KEYWORDS
Abstract
Aims: We studied the clinical and economic impact of bivalirudin in clinical practice.
Methods and results: Consecutive patients undergoing PCI via the common femoral artery for stable, unstable, or atypical angina, silent ischaemia, or non-ST-elevation myocardial infarction indications during 2007-2008 were prospectively studied. In-hospital bleeding events were systematically assessed and classified as either major or minor. Use of bivalirudin, vascular closure devices, heparin and/or glycoprotein (GP) IIb/IIIa inhibitor was at the operator’s discretion. Among 1,364 patients, 503 received bivalirudin and 861 received usual care consisting of either heparin monotherapy (n=687) or heparin+GP IIb/IIIa (n=174). Any post-PCI bleeding occurred in 356 (26.1%) patients, including 32 (2.3%) major and 324 (23.8%) minor events. Compared with usual care, bivalirudin was associated with reduced bleeding before adjustment (any: 17.3% vs. 31.2%, P<0.001; major: 1.2% vs. 3.0%, P=0.03; minor: 16.1% vs. 28.2%, P<0.01) and after propensity-matching (OR 0.46, 95% CI 0.34-0.63, P<0.001). Use of vascular closure devices was associated with an increase in any bleeding (32.2% vs. 17.7%, P<0.001), primarily due to an increase in minor bleeding (30.8% vs. 14.1%, P<0.001) while there was a significant decrease in major bleeding (1.4% vs. 3.7%, P=0.007). Bivalirudin was associated with total hospitalisation costs that were lower than usual care (mean cost savings, $463/patient; 95% CI 1,594 less to 621 more).
Conclusions: In this prospective PCI cohort, bivalirudin was associated with reduced major and minor bleeding without a significant increase in hospital costs compared with other anticoagulation regimens. Closure device use was associated with decreased major but increased minor bleeding.
Introduction
Bleeding following percutaneous coronary intervention (PCI) is associated with a higher frequency of morbidity, including myocardial infarction (MI)1,2 and death1,3-6. Bleeding also results in increased length of stay and hospital cost7-9. Current anticoagulation and antiplatelet therapies reduce post PCI ischaemic complications yet are associated with increased rates of bleeding10-12. In randomised controlled trials conducted in multiple clinical settings12-17, bivalirudin (Angiomax®, The Medicines Company, Parsippany, NJ, USA) has demonstrated efficacy in PCI patients. Bivalirudin is also associated with lower rates of bleeding13,17,18, shorter length of stay9, and lower in-hospital costs8. To date, much work has focused on bivalirudin in randomised controlled populations, with few studies assessing the association between bivalirudin and bleeding in actual clinical practice.
The purpose of this study was to prospectively assess the clinical and economic impact of bivalirudin in a clinical PCI practice, specifically with respect to in-hospital bleeding events and cost outcomes. Additional analyses were performed in various subgroups, including use/non-use of glycoprotein (GP) IIb/IIIa inhibitors and vascular closure devices.
Methods
Population and centres
Consecutive patients >18 years old undergoing PCI from May 2007 to October 2008 were prospectively studied at a single health system consisting of four PCI centres. Bivalirudin was not available for use in PCI patients in the time preceding study commencement. Indications for PCI were limited to stable, unstable, or atypical angina, silent ischaemia, or non-ST-elevation MI. Patients with ST-elevation MI were excluded. All patients underwent PCI via the common femoral artery. Data were collected using a standardised data collection form. This study was considered a quality improvement initiative and informed consent was waived19 by the Saint Luke’s Hospital Institutional Review Board.
