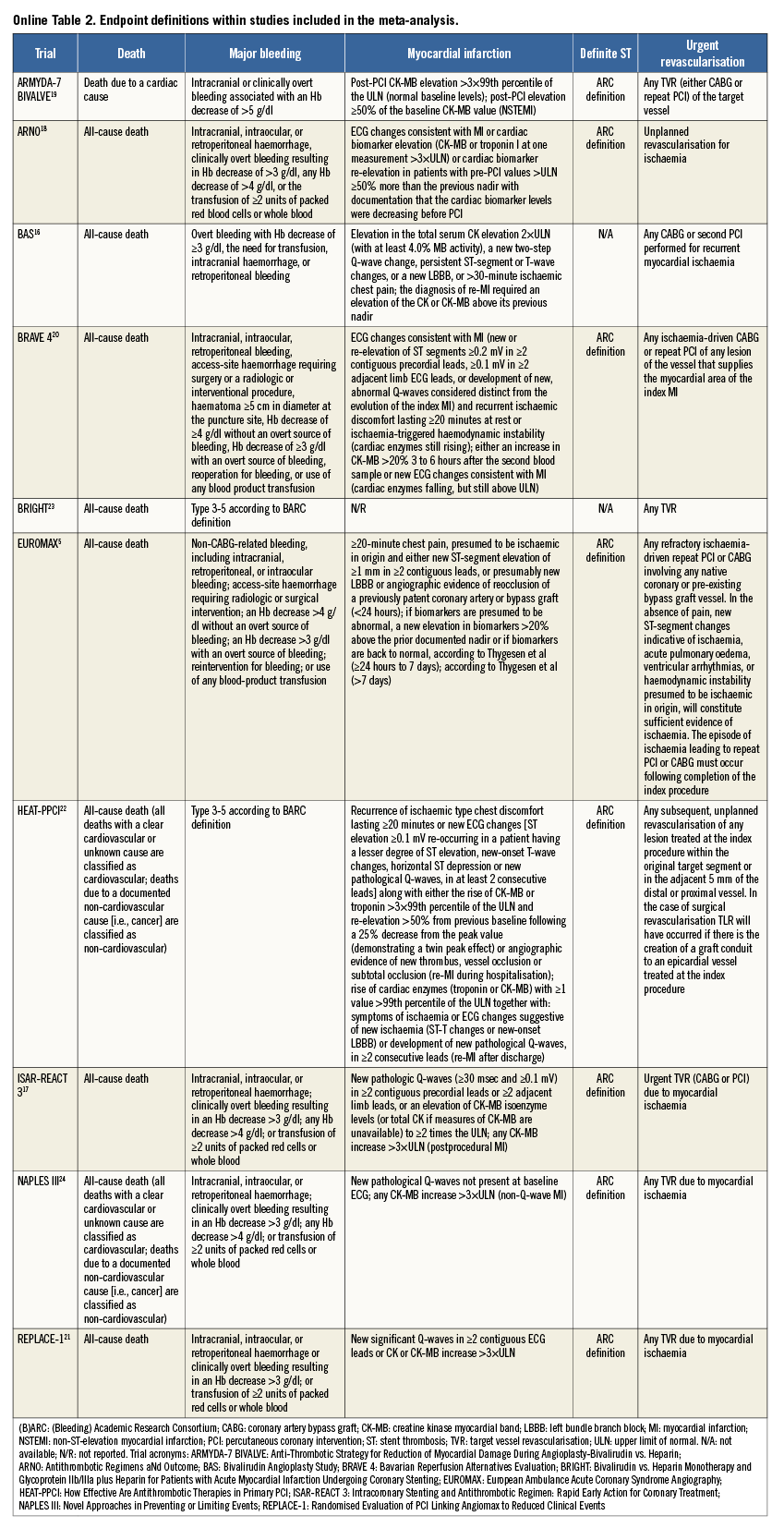
An overview of the definitions of the endpoints in the studies is reported in Online Table 2. In all trials except two16,21, the incidence of major bleeding was the primary outcome. Major bleeding was most frequently adjudicated according to the Thrombolysis In Myocardial Infarction (TIMI) definition25. Definite stent thrombosis was adjudicated according to Academic Research Consortium criteria26. The risk of bias among studies is reported in Online Table 3.
