
Abstract
Aims: Bivalirudin use as a procedural anticoagulant in patients undergoing percutaneous coronary intervention (PCI) is associated with a lower incidence of thrombocytopaenia compared to other antithrombotic agents. We aimed to evaluate the prognostic impact of baseline thrombocytopaenia and early changes in platelet counts among patients undergoing PCI with exclusive use of bivalirudin.
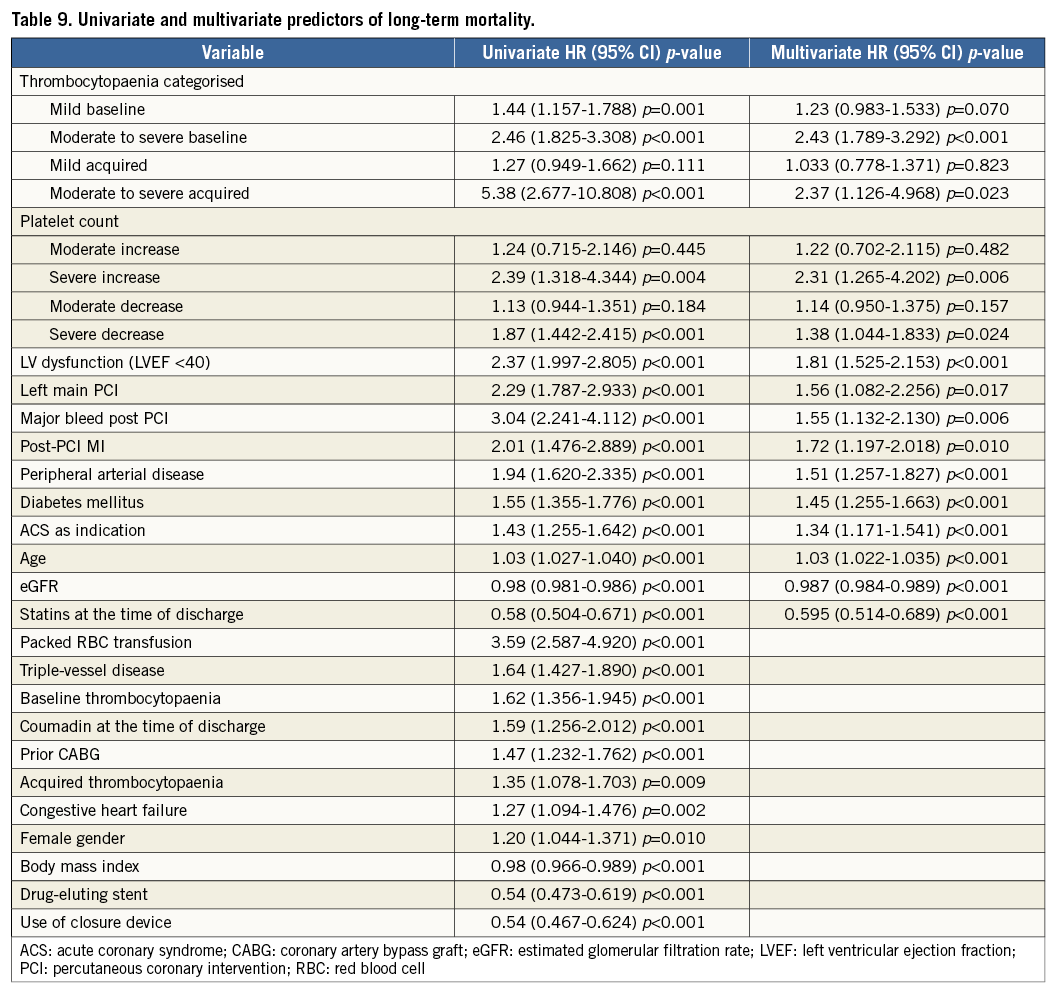
Methods and results: We evaluated 7,505 patients who underwent PCI over a period of eight years. Patients who received unfractionated heparin and glycoprotein IIb/IIIa receptor inhibitors were specifically excluded. Eight hundred and fifty-eight (11.4%) patients had baseline thrombocytopaenia and 451 (6.0%) developed acquired thrombocytopaenia. After adjustment for potential covariates, moderate to severe acquired thrombocytopaenia was the strongest independent predictor (HR 4.34, 95% CI: 2.13-8.84; p<0.001) of in-hospital net adverse clinical events, which included major adverse cardiac events and major bleeding complications. Age, male gender, baseline platelet count and intra-aortic balloon pump (IABP) insertion were independent predictors of in-hospital acquired thrombocytopaenia. After a mean follow-up of 2.6±1.7 years, moderate to severe baseline thrombocytopaenia (HR 2.42, 95% CI: 1.79-3.29; p<0.001), moderate to severe acquired thrombocytopaenia (HR 2.37, 95% CI: 1.13-4.97; p=0.02) and severe changes in platelet count (>67 k) were significant predictors of mortality.
Conclusions: In patients undergoing PCI with bivalirudin, moderate to severe baseline and acquired thrombocytopaenia along with severe changes in platelet count are associated with higher long-term mortality.
Introduction
It is well established that baseline and acquired thrombocytopaenia among patients undergoing PCI are associated with adverse in-hospital and 30-day outcomes1-8. A post hoc analysis of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial identified that baseline thrombocytopaenia was associated with higher 30-day cardiac and all-cause mortality among patients undergoing primary PCI for acute myocardial infarction (AMI)9. However, most clinical studies have excluded patients with moderate to severe baseline thrombocytopaenia, defined as a platelet count less than 100,000/mm3 1-3,10. Similarly, acquired thrombocytopaenia, defined as a drop in platelet count to <100,000-150,000/mm3 after PCI, is associated with increased length of hospital stay, in-hospital ischaemic and bleeding complications, need for in-hospital packed red blood cells transfusion, in-hospital death, and long-term mortality1-8.