Patient groups were defined according to anticoagulant use. Choice of anticoagulants was at the operator’s discretion. All patients received clopidogrel prior to PCI consisting of a 600 mg loading dose for patients undergoing catheterisation within six hours and 300 mg loading dose for all other patients. A 75 mg maintenance dose was continued for all patients. Patients either received usual care (heparin 70 u/kg with or without eptifibatide 180 mcg/kg bolus before PCI followed by a second 180 mcg/kg bolus 10 minutes later in all patients and 2 mcg/kg/min infusion up to 18 hours in patients with creatinine clearance >50 or 1 mcg/kg/min in patients with creatinine clearance <50) or bivalirudin (with or without IIb/IIIa inhibitor). In ACS patients, upstream use of eptifibatide was permissible in the bivalirudin arm. Bivalirudin was administered as a 0.75 mg/kg bolus in all patients followed by a 1.75 mg/kg/hr infusion for the duration of the procedure in patients with a glomerular filtration rate >30 mL/min/1.73 m2, 1.0 mg/kg/hr in patients with glomerular filtration rate <30 mL/min/1.73 m2, or 0.25 mg/kg/hr in chronic haemodialysis patients. The infusion of bivalirudin was stopped immediately post-procedure in all patients. Patients receiving usual care did not receive bivalirudin either in-lab or upstream at any time during the peri-PCI time period. The use of closure devices (Angio-Seal™, St. Jude Medical, St. Paul, MN, USA; or Perclose® A-T, Abbott Vascular, Abbott Park, IL, USA) was also at the discretion of the operator. A standard sheath removal protocol was used in patients receiving vascular closure devices, with the sheath removed two hours following discontinuation of bivalirudin and after activated clotting time <160 s.
Outcome measures and definitions
Post-PCI bleeding events were systematically assessed by trained healthcare providers in all patients and recorded using a standardised data collection tool. All providers were trained by single investigator (L.R.) Bleeding events were defined a priori as either major or minor. Major bleeding was identified using National Cardiovascular Data Registry® (NCDR®) CathPCI registry version 3.04 definitions (https://www.accncdr.com/webncdr/DefaultCath PCI.aspx), which includes events resulting in transfusion, prolonged hospital stay, and/or >3.0 g/dL decrease in haemoglobin during or after catheterisation until discharge. Major bleeding events are further classified by location at 1) percutaneous entry site and originating externally or manifesting as haematoma with size based on anatomic site (>10 cm for femoral, >5 cm for brachial, or >2 cm for radial); 2) retroperitoneal; 3) gastrointestinal; 4) genitourinary; or 5) other/unknown origin. Minor bleeding events were defined as 1) bruising with haematoma and sub-classified by size (<5 cm, 5-10 cm, or >10 cm), 2) bleeding requiring re-hold, or 3) bleeding event changing the course of hospitalisation as determined by clinical staff. Post-PCI adverse events and total hospital costs, including drug acquisition, in-lab, and post-lab costs, were also analysed. Other outcomes of interest included in-hospital mortality, procedural length of stay, urgent revascularisation (percutaneous or surgical), and post-PCI MI. Creatine kinase-MB was assessed every eight hours (available in 97% of patients). Periprocedural MI was defined as creatine kinase-MB >3x the upper limit of normal within 16 hours following PCI. Post-PCI renal failure was defined as an increase in creatinine of 0.5 g/dL. Total hospital costs, including anticoagulation, from date of PCI procedure to discharge were obtained from the hospital accounting system. Actual catheterisation laboratory costs were used in place of hospital estimates for procedural supplies and devices. Procedural supply costs, including balloons, stents, atherectomy devices, contrast dye and guidewires, were provided directly from the hospital’s catheterisation lab cost accounting system. Length of hospital stay and associated costs were adjudicated such that costs were included beginning on the date of the procedure and continuing until either discharge or three days from the procedure, whichever was earlier. Hospital costs beyond three days post-procedure were included only if adverse events or bleeding complications potentially related to the procedure were reported for the patient.