CLINICAL OUTCOMES
Among those randomised, a total of 18,032 patients (99.8%; bivalirudin, n=9,014, versus heparin, n=9,018) were available for 30-day outcome assessment.
The incidence of death at 30 days, the primary efficacy outcome, occurred in 234 patients (1.3%) (Figure 1A). Bivalirudin versus heparin showed a comparable risk of death (1.3% versus 1.2%; OR: 1.09 [0.83-1.41], p=0.54; I2=0%, p for heterogeneity - phet=0.70). Data regarding cardiac cause of death were available for four trials5,18-20 (n=3,993 patients). In these studies, cardiac death occurred in 88 patients (2.2%): bivalirudin versus heparin showed a comparable risk of cardiac death (1.7% versus 2.1%; OR: 0.85 [0.53-1.34], p=0.47; I2= 0%, phet=0.43).
Major bleeding, the primary safety outcome, occurred in 726 patients (4.0%). Bivalirudin versus heparin reduced the risk of major bleeding although there was high heterogeneity among studies (2.8% versus 5.2%; OR: 0.57 [0.40-0.80], p=0.001; I2=71%, phet=0.0003; NNT=41 [33-53]) (Figure 1B). Therefore, the analysis was restricted to those trials5,17,19,20,24 (n=8,550 patients) in which major bleeding was adjudicated according to TIMI definition. TIMI major bleeding occurred in 116 (1.3%) patients. Bivalirudin versus heparin reduced the risk of TIMI major bleeding without significant heterogeneity (0.8% versus 1.8%; OR: 0.51 [0.30-0.85], p=0.01; I2=31%, phet=0.21; NNT=108 [70-225]).
MI occurred in 606 patients (3.3%) (Figure 1C). Bivalirudin versus heparin showed a comparable risk of MI although there was moderate heterogeneity among studies (3.4% versus 3.2%; OR: 1.10 [0.83-1.46], p=0.50; I2=48%, phet=0.04).
Adjudication of definite ST was available for seven trials5,17-20,22,24 (n=11,212 patients). Definite ST occurred in 87 patients (0.7%) (Figure 1D). Bivalirudin versus heparin increased the risk of definite ST without significant heterogeneity (1.0% versus 0.5%; OR: 2.09 [1.26-3.47], p=0.005; I2=9%, phet=0.36; NNH=169 [107-366]). Data regarding the time point of definite ST were available for five trials5,17,18,20,22 (n=9,954 patients). Acute ST occurred in 43 patients (0.4%): bivalirudin versus heparin increased the risk of acute ST without significant heterogeneity (0.7% versus 0.2%; OR: 3.48 [1.66-7.28], p<0.001; I2=0%, phet=0.53; NNH=199 [131-408]). Subacute ST occurred in 44 patients (0.4%): bivalirudin versus heparin showed a comparable risk of subacute ST without significant heterogeneity (0.4% versus 0.4%; OR: 1.11 [0.60-2.03], p=0.74; I2=0%, phet=0.81). The time dependence of definite ST risk for bivalirudin versus heparin was supported by a significant interaction (p for interaction - pint=0.02).
Urgent TVR occurred in 388 patients (2.1%) (Figure 1E). Bivalirudin versus heparin showed a comparable risk of urgent TVR, although there was moderate-to-high heterogeneity among studies (2.1% versus 2.1%; OR: 1.20 [0.82-1.75], p=0.35; I2=50%, phet=0.04). Therefore, the trial of Bittl et al16, in which patients received balloon angioplasty only, was excluded. Among 16,720 patients available for further analysis, urgent TVR occurred in 176 patients (1.0%). Bivalirudin versus heparin showed a trend towards an increased risk of urgent TVR without significant heterogeneity (1.5% versus 1.0%; OR: 1.37 [0.96-1.96], p=0.08; I2=16%, phet=0.30).