Recently, the prevailing stance of improved outcome following PCI with bivalirudin11-13 has been challenged14,15. Bivalirudin reduces the incidence of acquired thrombocytopaenia compared to unfractionated heparin (UFH) and glycoprotein IIb/IIIa receptor inhibitors (GPI)7,11. The short- and long-term outcomes of patients with baseline and acquired thrombocytopaenia after PCI with bivalirudin have not been studied. We sought to evaluate the in-hospital outcomes and long-term mortality of patients with baseline and acquired thrombocytopaenia and correlate this to the magnitude of change in platelet count among patients undergoing PCI exclusively with bivalirudin. We also sought to identify the predictors of in-hospital acquired thrombocytopaenia in this group of patients.
Methods
PATIENT POPULATION
All patients undergoing PCI at the Mount Sinai Hospital (New York, NY, USA) from 1999 were enrolled in a prospectively followed database. For the present study, all patients who underwent PCI from Aug 2001 to May 2010 with bivalirudin as the sole antithrombotic agent were included (n=17,547). For patients undergoing repeat procedures during the follow-up period, only the index PCI was included (n=5,607 repeat procedures were excluded). Patients who received UFH (n=1,390) and those who received GPI (n=2,899) were also excluded, as the primary aim of the study was to evaluate the incidence and prognosis of baseline and acquired thrombocytopaenia among patients undergoing PCI exclusively with bivalirudin. Patients with a missing baseline and post-PCI platelet count were also excluded (n=269). After these exclusions, a total of 7,505 patients were available for analysis. Patients with a baseline platelet count <150,000/mm3 were included in the baseline thrombocytopaenia group. Patients with a baseline platelet count >150,000/mm3 with a subsequent drop to <150,000/mm3 were included in the acquired thrombocytopaenia group.
The institutional review board approved this study. Data were collected using standardised methods and recorded on case report forms of the PCI registry. Demographics and smoking status were based on patient self-reporting. Medical history and medications were ascertained through chart review conducted by trained research staff. Baseline routine laboratory values were measured before patients underwent PCI. After PCI, patients were followed every day by trained research staff, and all pertinent laboratory values and in-hospital complications were collected and recorded on PCI registry forms.
PERIPROCEDURAL MEDICATIONS
All patients were loaded with aspirin 325 mg and clopidogrel 300-600 mg prior to PCI. After prasugrel was approved by the Food and Drug Administration (FDA) in July 2009, loading with 60 mg of prasugrel was allowed at the discretion of the operator. Bivalirudin was administered as an intravenous bolus of 0.75 mg per kilogram, followed by an infusion of 1.75 mg per kilogram per hour and discontinued at completion of PCI in patients already on thienopyridine. From September 2008, bivalirudin infusion was continued for one hour post PCI in patients not already receiving clopidogrel (referred to as “clopidogrel naïve” in Table 1) in whom a loading dose was given periprocedurally. Bivalirudin dose was adjusted for renal impairment as recommended.
CORONARY INTERVENTION
Coronary interventional procedures were performed according to standard techniques via a femoral or radial approach. The choice of drug-eluting stent or bare metal stent implantation was at the discretion of the operator. Femoral vascular closure devices were used, unless contraindicated according to femoral arteriogram. Serial monitoring of complete blood count and cardiac biomarkers was performed every six hours (for two occurrences) after PCI. At the time of discharge all patients were recommended to take aspirin indefinitely and clopidogrel or prasugrel for at least one month for bare metal stents and one year for drug-eluting stents.
FOLLOW-UP
All-cause mortality was ascertained using the National Social Security Death Index and New York State interventional database by matching patients’ social security numbers to death index records. Only those patients for whom National Social Security Death Index data were available were included in the analysis. Follow-up data for secondary outcomes were available for those patients who re-presented to our hospital following the index revascularisation.
DEFINITIONS
Baseline and acquired thrombocytopaenia, as defined earlier, were further divided, according to the severity, into mild (100,000 to 150,000/mm3), moderate (50,000 to 100,000/mm3) and severe (<50,000/mm3). Furthermore, changes in platelet count after PCI were categorised into quintiles based on the magnitude of change in platelet count after PCI: minimal change (±33 k/uL), moderate increase (34 k-66 k/uL), severe increase (>67 k/uL), moderate decrease (34 k-66 k/uL), and severe decrease (>67 k/uL). Anaemia was defined according to the criteria of the World Health Organization (WHO), i.e., a haemoglobin level <13 g/dl in men and <12 g/dl in women16. Estimated glomerular filtration rate (eGFR) was calculated using the simplified Modification of Diet in Renal Disease (MDRD) formula: eGFRMDRD (ml/min)=186×(serum creatinine [mg/dl]–1.154×age [years])–0.203× (0.742 in women)17. Chronic kidney disease (CKD) was defined as an eGFR less than 60 ml/min/1.73 m2. Post-PCI myocardial infarction was defined