Statistical analysis
Continuous variables are described as mean±standard deviation and compared using unpaired t-tests. Categorical variables are described as counts and percentages and compared by χ2 or Fisher’s exact test. Multivariable logistic regression was performed to assess the impact of bivalirudin use on bleeding with results presented as odds ratios (95% confidence intervals). Covariates in the model were age, gender, body mass index, diabetes, previous MI, previous heart failure, coronary artery disease, peripheral vascular disease, chronic lung disease, hypertension, smoking, previous PCI, previous coronary artery bypass surgery, current heart failure, non-ST-elevation MI, and multivessel PCI. As a sensitivity analysis to assess the impact of bivalirudin on bleeding, a propensity model was developed for bivalirudin using the covariates listed above. Patients were matched 1:1 by propensity to receive bivalirudin with a 1% maximum difference allowed in the match. An additional exploratory analysis was performed to assess clinical and economic outcomes of bivalirudin and vascular closure devices in patients at high risk of bleeding according to a risk model developed using data from the NCDR20. Cost and length of stay data were compared by Wilcoxon rank-sum tests. Per patient costs attributable to bivalirudin and vascular closure devices were US $1,063 and $235, respectively. Due to the right-skewed nature of the distribution, length of stay and total costs greater than the 99th percentile in each treatment group were assigned to the 99th percentile for the group to reduce the impact of outliers on group means. Bootstrapping techniques with 1,000 replications using the percentile method21 were used to compute 95% percent confidence intervals around mean unadjusted differences in costs for groups of interest (bivalirudin vs. no bivalirudin, major vs. no bleeding event, minor vs. no bleeding event). Multivariable linear regression was used to adjust costs for in-hospital coronary artery bypass grafting, multivessel PCI, previous heart failure, acute coronary syndromes (ACS) on presentation, and peak creatine kinase-MB >3 times the upper limit of normal. All comparisons were two-tailed, with a P value <0.05 indicating statistical significance. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
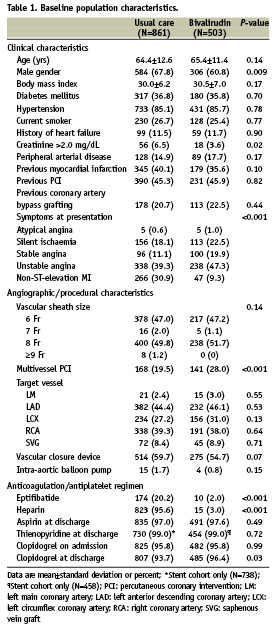
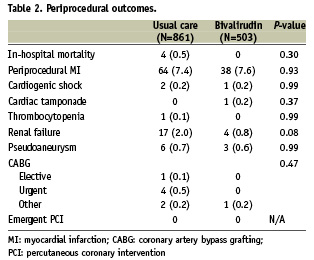
Baseline characteristics of patients receiving bivalirudin vs. usual care are shown in Table 1. Patients who did not receive bivalirudin were more likely to be male, present with renal failure, and undergo PCI for non-ST-elevation MI indications. Upstream heparin was used in 15 (3.0%) patients who were transitioned to bivalirudin in the catheterisation laboratory. No patients received low molecular weight heparin. There were no differences between patients receiving bivalirudin vs. usual care in post-PCI complications including periprocedural MI, cardiogenic shock, cardiac tamponade, emergent PCI, emergent/urgent coronary artery bypass grafting, and in-hospital mortality (Table 2).
Overall, bleeding occurred in 356 (26.1%) patients and was classified as major in 32 (2.3%) and minor in 324 (23.8%). Among patients with major bleeding events, 17 (53%) were at access site and 15 (47%) were at non-access sites. Patients with major bleeding events had significantly longer hospital stays compared with patients with minor bleeding events and no bleeding events (5.0±3.2 days vs. 1.7±1.7 days and 1.6±1.6 days, respectively, P<0.001).
Figure 1 compares rates of bleeding (any, major, and minor) for the overall cohort by use of usual care vs. bivalirudin (Figure 1A) and for subgroups according to the use of GP IIb/IIIa inhibitor (Figure 1B), and vascular closure devices (Figures 1C and 1D). Overall (N=1,364), bivalirudin was associated with a statistically significant 56% reduction in any bleeding event (OR 0.44, 95% CI 0.34-0.59, P<0.001) (Figure 2). In patients who did not receive GP IIb/IIIa inhibitor (N=1,180), 687 (58%) received heparin and 493 (42%) received bivalirudin. Among these patients, bivalirudin reduced overall risk of composite bleeding by an unadjusted 55% (OR 0.45, 95% CI 0.33-0.60, P<0.001), including a significant reduction in minor bleeding and a trend toward lower rates of major bleeding events (Figure 1B).
