Abstract
Aims: Fondaparinux is an indirect, Factor Xa inhibitor that requires co-administration of another anticoagulant with anti-Factor IIa activity for percutaneous coronary intervention (PCI) per guideline recommendations. In this setting, the use of bivalirudin, a direct Factor IIa inhibitor, is not well established.
Methods and results: Using the Premier hospital database, we identified 971 patients who underwent elective or urgent PCI after receiving fondaparinux as the initial anticoagulant. They were treated with either bivalirudin ± glycoprotein IIb/IIIa inhibitor (GPI) (Group A=618) or unfractionated heparin (UFH) ± GPI (Group B=353) during PCI. A 2:1 propensity score matching (PSM) process was performed to control for patient and hospital level characteristics. The primary endpoints were to determine in-hospital death, bleeding and post-PCI length of stay (LOS) between treatment groups. After PSM, 512 matched patients were analysed (Group A=348 and Group B=174). In-hospital death was 1.4% in Group A vs. 2.9% in Group B (p=0.26). Clinically apparent bleeding occurred in 4.0% of Group A vs. 9.2% of Group B patients (p<0.02). Clinically apparent bleeding requiring transfusion was lower in Group A patients (0.6% vs. 2.9%; p=0.04). Post-PCI LOS was 1.9±3.8 days for Group A and 2.4±5.8 days for Group B (p=0.36). GPI use during PCI occurred in 9.2% of Group A vs. 44.8% of Group B patients (p<0.0001).
Conclusions: After initial administration of fondaparinux, a bivalirudin-based strategy for PCI is associated with significantly reduced bleeding, with similar mortality and post-PCI LOS when compared with an UFH-based strategy.
Introduction
Fondaparinux is an indirect, Factor Xa inhibitor that requires additional anticoagulation with anti-Factor IIa activity, such as unfractionated heparin (UFH) ± a glycoprotein IIb/IIIa inhibitor (GPI), as advocated by professional society guidelines for percutaneous coronary intervention (PCI)1,2. Indeed, a fondaparinux alone strategy for PCI has been associated with an increased risk of catheter thrombosis, and a bolus of an agent with anti-IIa activity is mandatory for PCI with provisional GPI in high ischaemic risk patients3. Switching between anticoagulant strategies (unfractionated or low molecular weight) with the risk of over-anticoagulation during overlapping treatment has been discouraged in the current guideline recommendations (class III) based on the increased rates of bleeding complications, as observed in the SYNERGY trial4,5. On the other hand, bivalirudin, a direct, specific Factor IIa inhibitor without anti-Xa activity, has been consistently associated (with provisional use of GPI) with significantly reduced bleeding and similar ischaemic protection compared with UFH and GPI in high-risk patients undergoing PCI6,7. More recently, in non-ST-elevation myocardial infarction (non-STEMI) patients, abciximab and UFH, as compared to bivalirudin alone (without provisional use of GPI), failed to reduce the rate of net adverse cardiovascular events and increased the risk of bleeding within 30 days8. Additionally, in patients undergoing primary PCI for ST-segment elevation myocardial infarction (STEMI), the use of bivalirudin is associated with significant reductions in short and long-term mortality9,10. Bivalirudin has a short half-life, provides consistent intense Factor IIa inhibition, and carries a markedly reduced risk for bleeding. These advantages have prompted some to call for its routine use in patients pre-treated with fondaparinux11, but this strategy has not been systematically evaluated in randomised or observational studies. Accordingly, the aim of this analysis was to compare the in-hospital mortality, bleeding outcomes and post-PCI LOS in PCI-patients receiving bivalirudin±GPI versus UFH±GPI, after initial treatment with fondaparinux.
Methods
PATIENTS AND COHORT DEFINITION
We used the database maintained by Premier as described previously12. Premier maintains one of the largest US hospital clinical and economic databases. The data contained in the database at the time of this study were received from over 600 hospitals in the Premier healthcare alliance, representing all geographical areas of the USA, a broad range of size of hospitals according to total beds, teaching and non-teaching institutions, and urban and rural facilities. Nearly five million in-patient discharges and 30 million hospital out-patient visits are recorded annually in the Premier database. The database contains standard data elements available in most hospital discharge files, such as demographic data, diagnoses and procedures performed. In addition, the Premier database also contains patient-level, day-of-service billed items including procedures, medications, laboratory, and diagnostic and therapeutic services delivered during the hospitalisation. Premier receives hospital data on a quarterly or monthly basis, and hospitalisations are organised by month and year of admission. The data undergo quality checks and cost information is reconciled with the hospitals’ financial statements. Hospitals use these data to benchmark their clinical and financial performance against their peers.