according to the Third Universal Definition of Myocardial Infarction18. Bleeding complications were initially categorised according to TIMI and GUSTO definitions and retroactively adapted to HORIZONS-AMI definitions13. Major bleeding was defined as intracranial or intraocular haemorrhage; bleeding at the access site, with a haematoma which was 5 cm or larger or that required intervention; a decrease in the haemoglobin level of 4 g per decilitre or more without an overt bleeding source, or 3 g per decilitre or more with an overt bleeding source; reoperation for bleeding; or blood transfusion. Urgent revascularisation was defined as unplanned repeat revascularisation of a coronary artery or bypass graft following the index PCI. Stroke was defined as a focal neurological deficit lasting >72 hrs, resulting in irreversible brain damage or permanent impairment and confirmed by computed tomography scan or magnetic resonance imaging. A major adverse cardiovascular event (MACE) was defined as a combination of death, reinfarction, urgent repeat revascularisation and stroke. A net adverse clinical event (NACE) was defined as a combination of major bleeding and MACE.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±standard deviation (SD). Categorical variables are presented as percentages. Differences in categorical and continuous variables were assessed using Pearson’s chi-squared test and Student’s t-test, respectively. Non-parametric survival analyses with log-rank tests were conducted to find whether the potential covariates listed in Table 1-Table 4 had a univariate association with mortality. Left ventricular ejection fraction (LVEF) was categorised using a single cut-off of <40%. Age, body mass index, and glomerular filtration rate were introduced into the models as continuous variables. Semiparametric (Cox) survival analysis was conducted to assess the univariate effect of baseline and acquired thrombocytopaenia on mortality, using patients without thrombocytopaenia as reference category. The moderate and severe thrombocytopaenia subgroups were grouped together for survival analyses. For in-hospital outcomes, Pearson’s chi-squared test and gamma distribution were used to compare the differences between groups. To identify the predictors of in-hospital NACE, Mann-Whitney and Fisher’s exact tests were conducted to find whether predictors of interest and the same set of potential covariates (Table 1-Table 4) had a univariate association with NACE. Age, body mass index, race, female gender, hypertension, peripheral vascular disease, CKD, acute coronary syndrome (ACS) as indication for PCI, use of beta-blockers, left ventricular dysfunction and acquired thrombocytopaenia were significant univariate predictors of in-hospital NACE. Logistic regression analyses were then conducted to assess the univariate effect of acquired thrombocytopaenia and the extent of confounding or interaction of covariates that were statistically significant in univariate analysis. Similar analyses were repeated to identify the independent predictors of in-hospital acquired thrombocytopaenia. Covariates that showed significant univariate association in these analyses were age, male gender, race, body mass index, diabetes mellitus, prior coronary artery bypass grafting, CKD, ST-elevation myocardial infarction (STEMI) as indication for the procedure, left main disease, triple-vessel disease and left ventricular dysfunction. P-values of less than 0.05 were considered to indicate statistical significance. All statistical analyses were performed utilising SPSS software, Version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
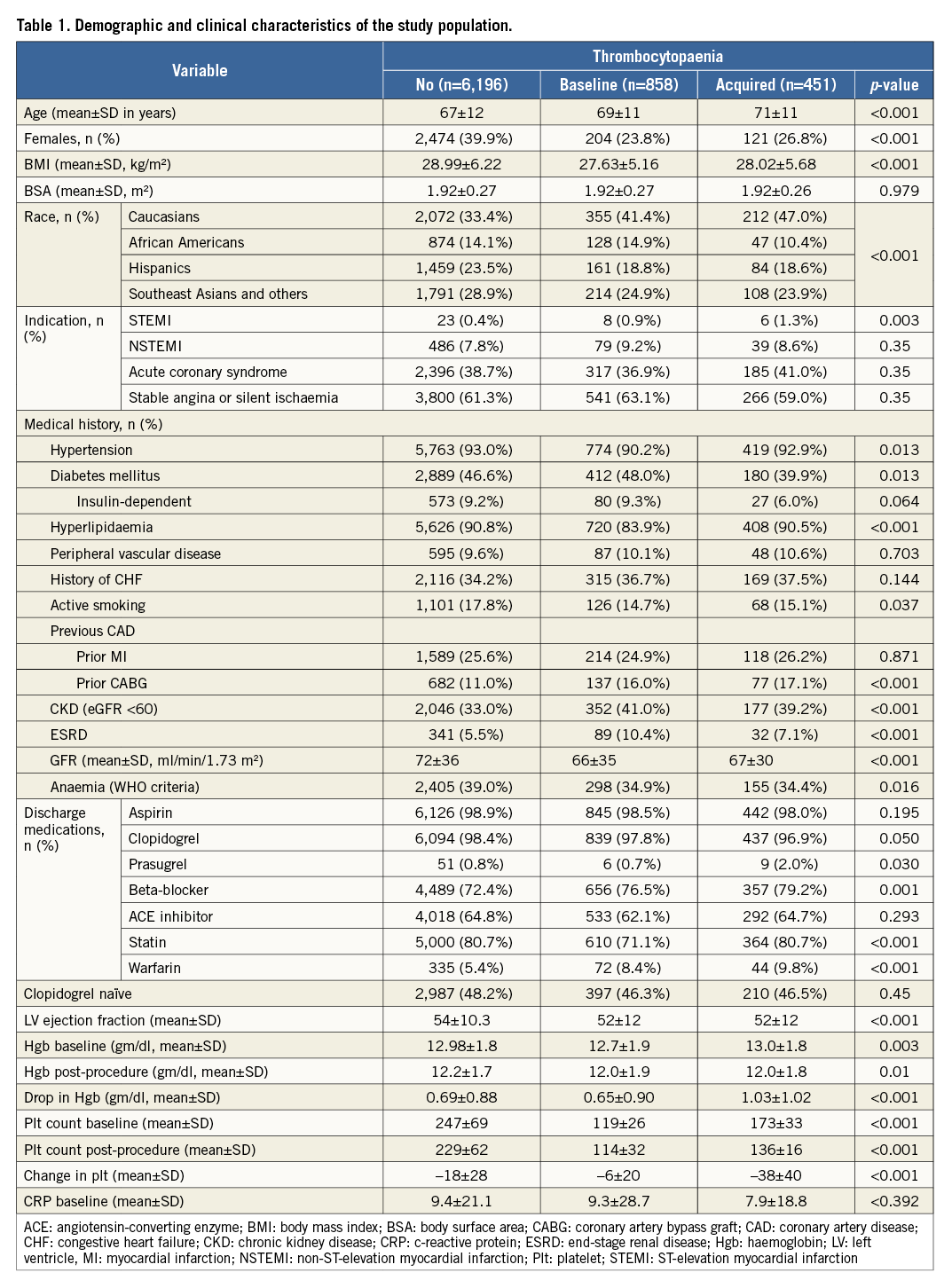
BASELINE CHARACTERISTICS
Among 7,505 patients included in the study, 858 (11.4%) patients had baseline thrombocytopaenia (9.2% mild, 2.07% moderate, 0.17% severe) and 451 (6.0%) patients developed acquired thrombocytopaenia (5.75% mild, 0.23% moderate, 0.03% severe). Details are shown in Table 1 and Table 2. Overall, patients with any form of thrombocytopaenia compared to those without tended to be older and more likely to be Caucasian, male, actively smoking and on warfarin therapy. They also tended to have a lower BMI and lower GFR, and were slightly less likely to have baseline anaemia. Within each category of thrombocytopaenia, patients with baseline thrombocytopaenia had lower rates of hypertension and hyperlipidaemia and were less likely to be on statin therapy but had a higher likelihood of having end-stage renal disease (ESRD) compared to patients without thrombocytopaenia (Table 1). Similarly, patients who subsequently developed thrombocytopaenia tended to have higher rates of STEMI as an indication for PCI, they were more likely to be on prasugrel therapy and had a higher drop in Hgb levels post procedure but they were less likely to have diabetes (Table 1). On the day of the procedure, patients with baseline thrombocytopaenia were found to have higher baseline creatinine and, as expected, received more bare metal stents (Table 2). Patients in the acquired thrombocytopaenia group had higher rates of triple-vessel disease, had longer hospital stay post procedure and were less likely to have a closure device used (Table 2). Compared to patients with baseline thrombocytopaenia, the acquired group were more likely to be dyslipidaemic and on statin therapy, less likely to be diabetic and had a greater drop in haemoglobin post PCI (Table 1).