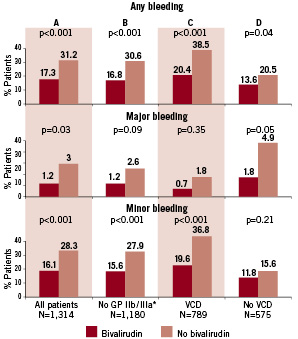
Figure 1. Major, minor, and composite rates of bleeding by bivalirudin use in (A) all patients and patient subgroups according to use of (B) heparin without a glycoprotein IIb/IIIa inhibitor, (C) use, and (D) non-use of a vascular closure device. *Bivalirudin monotherapy was administered to 493 patients. VCD: vascular closure device
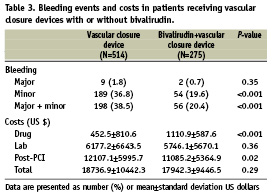
Among all patients, 789 (57.8%) received a closure device (Perclose: n=286 [36.2%]; Angio-Seal: n=491 [62.2%]; other: n=12 [1.5%]). Closure device use was associated with an increase in overall bleeding (32.2% vs. 17.7%, P<0.001). While there was a decrease in major bleeding (1.4% vs. 3.7%, P=0.007), there was a significant increase in the rate of minor bleeding (30.8% vs. 14.1%, P<0.0001) in patients receiving a closure device. Use of bivalirudin (n=275) vs. non-use (n=514) in the closure device cohort was associated with a significant reduction in overall bleeding events (OR 0.39, 95% CI 0.27-0.56, P<0.001), which was driven by a statistically significant reduction in minor bleeding events and a non-significant reduction in major bleeding (Figure 1C). In patients not treated with a closure device (n=575), bivalirudin use (n=228) was associated with significantly lower rates of overall bleeding (OR 0.60, 95% CI 0.38-0.95, P<0.001), driven by a significant reduction in major bleeds (Figure 1D).
Results from unadjusted, multivariable adjusted, and propensity matched logistic regression analyses examining the impact of bivalirudin on the risk of bleeding events are shown in Figure 2. The estimated reduction in the risk of bleeding associated with the use of bivalirudin was similar in magnitude and significance for each analysis.
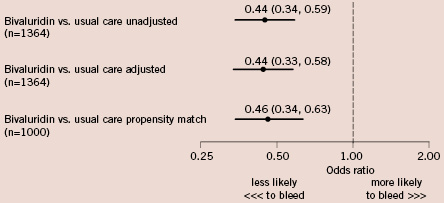
Figure 2. Unadjusted and adjusted odds ratios and 95% confidence intervals of bleeding associated with use of bivalirudin. A 1:1 propensity matching analysis was performed using covariates in the logistic regression model including age, gender, body mass index, diabetes, previous MI, previous heart failure, coronary artery disease, peripheral vascular disease, chronic lung disease, hypertension, smoking, previous PCI, previous coronary artery bypass surgery, current heart failure, non-ST-elevation MI, and multivessel PCI.
In-hospital costs
Incremental costs and cost offsets associated with the use of bivalirudin ($1,063 per patient) and the incremental costs associated with major and minor bleeding events are presented in Figure 3. Bivalirudin was associated with total hospitalisation costs that were $463 (95% CI 1,595 less to 621 more) lower than usual care. This was driven by a significant cost savings of $1,172 (95% CI 1814 less to 566 less) in post-PCI care but was partially offset by $630 (95% CI 554 more to 702 more) in greater costs to acquire bivalirudin (Figure 3A). Major bleeding events were associated with $11,266 (95% CI 5,961 more to 17,476 more) in excess hospital costs before adjustment, largely due to an increase of $9,700 (95% CI 5,927 more to 13,740 more) in post-PCI costs (Figure 3B). Major bleeding events were associated with significantly higher total hospital costs both before and after adjustment for in-hospital coronary artery bypass grafting, ACS, previous heart failure, multivessel PCI, and CK-MB >3 times the upper limit of normal. The increase in hospital costs associated with minor bleeding was not significant (Figure 3C). Bleeding events and costs associated with the use of vascular closure devices with or without bivalirudin are presented in Table 3.