Data from in-patient hospitalisations between January 2006 and June 2010 identified 530,842 PCI patients with known anticoagulant (bivalirudin, UFH, LMWH) use on the day of PCI suitable for screening. Inclusion criteria included: patients aged 18 years or older of known gender with a diagnosis of stable angina (SA), unstable angina (UA), acute myocardial infarction (AMI) or unknown diagnosis (462,840). Patients must have received fondaparinux on the day of or any day before their PCI (1,006), undergone an initial PCI but not undergone CABG during the same hospitalisation (971).
The final study population was analysed based on the anticoagulant regimen received on the day of PCI: (Group A) those receiving BIV±GPI (618 patients); (Group B) those receiving HEP±GPI (353 patients).
PRIMARY ENDPOINTS AND DEFINITIONS
The primary endpoints examined were in-hospital mortality, bleeding and post-PCI LOS. Follow-up for outcomes started on the index PCI day and until the end of the index hospitalisation. In-hospital mortality was defined as death occurring in hospital, determined from the recorded discharge status. Because we did not have the data elements to use an established definition of bleeding, we further separated bleeding events into “clinically apparent bleeding” and “clinically apparent bleeding requiring transfusion”. These bleeding events were prospectively defined as an ICD-9 diagnosis code for bleeding with or without transfusions. Transfusion events were identified using administrative billing data for whole blood or packed red blood cells. Post-PCI LOS was defined as the date of discharge subtracted from the date of the index PCI.
STATISTICAL ANALYSIS
Discrete data were reported as frequencies and percentages, continuous data as mean and standard deviation. Because the choice of anticoagulant was not randomised and we observed imbalances in the baseline characteristics of the bivalirudin and unfractionated heparin (UFH) patients, a propensity score matching strategy was utilised to reduce the influence of observed imbalances. PSM was accomplished by first developing a list of patient and hospital variables thought to influence both treatment choice and patient outcome. A logistic regression model was used to estimate the log-odds (logit) of receiving UFH for each patient while controlling for the patient and hospital-level characteristics described above. We next used a Greedy matching algorithm without replacement to match one UFH patient to two bivalirudin patients to increase statistical efficiency. We accepted as matches only those bivalirudin patients who were within 0.6 of a standard deviation of the UFH patient’s estimated log-odds of receiving UFH. The 0.6 value was selected as it has been shown to eliminate approximately 90% of the bias in observed confounders. Statistical differences between the treatment groups before and after propensity score matching were determined using the Chi-square test for discrete data and Wilcoxon-Mann-Whitney test for continuous data.
Results
From the Premier database we identified 971 patients who underwent elective or urgent PCI in 80 US hospitals who were treated with fondaparinux before or on the day of PCI and administered either bivalirudin±GPI (Group A=618) or UFH±GPI (Group B=353) on the day of PCI.
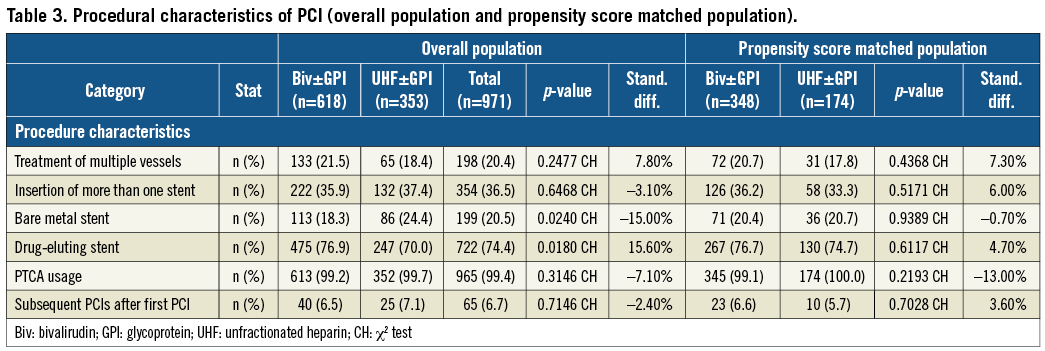
Baseline clinical characteristics of the study population before and after propensity score matching are shown in Table 1. The mean age of the overall population was 65±12 years with a majority of male patients. One third of the PCIs were performed in ACS patients including 7.3% STEMI patients and 26.9% of non-ST-segment myocardial infarction (NSTEMI) patients. Medications given to the patients before, during and after PCI are shown in Table 2. As expected, the use of GPI was lower in the bivalirudin treatment group (Group A) compared to the UFH-based strategy (Group B) (49.3% versus 9.2%, respectively; p <0.0001). The procedural characteristics of PCI are summarised in Table 3. Drug-eluting stents were used in 74.4% of cases.
