Abstract
Aims: Our study sought to evaluate mechanisms of the current strategies for optimal anticoagulation during percutaneous coronary intervention (PCI).
Methods and results: Thirty-two high risk acute coronary syndrome patients were randomised to bivalirudin and provisional GPIIb/IIIa inhibition (GPIIb/IIIa) or unfractionated heparin (UFH) and mandatory GPIIb/IIIa. Flow cytometric measurements immediately after anticoagulation showed that, unlike UFH, bivalirudin did not activate platelets as indicated by P-selectin expression and fibrinogen binding while decreasing platelet-monocyte aggregates and monocyte expression of tissue factor. UFH released tissue factor pathway inhibitor (TFPI) during and immediately after PCI while bivalirudin (irrespective of GP IIb/IIIa) did not. Lower levels of TFPI with bivalirudin were seen during and immediately after PCI (P<0.01). Thrombin generation as indicated by prothrombin fragment F 1+2 levels was reduced during PCI in the UFH group (P<0.01) but not with bivalirudin. Soluble CD40 ligand is associated with thrombosis and levels were higher in the bivalirudin group irrespective of GPIIb/IIIa at the same stages (P<0.05).
Conclusions: Bivalirudin has some early advantages on platelet activation when compared to UFH. However, there are significant limitations in its mechanism of action, particularly a lack of release of tissue factor pathway inhibitor.
Introduction
We sought to evaluate the underlying mechanisms of action of the current strategies for optimal anticoagulation during intracoronary stenting in patients with high risk acute coronary syndrome (ACS).
Platelet inhibition prior to percutaneous coronary intervention (PCI) is generally provided by aspirin and clopidogrel and additionally during PCI by glycoprotein IIb/IIIa inhibition (GPIIb/IIIa).
Studies of bivalirudin1-3 suggest that bivalirudin and provisional GPIIb/IIIa is as effective as UFH and mandatory GPIIb/IIIa at preventing the ischaemic sequelae of ACS with fewer bleeding complications. However, the ACUITY study showed that when bivalirudin or UFH were both administered with GPIIb/IIIa there were similar rates of major and minor bleeding problems as well as composite ischaemic endpoints2. Additionally, another study of bivalirudin compared to UFH where most platelet inhibition was provided by clopidogrel showed the bivalirudin provided no clinical benefit at a substantially higher cost4, the reduced bleeding possibly being an UFH dosing issue5. The claimed benefits for bivalirudin are therefore the subject of debate.
Our study aimed to investigate the differences in mechanisms of action of the strategies used in the REPLACE-21, ACUITY2 and HORIZONS-AMI3 studies by measuring: (a) platelet activation, (b) monocyte expression of tissue factor and P-selectin glycoprotein ligand-1 (PSGL-1) and (c) inhibition of the coagulation cascade amongst high risk ACS patients randomised to bivalirudin with provisional GPIIb/IIIa or UFH and mandatory GPIIb/IIIa.
Methods
Study population and design
Approval to conduct this study was obtained from the hospital Human Research and Ethics Committee and patients consented to participate. In this non-blinded study, all PCI patients had a non-ST elevation myocardial infarction or ST elevation myocardial infarction within the previous seven days. All were receiving aspirin and either 75 mg/day of clopidogrel and/or a 300 mg or 600 mg loading dose two to 12 hours before PCI. Exclusions were: active bleeding, a history of intracranial bleeding, creatinine clearance < 60 mL/min, platelet count < 100x109/L, thrombolysis within previous 24 hours, receiving low molecular weight heparin within previous eight hours, poorly controlled hypertension, PCI within prior month, GPIIb/IIIa within previous seven days, possible urgent cardiopulmonary bypass surgery or surgery within prior six weeks.
The sample size was calculated on the number of patients required to show a 35% difference in platelet P-selectin expression from 4.0% with UFH to 2.6% with bivalirudin with a standard deviation of 1.3. At a confidence level of 0.95, a power of 0.80 and a ratio of 1.0 a total sample size of 30 patients was required. Thirty-two patients were randomly allocated to one of two groups, bivalirudin and provisional GPIIb/IIIa or UFH with mandatory GPIIb/IIIa.
Provisional GPIIb/IIIa was provided by HD-tirofiban and was administered in situations such as prolonged ischaemia or distal embolisation.