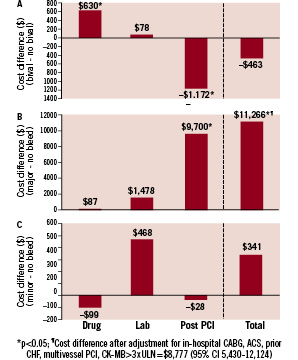
Figure 3. Unadjusted differences in total hospitalisation costs by (A) bivalirudin use, (B) major bleeding events, and (C) minor bleeding events. Drug, laboratory, and post-procedural cost categories were used to calculate totals. Statistical significance of cost differences derived from bootstrap re-sampling (1,000 replicates). Lab: catheterisation laboratory costs
Treatment by bleeding risk
In an exploratory analysis, we used a published model that estimates bleeding risk rates20 to evaluate the efficacy of bivalirudin and closure device use in patients at highest risk for bleeding. There were 323 patients classified as high risk (expected rate of bleeding >3%). The overall rate of major bleeding was 5.0% in this group; the highest rate was observed in patients receiving standard of care (Figure 4A). Compared with no treatment, the use of bivalirudin, vascular closure devices, or both was associated with a statistically significant reduction in major bleeding (P=0.02). A similar exploratory cost analysis of bivalirudin usage by bleeding risk is shown in Figure 4B.
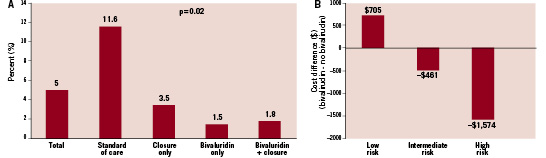
Figure 4. (A) Exploratory analysis of patients estimated to be at >3% risk of bleeding events calculated using a validated risk prediction algorithm20. Actual rates of major bleeding events are presented for all high risk patients and for patient subgroups defined according to use of specific bleeding avoidance therapies. (B) Estimated incremental hospital costs are presented for patients at low (<1.0%), medium (1.0-3.0%), and high (>3.0%) risk for a bleeding event. Statistical significance of cost differences derived from bootstrap re-sampling (1,000 replicates).
Discussion
In this study, bivalirudin use was associated with a significant reduction in post-PCI bleeding in the overall cohort, as well as in a large cohort receiving heparin monotherapy, which is an under-represented group in clinical trials. Vascular closure device use was associated with reduced major and increased minor bleeding rates. In vascular closure device patients bivalirudin was associated with lower rates of minor bleeding compared with the usual care group. In patients not treated with vascular closure devices, bivalirudin use was associated with a statistically significant reduction in major bleeding compared with the usual care group and a non-significant reduction in minor bleeding. Finally, the combination of bivalirudin and a closure device may be particularly efficacious in patients at heightened risk for bleeding as determined by a clinically applicable risk prediction algorithm.
In the United States, nearly one million individuals undergo PCI procedures annually22. Although the safety of PCI has improved in terms of low rates of in-hospital mortality, acute vessel closure, and need for repeat urgent revascularisation, bleeding rates vary considerably both across PCI centres and by clinical syndromes3,5,23. Bleeding is also morbid and costly. Major bleeding events result in a three to four day increase in length of stay7, in turn increasing hospitalisation costs by $6,000-8,0009. Bleeding is strongly associated with early and late mortality4,24, non-fatal MI, stroke, and need for blood transfusions5. Importantly, in ACS patients, major bleeding is associated with a prolonged excess risk of mortality over one year after the index procedure, and death attributable to major bleeding events is similar to that related to non-fatal MI25.
According to the Agency for Healthcare Research and Quality Healthcare Cost and Utilisation Project (HCUP), a database of community hospitals in the United States, (http://www.ahrq.gov/ data/hcup/datahcup.htm) one-half of all PCI-related bleeding events require blood transfusions, translating into 1.7% of 830,000 PCI procedures in 2006. In HCUP data, PCI-related bleeding with blood transfusion was associated with greater median length of stay (2.0 vs. 6.0 days) and mean hospital charges ($48,000 vs. $85,000), resulting in approximately $1 billion in excess healthcare expenditures for PCI-related blood transfusions. Patients requiring blood products are 50% less likely to be discharged directly to home and require additional medical services including nursing home, skilled nursing facilities, rehabilitation services, and home health care23,26. Our results are consistent with the ACUITY investigators who have shown bivalirudin to be associated with greater savings in costs of hospitalisation due to avoidance of major and minor bleeding events9. We believe bleeding and its associated morbidities are a valid target of therapy. Avoidance of bleeding events addresses two important aspects of health care delivery: cost reduction and improvement in PCI quality.