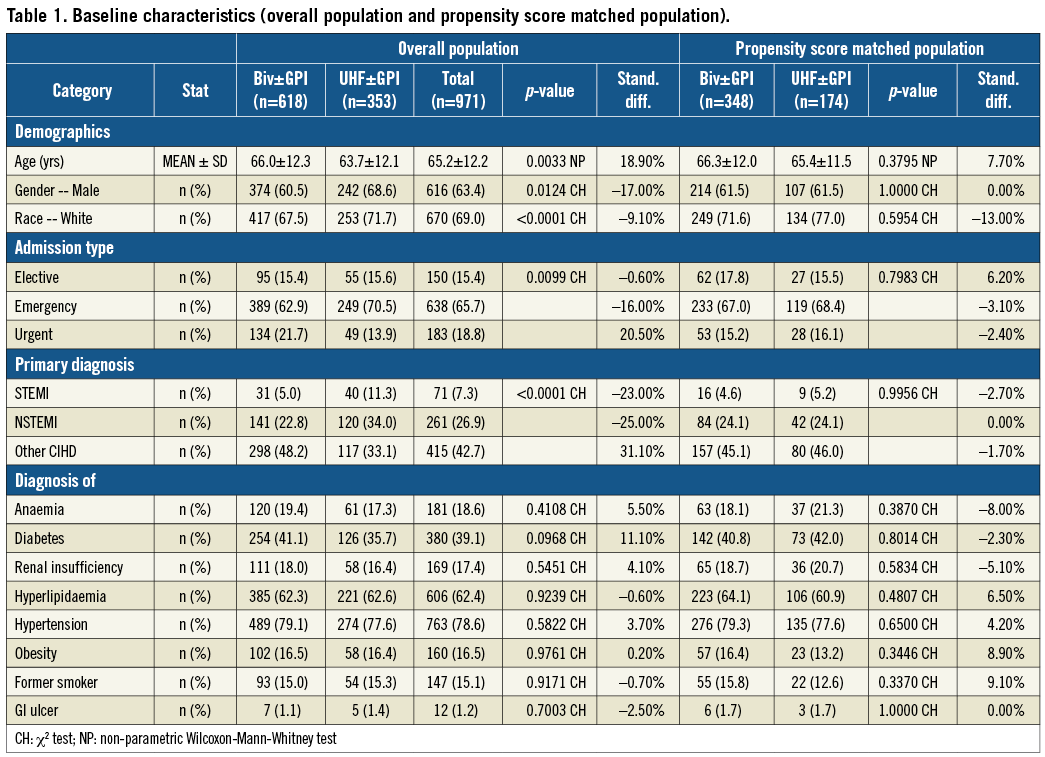
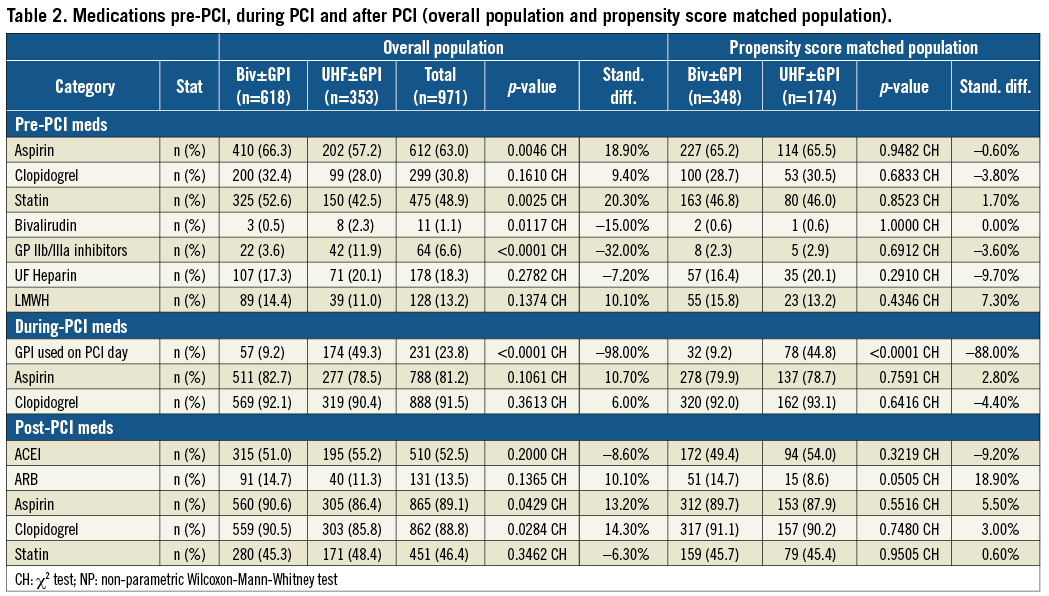
Primary outcomes are shown in Table 4. Before PSM, all outcomes were lower in Group A vs. Group B, with significant differences seen in clinically apparent bleeding and post-PCI LOS. In-hospital death was 1.5% in Group A vs. 2.8% in Group B (p=0.14). Clinically apparent bleeding occurred in 4.5% of Group A vs. 8.8% of Group B patients (p<0.01). Clinically apparent bleeding requiring transfusion was 1.1% in Group A and 2.5% in Group B (p=0.10) (Figure 1A). Post-PCI LOS was 1.7±4.0 days for Group A and 2.4±5.1days for Group B (p<0.001).