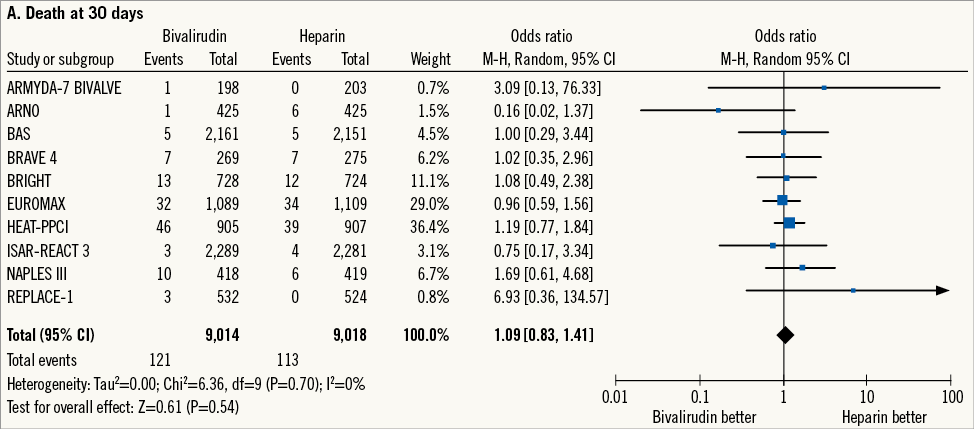
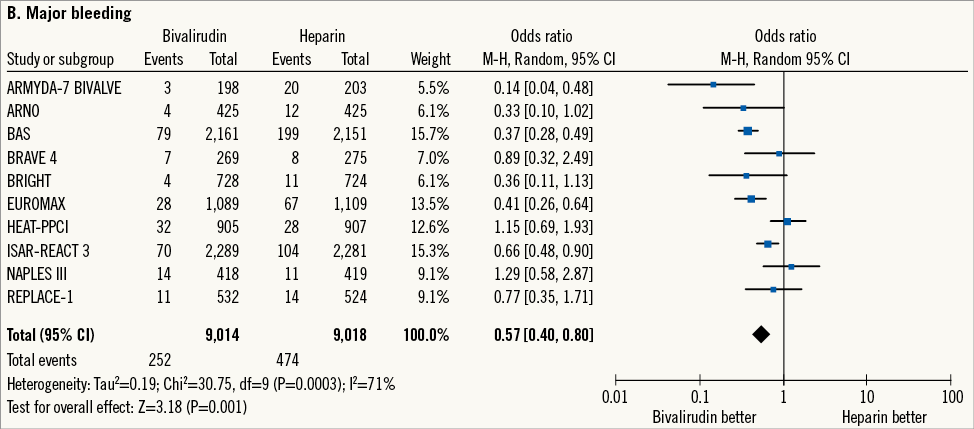
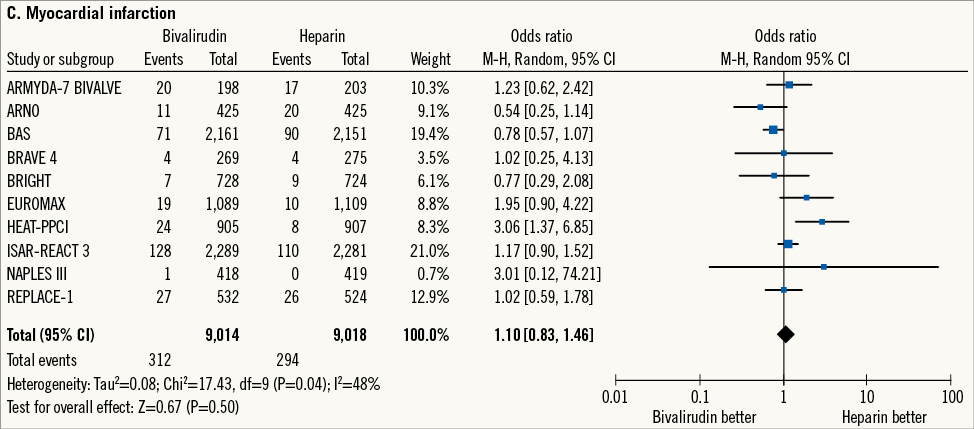
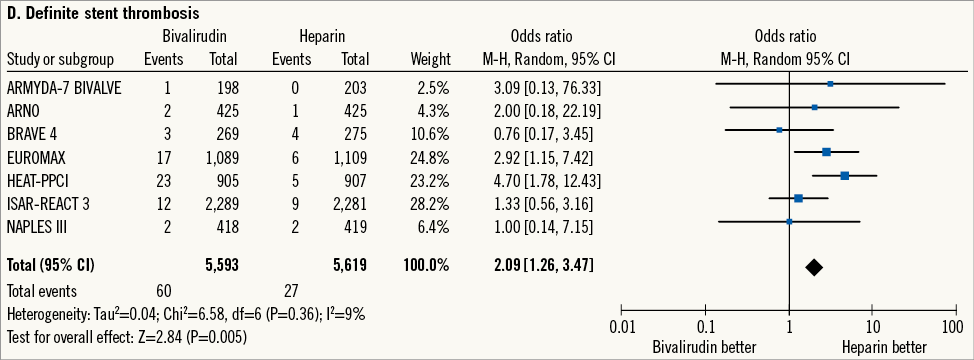
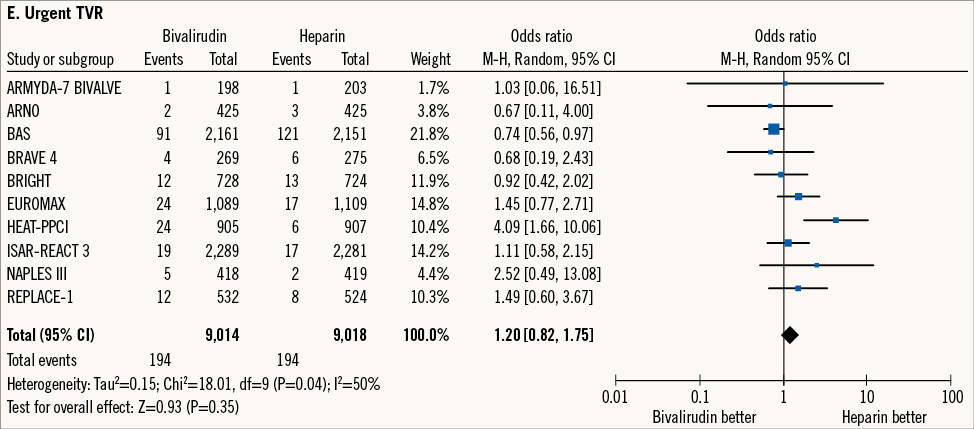
Figure 1. Risk estimates of primary and secondary outcomes at 30-day follow-up for bivalirudin versus heparin in patients treated with PCI. Plot of odds ratio for primary efficacy (A) and safety outcomes (B) as well as for secondary outcomes (C-E) associated with bivalirudin versus heparin. The diamond indicates the point estimate and the left and the right ends of the line the 95% confidence interval (CI). M-H: Mantel-Haenszel; TVR: target vessel revascularisation
Overall, clinical follow-up was reported to 105 days (30-360), with five trials16-18,23,24 evaluating >30-day outcomes. During this period, death occurred in 400 patients (2.2%) (Figure 2). Bivalirudin versus heparin showed a comparable risk of death without significant heterogeneity (2.3% versus 2.1%; OR: 1.08 [0.89-1.32], p=0.44; I2= 0%, phet=0.74).