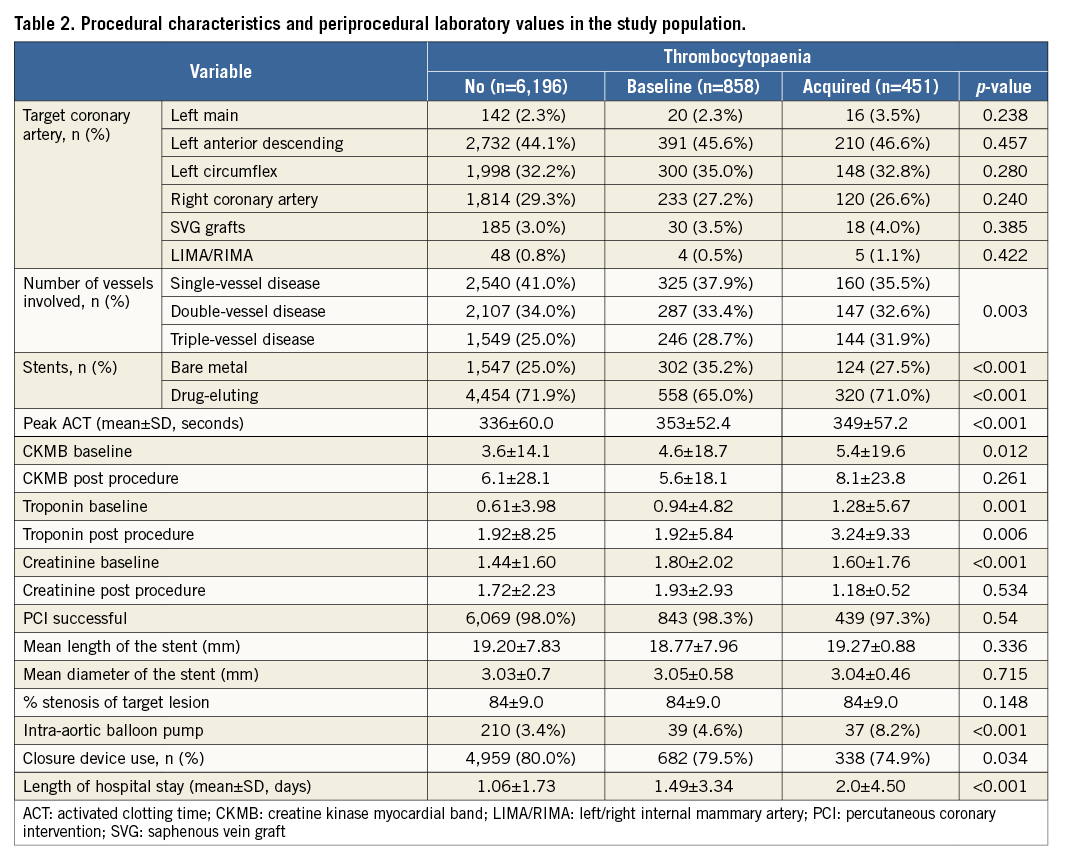
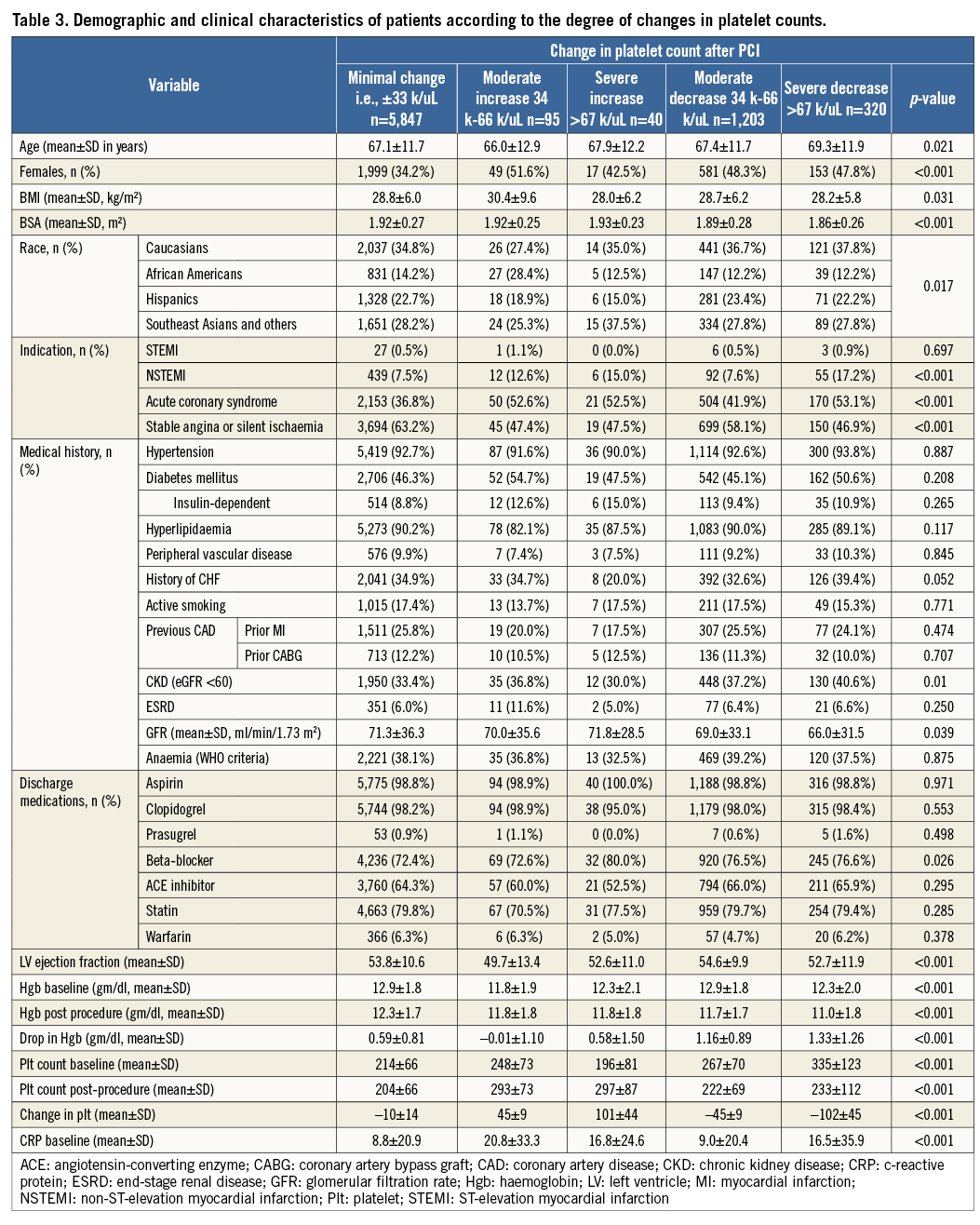
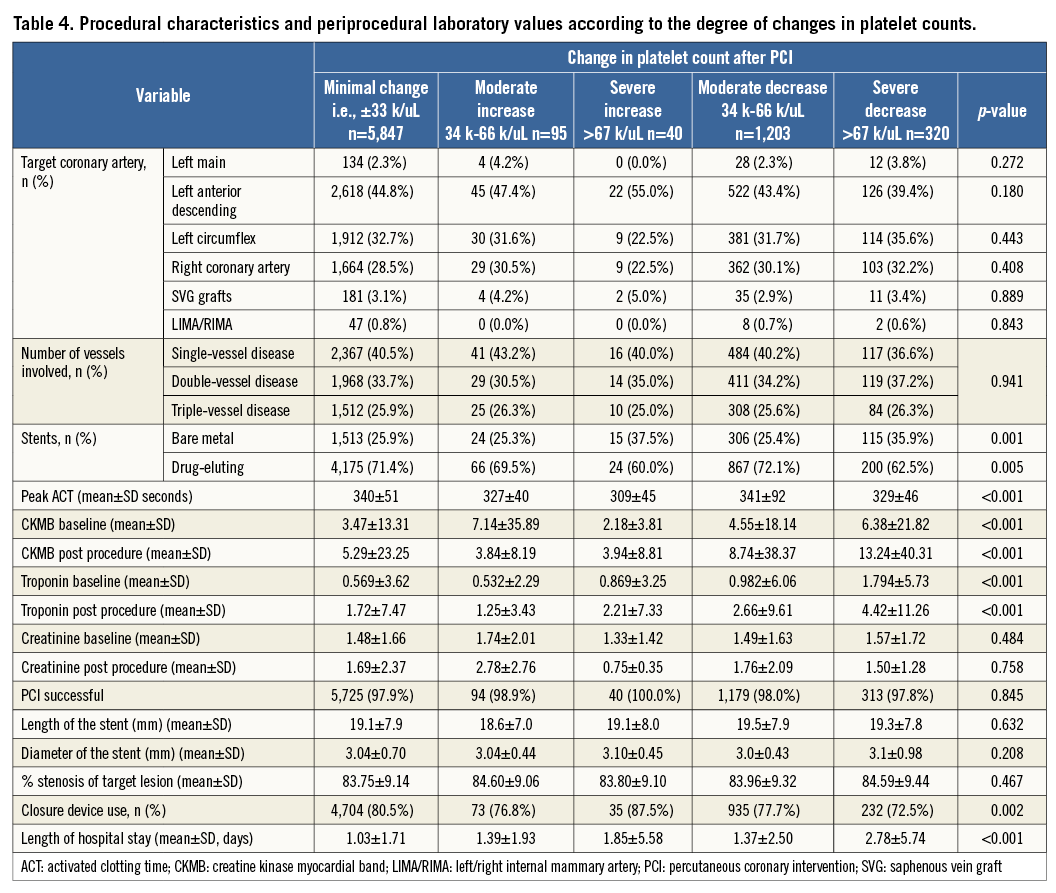
With regard to the magnitude of changes in platelet count after PCI, 5,847 (77.9%) of patients had minimal change, whereas 1,203 (16.3%) had a moderate decrease and 320 (4.3%) had a severe decrease in counts, while 95 (1.3%) had a moderate increase and 40 (0.5%) had a severe increase in platelet counts, Severe changes in platelet counts were more common in patients who presented with NSTEMI/ACS, which might reflect a group of patients with higher risk, patients implanted with bare metal stents, and patients with longer hospital stays (Table 3, Table 4).
PREDICTORS OF ACQUIRED THROMBOCYTOPAENIA
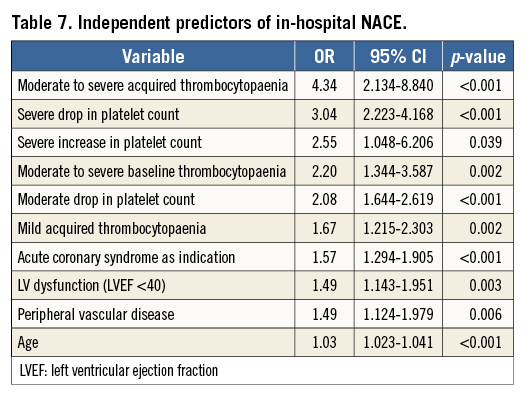
On multivariate analysis, age, male gender, baseline platelet count and IABP insertion periprocedure were independent predictors of in-hospital acquired thrombocytopaenia (Table 5). STEMI as an indication for PCI had a borderline association with acquired thrombocytopaenia.