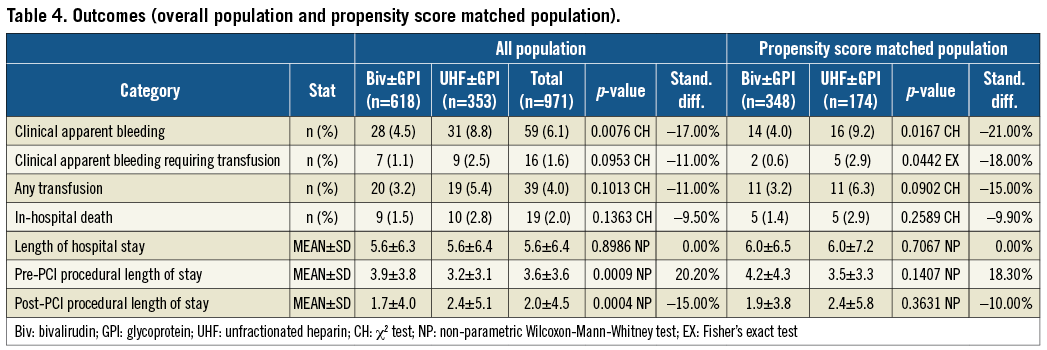
After PSM, 512 matched patients were analysed (Group A=348 and Group B=174). Again, all values were lower for Group A vs. Group B, with statistical differences now evident in clinically apparent bleeding with or without transfusion. In-hospital death was 1.4% in Group A vs. 2.9% in Group B (p=0.26). Clinically apparent bleeding occurred in 4.0% of Group A vs. 9.2% of Group B patients (p<0.02). Clinically apparent bleeding requiring transfusion was lower in Group A patients (0.6% vs. 2.9%; p=0.04) (Figure 1B). Post-PCI LOS was 1.9±3.8 days for Group A and 2.4±5.8 days for Group B (p=0.36).