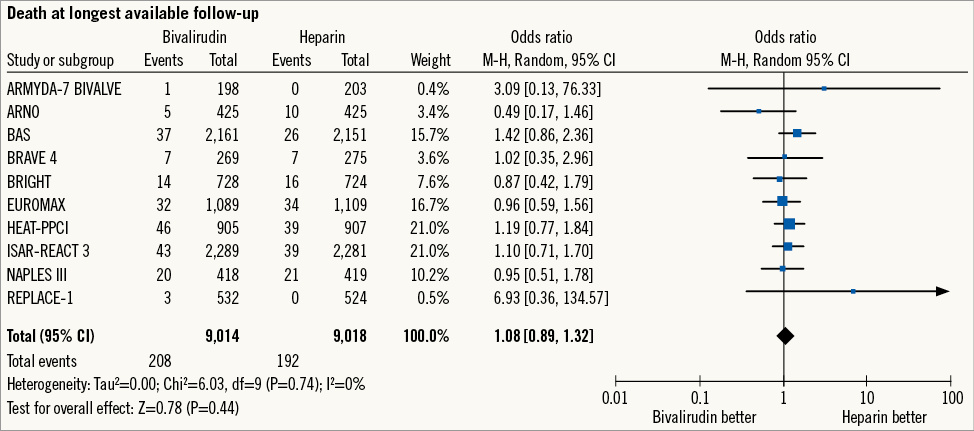
Figure 2. Risk estimates of overall mortality for bivalirudin versus heparin in patients treated with PCI. Plot of odds ratio for overall mortality associated with bivalirudin versus heparin. The diamond indicates the point estimate and the left and the right ends of the line the 95% confidence interval (CI). M-H: Mantel-Haenszel
SMALL STUDY EFFECTS, INFLUENCE AND SENSITIVITY ANALYSES
Funnel plot distribution of outcomes of interest was derived from the standard error of the logarithm OR plotted against the OR of outcomes of interest (Online Figure 2). Of note, the absence of bias due to small study effects was confirmed both visually and mathematically. Additionally, the influence analysis demonstrated that no single study significantly altered the summary OR for outcomes of interest (data not shown).
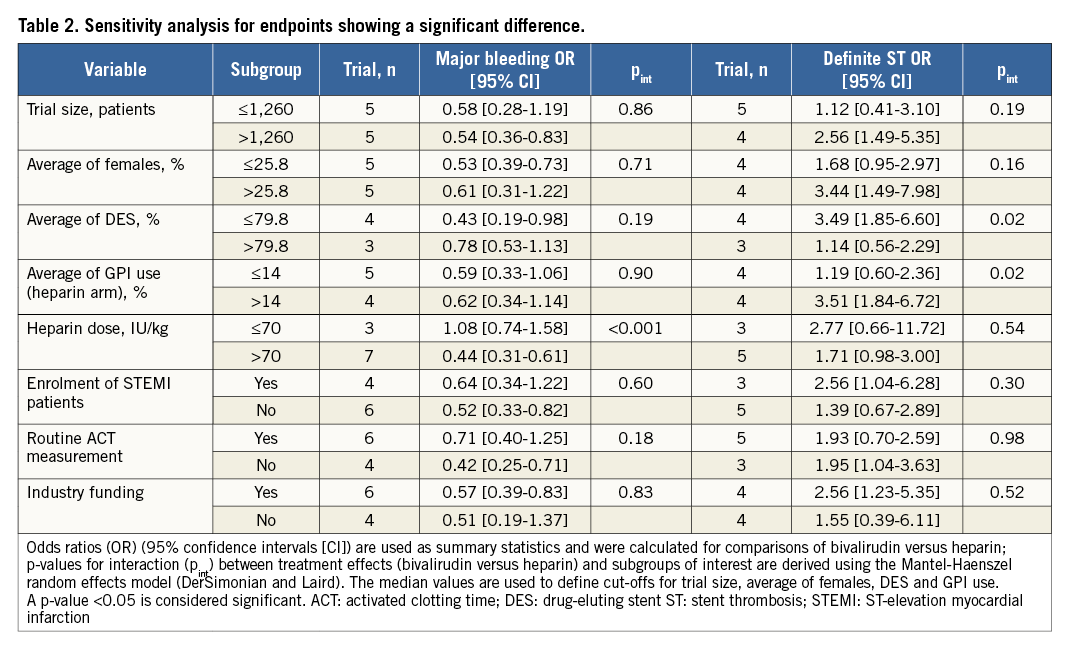
Sensitivity analyses were conducted for those outcomes showing a significant difference (Table 2). The risk of major bleeding was increased by high doses of heparin (pint<0.001). The risk of definite ST was higher with bivalirudin in combination with less frequent DES use (pint=0.02); similarly, bivalirudin showed a higher risk of definite ST in comparison with heparin and more frequent GPI use (pint=0.02). The size of studies, the average proportion of females, the enrolment of STEMI patients, the use of routine ACT measurement and the nature of trial sponsorship did not influence the risk estimates for major bleeding nor for definite ST.