Bivalirudin was administered as an IV bolus of 0.75 mg/kg prior to the PCI followed by a continuous infusion of 1.75 mg/kg/hr until the end of the PCI1. No monitoring of anticoagulation with the activated clotting time (ACT) was employed and the sheath was removed two hours after the end of the procedure. UFH was administered as an IV bolus dose of 65 U/kg (maximum 7000 U) prior to the start of the PCI. Further UFH was administered as required to maintain an ACT >250 sec. GPIIb/IIIa was provided by HD-tirofiban or abciximab, given once the guidewire crossed the lesion. HD- tirofiban was given as a bolus of 25 µg/kg of bodyweight followed by an infusion of 0.15 µg/kg/min for 18 to 24 hours6. Abciximab was administered as a bolus followed by infusion at the recommended weight-adjusted regimen7.
The endpoints of the study were: (a) to determine any different effect on platelets and monocytes of bivalirudin or UFH five minutes after administration and (b) to evaluate any differences over a 24 hour period in the effect of bivalirudin with or without GPIIb/IIIa compared to UFH with GPIIb/IIIa on the coagulation cascade.
Blood samples
Blood samples for flow cytometry were collected via a 7 Fr arterial sheath at: (a) baseline immediately before anticoagulation with bivalirudin or UFH, (b) five minutes later to measure the direct impact of the anticoagulants on the platelets and monocytes. The initial 10 ml of blood was discarded. The sample was anticoagulated with 100 µM PPACK Dihydrochloride (Calbiochem, Darmstadt, Germany)8. To avoid in vitro artefactual changes, the blood samples were processed and counted by flow cytometry immediately, following the recommendations of European Working Group on Clinical Cell Analysis9.
Blood to investigate coagulation was collected into 0.109 M trisodium citrate for tissue factor pathway inhibitor (TFPI) and prothrombin fragment F 1+2 (F1+2) assays and into lithium heparin for soluble CD40 ligand (sCD40L) assays. To observe these levels over a longer time frame, samples were collected at: (1) baseline, (2) 10 minutes after the wire crossed the lesion and any GPII/IIIb administered (10 minutes), (3) two hours after anticoagulation (two hours) and (4) the next day (24 hours). For patients who received GPIIb/IIIa, the latter sample was taken more than six hours after the end of the infusion. These bloods were centrifuged at 3000 g for 10 minutes, the plasma removed and spun again at 10,000 g at four degrees centigrade for 20 minutes. Plasmas were frozen in liquid nitrogen until the assays were performed.
Flow cytometric measurements
A whole blood method was followed as previously described10. An aliquot of diluted anticoagulated blood was added to CD42a Peridinin Chlorophyll Protein (PerCP) (Becton Dickinson, San Jose, CA, USA) (BD), CD62 Phycoerythrin (PE) (BD) and anti-human fibrinogen fluorescein isiothyocyanate (FITC) (Gentaur, Brussels, Belgium). CD42a is directed against GP IX and GP 1b-IX-V, these being present on all platelets. CD62 detects P-selectin expressed on the platelet surface. Counts were performed after seven minutes staining. Platelets were selected by logarithmic forward scatter (FSC) and side scatter (SSC) as well as being positive for CD42a and the percentage expressing P-selectin and fibrinogen was recorded. Three thousand cells were counted.
Another aliquot of blood was added to CD14 PerCP (BD), and either CD42a FITC (BD), CD162 PE (PSGL-1) (BD) or anti-human tissue factor (FITC) (American Diagnostica Inc., Stamford, CT, USA). After seven minutes incubation FACS Lysing Solution (BD) was added and the suspension counted. SSC of 700 was used as the threshold. Monocytes were selected on the basis of being CD14 positive and their size according to linear FSC. The percentage of monocytes positive for CD42a (platelet-monocyte aggregates) (PMA), PSGL-1 or tissue factor was recorded. The degree of expression of each marker was measured against appropriate isotype negative controls of matched protein concentration.
Coagulation assays
Enzyme-linked sandwich immunoassays were employed. The TFPI assay (IMUBIND American Diagnostica, Stamford, CT, USA) detects intact and truncated TFPI as well as TFPI bound to tissue factor, factor VIIa and factor Xa. The F1+2 assay utilised mouse monoclonal antibodies to F1+2 (Enzygnost, Dade-Behring Marburg GmbH, Marburg, Germany) and the sCD40L assay (R&D Systems, Inc., Minneapolis, MN, USA) utilised polyclonal antibodies to sCD40L.