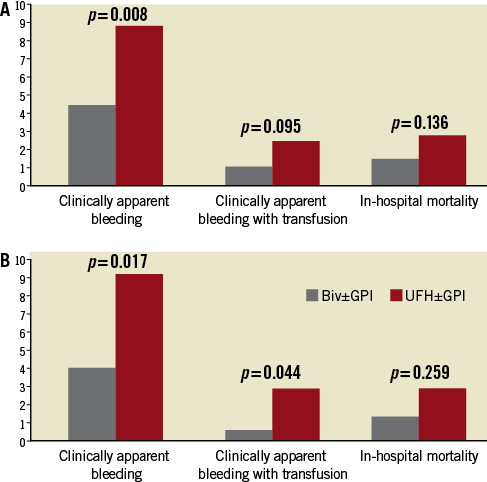
Figure 1. In-hospital events (clinically apparent bleeding, clinically apparent bleeding requiring transfusion and in-hospital mortality) in PCI-patients comparing bivalirudin±GPI versus UFH±GPI after prior administration of fondaparinux. Results in the whole population (A) and in the propensity matched population (B).
Discussion
After initial administration of fondaparinux, a bivalirudin-based strategy for PCI compares favourably to the conventional use of UFH±GPI. In our non-randomised analysis of PCI patients, after adjusting for baseline imbalances, neither in-hospital mortality nor post-PCI length of stay was different between the groups (but trended lower with bivalirudin therapy), while statistically significant reductions in clinically apparent bleeding and clinically apparent bleeding requiring transfusion were observed for patients treated with bivalirudin±GPI compared with UFH±GPI.
Treatment pathways for NSTEMI or STEMI patients frequently include some pharmacologic therapy at first medical contact, including anticoagulant and/or antiplatelet drugs. Therefore, interventionalists routinely encounter patients who have received one or more of these drugs and must make the decision whether to continue, stop or switch to other agents for angiography and PCI. In the recently published ESC guidelines on myocardial revascularisation, the golden rule to continue the initial therapy and avoid switching heparin therapy has been underlined with the exception of fondaparinux1. In the OASIS-5 trial, fondaparinux was compared to enoxaparin in ACS patients treated with an initial conservative strategy: fondaparinux use resulted in a similar rate of composite ischaemic events while halving severe bleeding complications3. However, higher rates of catheter thrombosis were observed with fondaparinux alone in the early stages of the trial and the protocol was amended to mandate a bolus of UFH for patients who underwent angiography and PCI. In the FUTURA/OASIS-8 trial, comparing the safety of two UFH regimens (low and standard doses) during PCI of high risk NSTE-ACS initially treated with fondaparinux, similar peri-PCI bleeding and vascular access-site complications were found in both groups13. Rates of catheter thrombosis were very low with a trend for reduction in the standard dose (0.5% in the low-dose group versus 0.1% in the standard-dose group, p=0.15)13. An important consideration is that catheter thrombosis is probably under-reported in clinical trials except in the series of trials with fondaparinux. However, the degree to which thrombus formation on guiding catheters, guidewires, balloon catheters and stents may contribute to periprocedural ischaemic events including myocardial infarction, silent stroke and the no-reflow phenomenon is largely unknown14. In vitro experiments investigating contact-induced thrombosis have shown that treatment with fondaparinux alone failed to prevent catheter thrombosis during continuous perfusion of blood within in vitro circuit while patency of the circuit was effectively maintained with simultaneous addition of UFH15. Bivalirudin, a direct thrombin inhibitor, alone or with provisional use of GPI, was compared with UFH+GPI in intermediate and high risk NSTE-ACS and in STEMI patients6-8. Bivalirudin therapy consistently reduced bleeding while conferring similar ischaemic protection compared with UFH+GPI. It is important to remember that a multitude of trials have demonstrated the need for GPI use in patients with high risk ACS (NSTEMI, STEMI) treated with an indirect thrombin inhibitor such as UFH or LMWH in order to achieve adequate ischaemic protection16,17. In that regard, the higher percentage of GPI use among the UFH-treated patients in our study, that included a significant portion of ACS patients, is to be expected. Importantly, in STEMI patients, the favourable net clinical outcome of bivalirudin therapy translated into significant reductions in both cardiac and overall mortality that remained robust up to three years of follow-up18.