Discussion
We undertook this meta-analysis to investigate the relative efficacy and safety of bivalirudin versus heparin in patients treated with PCI without planned use of GPI. The main findings of this systematic review of randomised trial data are that bivalirudin in comparison with heparin i) does not reduce mortality, ii) decreases the risk of major bleeding, and iii) increases the risk of acute ST.
Anticoagulation is an integral part of interventional therapy of coronary lesions in order to avoid intravascular or device-related clot formation. Heparin has, for a long period of time, been the dominant anticoagulant used in PCI and still maintains a class I recommendation for use1,4. In the last decade, the use of bivalirudin, a direct thrombin inhibitor, has attracted considerable interest: the lack of protein binding, ensuring a more predictable pharmacokinetic together with both anticoagulant and antiplatelet effects, has supported bivalirudin as a valuable therapy alternative to heparin2.
Earlier large-scale randomised trials3,27,28 and meta-analyses8-10 have shown that bivalirudin significantly reduces bleeding-related complications in patients receiving PCI. According to this evidence, bivalirudin has received a class I recommendation as anticoagulant for PCI1,4. Importantly, this recommendation is largely based on trials in which the comparator was the fixed combination of heparin and GPI27,28. Since GPI use for patients undergoing PCI is nowadays recommended primarily as a bail-out rather than a planned routine strategy, the applicability of these results in the current era is open to question. Moreover, the impact of the increasing use of more potent oral antiplatelet inhibitors must also be considered. In addition, some recent data have suggested that bivalirudin may increase the risk of ischaemic complications, calling into question the benefit of this direct thrombin inhibitor in patients undergoing PCI5,22.
The present analysis pooled study-level data from 10 randomised trials including more than 18,000 PCI-treated patients and investigated the efficacy and safety of bivalirudin versus heparin. In contrast to previous meta-analyses on the same topic7-10,29, but in accordance with recommendations of guideline-writing authorities and current clinical practice, the present study included only randomised trials without planned routine use of GPI1,4.
The findings of the current report may be considered important for a number of reasons. Firstly, in our analysis bivalirudin versus heparin showed no sign of mortality benefit. In fact, the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial is to date the only large-scale randomised trial in which the use of bivalirudin as compared with heparin plus planned use of GPI for PCI has reduced mortality3. Although these results were based on wide confidence intervals and were regarded as hypothesis-generating, the lower mortality associated with bivalirudin was supposed plausible as a consequence of bleeding reduction. Indeed, the higher risk of death in patients who bleed after PCI remains undisputed30,31. However, it should be noted that GPI was routinely applied in the control arm of the HORIZONS-AMI trial and that the bleeding-related deaths accounted only in part for the survival difference observed for bivalirudin32,33.
Secondly, the present meta-analysis reports that bivalirudin versus heparin during PCI significantly reduces the risk of major bleeding at 30-day follow-up. Although this result is consistent with all previous studies and meta-analyses9,10, a high degree of heterogeneity was observed, mainly due to different protocol definitions of major bleeding across trials. However, in the meta-analysis of those trials adjudicating major bleeding according to the TIMI definition, bivalirudin remains superior to heparin in terms of bleeding reduction in the absence of significant heterogeneity. Additional sensitivity analysis shows that the bleeding reduction with bivalirudin is more evident when high doses of heparin are used as comparator treatment. This is important, since it has been shown that in PCI patients a low dose as compared with a high dose of heparin reduces bleeding without increasing the risk of thrombotic events34.