Data establishing reductions in access site bleeding associated with the use of vascular closure devices are heterogeneous and mostly from non-randomised studies. The few randomised controlled trials are limited methodologically and were designed to assess the relationship between closure devices and ambulation time rather than bleeding. A meta-analysis of 30 clinical studies including 4,000 patients showed a trend for reduced vascular complications associated with the Angio-Seal device (OR 0.46, 95% CI 0.20-1.04, P=0.06)27. However, the lack of systematic ascertainment of both major and minor bleeding limits prior study results. Data from a recent prospective registry demonstrated vascular closure devices to be associated with a 42% reduction in vascular complications following PCI, which persisted following multivariable and propensity analyses and was driven in part by reductions in bleeding and haematoma28. In the present study, vascular closure devices were associated with a significant reduction in major bleeding (1.4% vs. 3.7%, P=0.007) but an increase in minor bleeding compared with manual pressure (30.8% vs. 14.1%, P<0.0001).
Prior data are also limited comparing heparin monotherapy with bivalirudin. ISAR-REACT 3 investigators randomised over 4,000 elective PCI patients to bivalirudin or heparin, of which 0.2% in each arm also received GP IIb/IIIa inhibitors17. There was no reduction in the composite endpoint of death, MI, or urgent revascularisation associated with bivalirudin, although there was a reduction in major bleeding (3.1% vs. 4.6%, P=0.008). While it is difficult to compare our bleeding rates to those reported by others due to heterogeneity in bleeding definitions, the present data also suggest that reductions in bleeding can be expected with bivalirudin compared with heparin monotherapy. Therefore these findings are generalisable to PCI practices with lower rates of GP IIa/IIIb inhibitor use.
Future applicability
To our knowledge, there are no data evaluating the efficacy of bivalirudin and vascular closure devices in patients at high risk for bleeding. Using data from the NCDR, we previously developed and validated a model to estimate risk of bleeding20. From a set of multivariable predictors of bleeding, this model was simplified into a clinically useful algorithm consisting of the 10 most significant covariates. This model identifies three risk categories of bleeding (<1.0%, 1.0-3.0%, and >3.0%)20. In the present study, vascular closure devices and bivalirudin were both associated with substantial reductions in observed bleeding rates among patients at high risk for bleeding calculated using this risk algorithm. Although exploratory, these findings suggest bleeding avoidance therapies may exert differential effects based on patient risk for PCI-related bleeding. Future comparative effectiveness work should focus on determining the potential to target these expensive therapies to patients most likely to benefit.
Limitations
The present study was an observational design rather than a randomised trial, thus selection bias likely exists. As shown in Table 1, pre-selection to heparin was seen in the non-ST-elevation MI cohort, as administration of upstream heparin with provisional GP IIb/IIIa receptor antagonists is consistent with our institutional practice. However, other measured covariables were reasonably balanced. It is also noteworthy that following propensity matching the point estimate favouring bivalirudin for bleeding reduction was nearly equivalent to the unadjusted point estimate. Since patients with ST-elevation MI were excluded from the present study, these findings cannot be extended to this patient subset. Economic outcomes were not the primary endpoints, therefore reported cost effectiveness comparisons are likely underpowered to draw any definite conclusions and require confirmation in a larger study. Since the present study consisted of only patients undergoing femoral access PCI, our findings may not be generalisable to centres performing a majority of procedures via radial access. It should be noted, however, that femoral access is the dominant PCI strategy in the United States. Lastly, the associations between vascular closure devices and major and minor bleeding as well as the large effect of combined use of bivalirudin and vascular closure devices on reductions in major bleeding events in high risk patients should be considered exploratory. These treatment effects will be evaluated in a future large-scale comparative effectiveness study.
Conclusions
In a consecutive PCI population, bivalirudin was associated with significantly lower major and minor bleeding rates and similar total hospital costs compared with patients receiving other anticoagulation regimens. Restricted to patients receiving heparin monotherapy, bivalirudin was also associated with reduced bleeding rates.
Acknowledgements
We thank Jose Aceituno and Joseph Murphy for publication assistance.