The ability to switch safely to bivalirudin at the time of PCI in patients previously treated with UFH or LMWH has been previously reported in both stable and NSTE-ACS and STEMI patients19-21. Specifically, in the HORIZONS-AMI trial, the use of UFH prior to randomisation was a pre-specified analysis and this practice was observed in two-thirds of the overall study population. Importantly, within this pre-specified subgroup, switching to bivalirudin resulted in significant reduction in bleeding and improved early and late cardiac survival compared to UFH+GPI21. The benefit of bivalirudin was consistent regardless of the ACT at the onset of PCI (either therapeutic [>250 seconds] or sub-therapeutic [<200 seconds]) suggesting that the time interval between the administration of the UFH bolus and the initiation of bivalirudin is not clinically relevant. In all-comer PCI-patients treated with fondaparinux the use of bivalirudin in the present analysis also seems to provide effective and safe outcomes comparing favourably to the conventional use of UFH-based PCI anticoagulation strategy.
The lower rate of GPI use in the bivalirudin group may in part explain the reduction in bleeding and transfusion requirements compared to the UFH group. Given the rate of ACS patients in the present cohort, the use of GPI in the UFH group (49%) was expected, and the addition of GPI in the bivalirudin group (9%) was slightly higher than rates observed in randomised studies6,7,9. The bleeding risk assessment and how this potential factor has influenced the anticoagulant option chosen by the physicians for PCI in this patient cohort is unknown because planned vs. bail-out use of GPI is not recorded in the database.
Several important limitations of the present analysis warrant discussion. This study is an observational retrospective analysis from the Premier database. Differences were present between the two groups at baseline that may have impacted on the results, and potential unmeasured confounders may be present which cannot be adjusted by propensity analysis. Thus, our results should be considered hypothesis-generating. Prospective, randomised trials are needed to evaluate further the impact on patients’ outcomes of bivalirudin as the default antithrombin strategy in high ischaemic risk PCI-patients pretreated with fondaparinux.
In conclusion, in our prospective, contemporary, hospital-database-derived PCI patient population, and after adjusting for observed baseline imbalances, we observed significant reductions in clinically apparent bleeding and clinically apparent bleeding requiring transfusion for patients treated with bivalirudin±GPI compared with UFH±GPI. Although mortality rates and LOS also were numerically lower in bivalirudin-treated patients, these outcomes did not achieve statistical significance. After initial administration of fondaparinux, a bivalirudin-based strategy for PCI seems to be a valuable alternative to an UFH-based anticoagulation strategy.
Conflict of interest statement
M. Hamon has received fees for lectures, advisory board or consulting services in the last two years from Cordis, Terumo, The Medicines Company, Lilly, Biotronik, Medtronic and research grants from The Medicines Company, GlaxoSmithKline and Lilly. S.V. Rao is a consultant for The Medicines Co, Eli Lilly, Terumo and ZOLL; he is on the Speakers’ Bureau for The Medicines Co and Boehringer Ingelheim and has received research funding from Ikaria and Sanofi-Aventis. P.G. Steg has received research grants (to INSERM U698) from the NYU school of Medicine, Sanofi and Servier; he is a speaker for or has received consulting fees from Ablynx, Amarin, Amgen, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Daiichi- Sankyo-Lilly, Eisai, GSK, Medtronic, Merck, Novartis, Otsuka, Pfizer, Roche, Sanofi-Aventis, Servier, The Medicines Company; and he is a stockholder of Aterovax. M Valgimigli has received honorarium as a public speaker from the following companies: Terumo, The Medicines Company, Medtronic, Iroko, Merck, Abbott, Ely Lilly, Astra Zeneca, Cordis, CID and Bayer. F. Verheugt has received educational and research grants from Bayer Healthcare, Roche, Eli Lilly and Boehringer Ingelheim and received honoraria for consultancies from Daiichi-Sankyo, Eli Lilly, Merck, The Medicines Company and Bayer Healthcare. S. Marso reports no personal conflicts of interest during the previous 12 months. All compensation for his research activities, including research grants and consulting fees from The Medicines Company, Novo Nordisk, Abbott Vascular, Amylin Pharmaceuticals, Boston Scientific, Volcano Corporation, and Terumo Medical, are paid directly to the Saint Luke’s Hospital Foundation of Kansas City. A. Gerschlick has received honoraria for lectures and is on the Advisory Board of Medtronic, Abbott Vascular, Boston Scientific,The Medicines Company and Daiichi-Sankyo. Y. Wang and E. Deliargyris are employees of The Medicines Company.