Thirdly, in this analysis bivalirudin versus heparin significantly increased the risk of definite ST at 30 days. The large majority of these events occurred within 24 hours after PCI. This issue potentially represents the major drawback of bivalirudin. Indeed, even in the current era of rapid-onset more potent thienopyridine therapy, full antiplatelet inhibition is not achieved until some hours after the administration of the loading dose35. For this reason, insufficient antithrombotic protection in the early phase after revascularisation may expose patients to a higher risk of abrupt vessel closure. Moreover, the sensitivity analysis for definite ST showed that the combination of bivalirudin with less frequent DES use significantly increased the risk of definite ST. The large majority of trials included in this analysis have been performed in the current era of safer DES which have demonstrated a lower risk of ST versus bare metal platforms36. In this respect, it might be speculated that the use of bivalirudin may less effectively neutralise the intrinsic thrombotic risk of platforms other than current DES. Moreover, the sensitivity analysis for definite ST showed that bivalirudin is associated with a higher risk of definite ST when compared with heparin and higher percentages of GPI use. Per protocol, the trials included in the present meta-analysis allowed the use of GPI only as bail-out or provisional indication: on the one hand, a higher percentage of GPI use indicates a higher thrombotic risk; on the other hand, the higher antithrombotic potency of the combination of heparin plus GPI cannot be neglected. Unfortunately, the lack of a mechanistic explanation precludes further speculations concerning the efficacy of bivalirudin in preventing ST in those patients at higher risk for this complication. Surprisingly, although the use of bivalirudin increased the risk of acute ST it did not impact on mortality hazard at 30-day follow-up. This is consistent with previous observations3,5 and is possibly due to the predominant in-hospital occurrence of ST, which is amenable to prompt invasive management.
Finally, at 30-day follow-up, bivalirudin versus heparin increased the risk of urgent TVR in patients receiving stents. However, the risk of MI was not affected. On the one hand, it is intuitive that a higher risk of thrombotic occlusion of the stented vessel carries a parallel increase in the number of urgent (ischaemia-driven) TVR. On the other hand, the moderate heterogeneity reported, due to the different definitions of MI used in the original trials, precludes definitive conclusions.
STUDY LIMITATIONS
The current study has some important limitations. Firstly, this meta-analysis is based on study-level rather than individual patient-level data and shares the limitations of the original trials. Secondly, we elected to include both published and unpublished trials. However, in our opinion the inclusion of “grey literature” is the preferred approach, as prior investigation has suggested that treatment effects may be exaggerated when grey literature is excluded37. Thirdly, different thienopyridines have been used among treatment arms and may have been administered at different intervals before PCI; however, a possible influence of antiplatelet regimens on the risk of thrombotic as well as bleeding complications cannot be addressed in the context of this analysis. Fourthly, the majority of the studies included were powered for composite endpoints of ischaemic and bleeding outcomes: for this reason, analysis regarding relatively infrequent adverse events such as ST should be interpreted with caution. Moreover, although the sources of heterogeneity observed in the risk estimates have been investigated thoroughly, unknown sources of heterogeneity cannot be definitively excluded. Finally, the majority of trials included in the present analysis used the femoral route to perform PCI; the widespread adoption of radial access and vascular closure devices makes further evaluation in these settings a relevant undertaking.
Conclusions
In comparison with the currently used standard regimen of heparin, administration of bivalirudin does not reduce mortality in patients undergoing PCI. However, bivalirudin as compared with heparin appears to reduce the risk of major bleeding at the expense of a higher risk of acute stent thrombosis.
| Impact on daily practice The role of bivalirudin in patients undergoing PCI without planned glycoprotein IIb/IIIa inhibitor remains unclear with four recent clinical trials providing conflicting results. This meta-analysis shows that in patients treated with PCI receiving bail-out or provisional use of glycoprotein IIb/IIIa inhibitor, bivalirudin has no advantage over the currently used standard regimen of heparin in terms of mortality. Although lower rates of bleeding are seen with bivalirudin, the rates of stent thrombosis are higher. |
Conflict of interest statement
R.A. Byrne has received lecture fees from B. Braun and Biotronik. A. Kastrati has submitted patents in relation to a number of DES technologies and has received consulting or lecture fees from Abbott, Biosensors and Biotronik. The other authors have no conflicts of interest to declare.