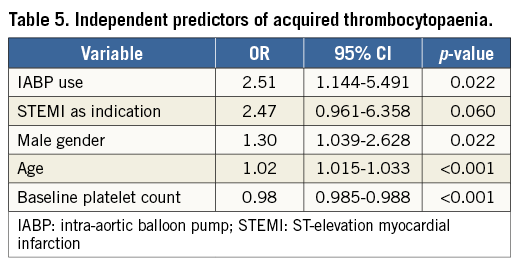
IN-HOSPITAL OUTCOMES
The incidence of MACE and NACE was significantly higher in the acquired thrombocytopaenia group compared to the baseline and no thrombocytopaenia groups, driven largely by increased post-PCI MI, major bleeding and PRBC transfusion (Table 6). NACE was more common in patients with severe changes in platelet count driven by increased major bleeding, which was mainly in non-access sites, and PRBC transfusion (Table 6).
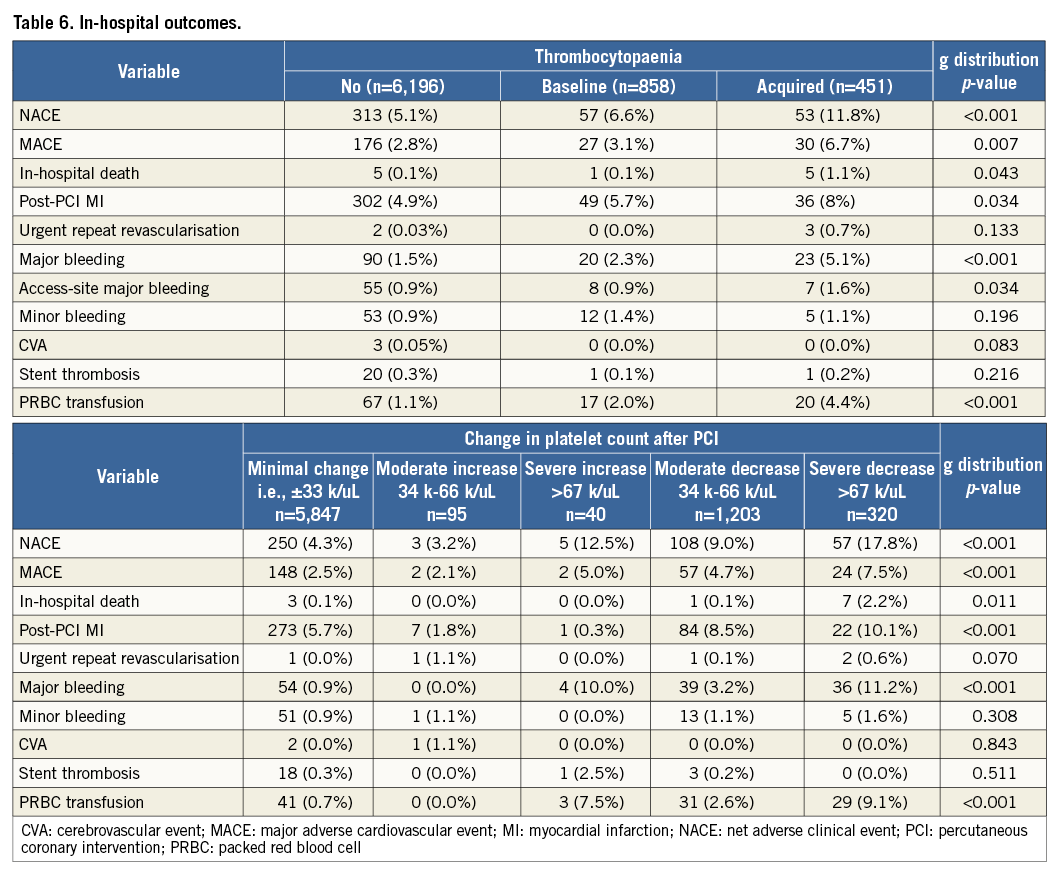
After adjustment for potential covariates, moderate to severe acquired thrombocytopaenia was the strongest independent predictor of in-hospital NACE (HR 4.34, 95% CI: 2.13-8.84; p<0.001). A severe drop in platelet count (HR 3.04, 95% CI: 2.22-4.16), moderate drop (HR 2.08, 95% CI: 1.64-2.62) and ACS as indication for PCI (HR 1.57, 95% CI: 1.29-1.90) were also strong predictors (Table 7). Among other predictors, moderate to severe baseline thrombocytopaenia (HR 2.20, 95% CI: 1.34-3.60; p=0.002), mild acquired thrombocytopaenia (HR 1.67, 95% CI: 1.22-2.30; p=0.002), and severe increase in platelet count (HR 2.55, 95% CI: 1.05-6.21; p=0.039) were also significant (Table 7).
LONG-TERM MORTALITY
After a median follow-up of 2.6 years (interquartile range 1.5 to 3.7 years), there were a total of 842 (11.2%) deaths. When analysed by follow-up years, the overall incidence of mortality was highest during the first two years of follow-up (Table 8). Moreover, analysis according to baseline platelet count showed that the survival rate was 89.7% in the no thrombocytopaenia group, 86.5% in the mild baseline thrombocytopaenia group, 72.0% in the moderate to severe baseline thrombocytopaenia group, 87.7% in the mild acquired thrombocytopaenia group, and 57.9% in the moderate to severe acquired thrombocytopaenia group at the end of the follow-up (Figure 1). Survival differences were also noted according to the magnitude of change in platelet count with the minimal change group showing the highest survival (89.7%), followed by moderate increase or decrease (86.3% and 87.6%, respectively). The groups with the lowest survival were the ones showing severe increases or decreases in platelet counts (72.5% and 80.0%, respectively) (Figure 2). After adjustment for baseline clinical and procedural characteristics using the Cox proportional hazards model, mild baseline and acquired thrombocytopaenia were not significant predictors of mortality. However, moderate to severe baseline thrombocytopaenia (HR 2.42, 95% CI: 1.79-3.29; p<0.001) and moderate to severe acquired thrombocytopaenia (HR 2.37, 95% CI: 1.13-4.97; p=0.023) were significant independent predictors of long-term mortality. Interestingly, of the 842 deaths in the follow-up, 133 patients had major bleeds in hospital of which 73% were non-access-site bleeds. While moderate increases or decreases in platelet count were not statistically significant, severe changes in platelet count (>67 k) were significant independent predictors of long-term mortality. Interestingly, a >67 k increase was a stronger predictor (HR 2.31, 95% CI: 1.27-4.20; p=0.006) than >67 k decrease (HR 1.38, 95% CI: 1.04-1.83; p=0.024). Univariate and multivariate predictors of long-term mortality are listed in Table 9.
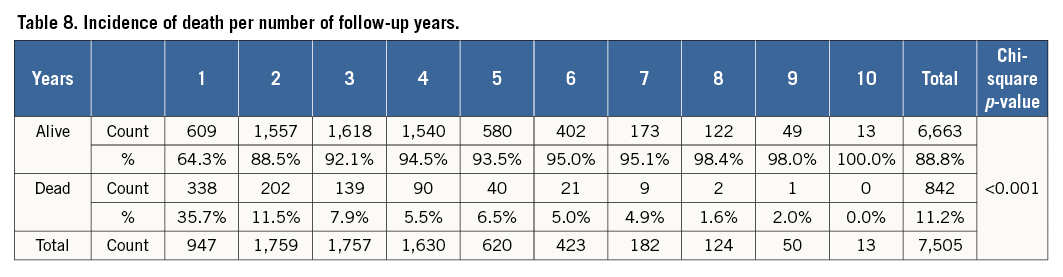
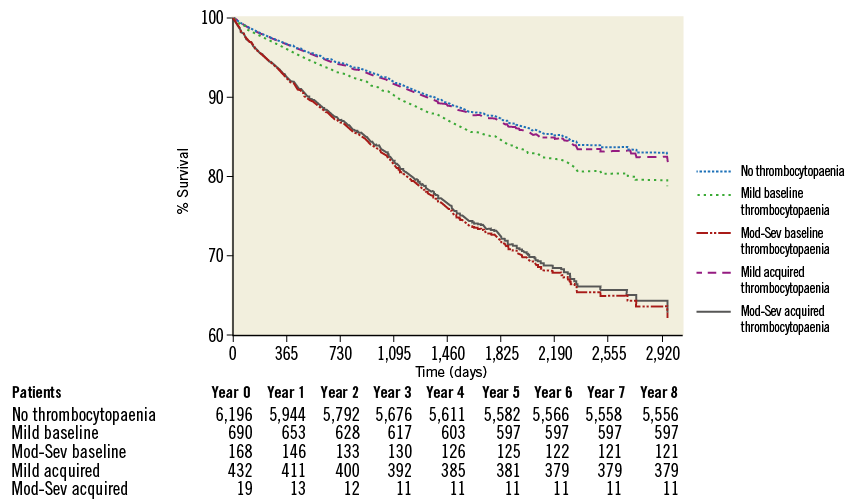
Figure 1. Survival analysis based on degree and type of thrombocytopaenia.
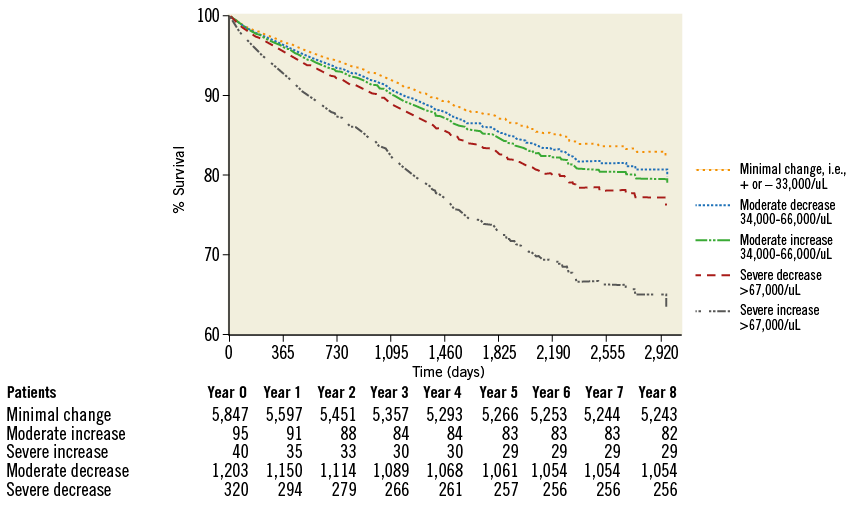
Figure 2. Survival analysis based on changes in platelet count after PCI.
Discussion
We evaluated the effects of thrombocytopaenia and acute changes in platelet counts on long-term mortality and in-hospital NACE in patients anticoagulated with bivalirudin and report a number of important findings. First, mild acquired thrombocytopaenia was relatively common compared to moderate to severe acquired thrombocytopaenia, even with sole use of bivalirudin. Second, acquired thrombocytopaenia of any degree was a strong and independent predictor of in-hospital NACE. Third, moderate to severe acquired thrombocytopaenia and moderate to severe baseline thrombocytopaenia were strong and independent predictors of long-term mortality. Fourth, only severe changes in platelet counts led to a significant increase in mortality.