Online Appendix. PRISMA research checklist.
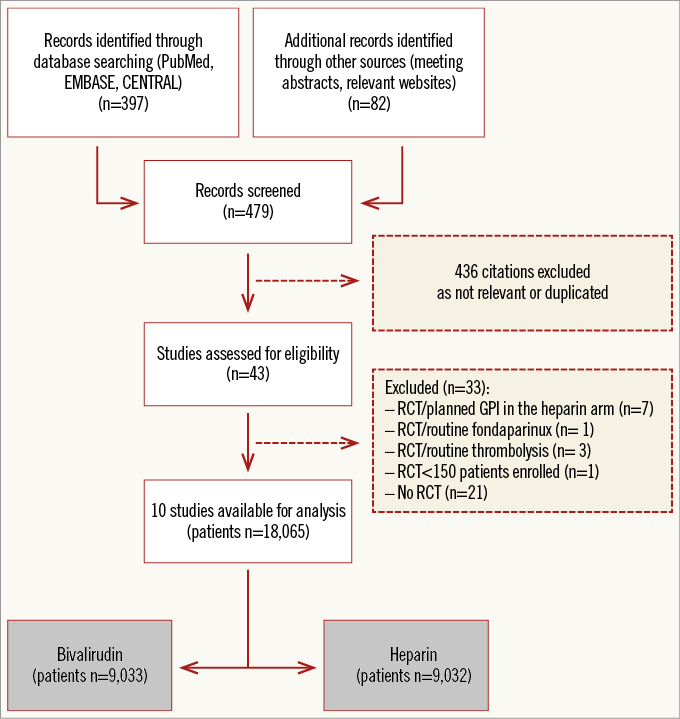
Online Figure 1. PRISMA flow chart for the study selection process. PRISMA: preferred reporting items for systematic reviews and meta-analyses.
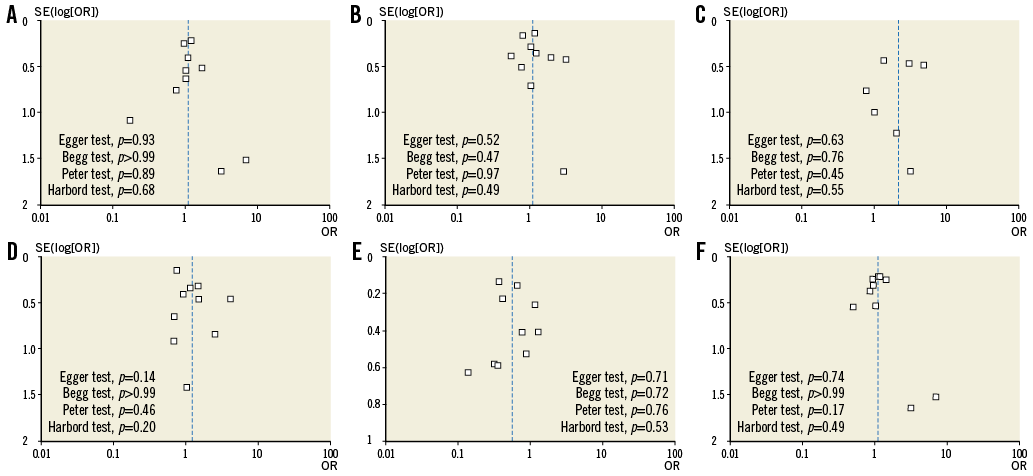
Online Figure 2. Funnel plot distribution of studies included in the meta-analysis according to outcomes of interest. The standard error (SE) of the logarithm of odds ratio (OR) - SE(log[OR]) - is plotted against the OR at 30-day follow-up of death (A), major bleeding (B), myocardial infarction (C), stent thrombosis (D), urgent target vessel revascularisation (E), as well as against the OR of overall death (F). The absence of publication bias can be evaluated both visually and mathematically. A p-value <0.05 indicates significance.