The incidence of moderate to severe acquired thrombocytopaenia post PCI in the UFH and GPI era has been reported as 0.7% to 3.9%2-6,19. With bivalirudin, the incidence is lower7,11. In the REPLACE-211 and ACUITY12 trials, the incidence was reported as 0.7% and 0.5%, respectively8. In our population the incidence of moderate to severe acquired thrombocytopaenia was only 0.26%. We attribute this reduction to the 3.5% utilisation of GPI in REPLACE-211 and 9.1% provisional use in ACUITY12. Interestingly, in ACUITY the UFH+GPI and bivalirudin+GPI arms had similar rates of 30-day NACE (11.8% versus 11.7%) and major bleeding (5.3% vs. 5.7%) while the bivalirudin monotherapy strategy was superior to either GPI arm driven by a significant reduction in major bleeding. The fact that the combination of bivalirudin and GPI did not seem to reduce bleeding risk suggests that the addition of GPI to any antithrombin agent increases bleeding risk through potent platelet inhibition as well as induction of thrombocytopaenia. Compared to these previous studies, our findings of lower rates of moderate to severe acquired thrombocytopaenia in patients treated solely with bivalirudin may explain, at least partly, why there is no safety advantage of bivalirudin in the presence of concomitant GPI.
Similar to previous reports9,20, baseline thrombocytopaenia was a significant predictor of in-hospital NACE in our study. Overgaard et al and HORIZONS-AMI showed baseline thrombocytopaenia as a predictor of in-hospital mortality in a patient population undergoing urgent PCI. We have now observed this in patients presenting with chronic stable angina and exclusive anticoagulation with bivalirudin. Furthermore, our results can be generalised to a wider population since patients with platelet counts <100,000/mm3 were excluded in HORIZONS-AMI. Although mild thrombocytopaenia was not associated with an increased risk of long-term mortality, patients with moderate to severe baseline thrombocytopaenia had significantly higher long-term mortality. Although such a degree of baseline thrombocytopaenia may indicate a higher risk of subsequent adverse cardiovascular events in patients undergoing PCI, it may also reflect the presence of other comorbidities that could independently contribute to the higher mortality observed in this group of patients. Overall, baseline platelet count can be an easily obtainable and useful haematologic parameter for risk stratification of patients undergoing PCI. In our hospital, based on the results of the present analysis, patients with moderate to severe baseline thrombocytopaenia are identified pre-procedurally, and additional considerations, such as pharmacotherapeutic strategy and radial or micropuncture vascular access, are employed.
Acquired thrombocytopaenia was an independent predictor of in-hospital events in our study. Major bleeding events were elevated 1.5-fold in baseline and 3.4-fold in acquired thrombocytopaenia patients, translating to a higher incidence of NACE. The hazard ratio for in-hospital NACE associated with moderate to severe acquired thrombocytopaenia was 4.3, higher than the hazard from conventional mediators of adverse in-hospital events including LV dysfunction, acute coronary syndrome and peripheral vascular disease. Multivariate analysis identified that moderate to severe acquired thrombocytopaenia was the most powerful independent predictor of long-term all-cause mortality. This finding is in keeping with those presented in CADILLAC, ACUITY and the study by De Labriolle et al who also showed that acquired thrombocytopaenia is associated with higher long-term mortality3,7,8. These studies included patients receiving UFH with or without GPI and concluded that bivalirudin is associated with a lower incidence of acquired thrombocytopaenia. Although based on these previous data it can be proposed that the incidence of acquired thrombocytopaenia can be reduced by avoiding agents like UFH and GPI, those who develop thrombocytopaenia on bivalirudin continue to have a higher risk of in-hospital NACE and long-term mortality.
While investigating the effects of the magnitude of platelet change on long-term survival we noted that the hazard ratio for a severe decrease in platelet count (>67 k drop) was second in magnitude only to development of moderate to severe acquired thrombocytopaenia. At the other extreme, patients with severe increases (>67 k) in platelet count also showed higher mortality (Figure 2). Interestingly, although the majority of this group of patients had no or only mild baseline thrombocytopaenia, they also had the lowest mean platelet count prior to PCI (mean: 196, SD: 81). A tendency for higher C-reactive protein levels (mean: 16.8, SD: 24.6) in this group might reflect higher levels of systemic inflammation that can both cause reactive thrombocytosis and affect the outcomes independently. Nevertheless, the increase in platelets from baseline to post procedure (101±44) suggests a mobilisation from the bone marrow. Indeed, previous studies have shown that these bone marrow-derived platelets are hyperreactive21 and furthermore that residual platelet activity from these platelets can negatively affect outcome22-24. Further investigations are needed to elucidate how acute changes in platelet count post PCI with bivalirudin as the procedural anticoagulant occur and how this can affect short- and long-term outcomes.
Limitations
The present study has a number of important limitations. First, although we believe our findings are robust, the low incidence of severe thrombocytopaenia posed a statistical challenge and, to remedy this, moderate and severe thrombocytopaenia patients needed to be grouped together. Second, although our population of patients receiving only bivalirudin was unselected, they largely comprised a cohort of stable patients. Third, although we used multivariate analysis to adjust for confounding variables, unmeasured variables could affect the relationship between thrombocytopaenia and long-term mortality. As the cause of death was not available, only all-cause mortality was presented. Fourth, no data were available on long-term medical therapy or drug discontinuation. Fifth, this is a single-centre study. Patients who re-presented to our institution with the defined adverse events post PCI were captured in the follow-up. As such, completeness of follow-up cannot be determined. Sixth, the current study examined the effects of baseline thrombocytopaenia and early changes in the platelet counts on short- and long-term outcomes and did not take into account changes in platelets that might have occurred during the follow-up period. Finally, our study is observational in design. Despite these limitations, we conclude that moderate to severe baseline and acquired thrombocytopaenia along with severe changes in platelet counts, among patients undergoing PCI with bivalirudin, are associated with higher long-term mortality.
| Impact on daily practice Bivalirudin as the procedural anticoagulant in PCI has been reported to cause lower rates of acquired thrombocytopaenia. This study of a large cohort of patients undergoing PCI in a real-world setting shows that mild acquired thrombocytopaenia is also common with the sole use of bivalirudin and is associated with adverse short-term clinical outcomes. Moreover, moderate to severe baseline and acquired thrombocytopaenia in patients treated with bivalirudin are predictors of worse long-term outcomes. Therefore, practical approaches other than bivalirudin monotherapy should be considered in patients with moderate to severe thrombocytopaenia undergoing PCI to reduce the risk of bleeding and adverse clinical outcomes. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