Statistical analysis
Statistical analysis was performed using the SPSS statistical package version 15.0 (SPSS Inc., Chicago, IL, USA). Non-parametric analysis was used as all data were not distributed normally. Data for continuous variables were expressed as medians with 25th and 75th percentiles. Mann-Whitney Test was used to measure differences between groups. Wilcoxon Signed Ranks Test was used for paired analysis. Pearson Chi-square or Kruskal-Wallis test was used to detect differences in demographic values. Correlations were tested with Spearman’s correlation co-efficient. Measurements were regarded as significant when P <0.05.
Results
Demographics of study population
Of the 32 patients randomised to bivalirudin or UFH, seven of the 16 bivalirudin patients received provisional HD-tirofiban (Table 1).
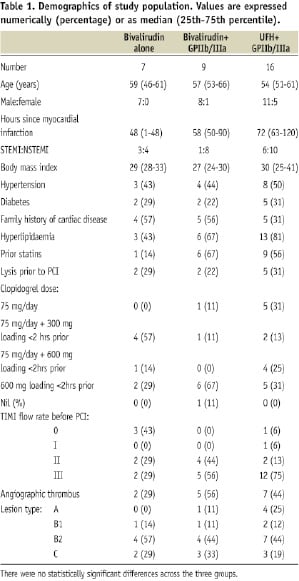
The characteristics were similar between the groups.
Clinical outcomes
All PCI procedures were successful in restoring TIMI III blood flow rate. However, one patient in the bivalirudin with no GPIIb/IIIa group developed an abrupt stent thrombosis one hour after PCI. No patient had a myocardial infarction. There were no major or minor bleeding complications in either group.
Effect of anticoagulation regime on platelets and monocytes
The bivalirudin and UFH groups were compared. The initial effect of the anticoagulant alone can best be seen by comparing the measurements on the blood samples taken five minutes after bivalirudin or UFH administration to those taken at baseline (Figure 1).
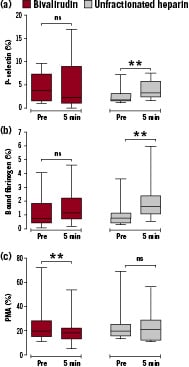
Figure 1. Box plots of the percentage of: (a) platelets expressing P-selectin, (b) platelets binding fibrinogen and (c) platelet-monocyte aggregates (PMA) immediately before administration of bivalirudin or UFH (Pre) and five minutes afterwards (5 min). The bar represents the median, the box the 25th and 75th percentiles, and the whiskers the range. The statistical significance refers to the difference in expression from Pre using paired analysis (Wilcoxon Signed Ranks test). ns=not significant, ** P <0.01.
Platelet expression of P-selectin did not change with administration of bivalirudin but increased significantly with UFH (P<0.01) (Figure 1 a).
Similarly, the percentage of platelets binding fibrinogen did not change with bivalirudin but increased significantly with UFH (P<0.01) (Figure 1 b).
Including all patients after anticoagulation, platelet P-selectin expression correlated with the fibrinogen binding (r=0.48, P<0.01).
The percentage of PMA decreased significantly five minutes after administration of bivalirudin (P<0.01) but did not change with UFH (Figure 1 c).
PSGL-1 expression on monocytes was not affected by bivalirudin but was increased five minutes after UFH (P<0.01).
With the decrease in PMA associated with bivalirudin administration there was a parallel decrease in the percentage of monocytes expressing tissue factor from 0.60 (0.25-1.35) % at baseline to
0.40 (0.15-0.70) % five minutes later (P<0.05). There was no such change in the UFH group.
Effect of anticoagulants on the coagulation cascade
The groups compared were bivalirudin alone, bivalirudin with GPIIb/IIIa and UFH with GPIIb/IIIa. Anticoagulation with UFH was associated with an increase in TFPI levels, these becoming higher than either bivalirudin group at 10 minutes and two hours, then decreasing with time to be no different at 24 hours (Figure 2 a).
sCD40L levels in the UFH group were significantly lower than either bivalirudin group at 10 minutes and two hours (Figure 2 b).
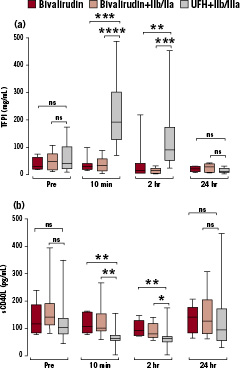
Figure 2. The levels of (a) tissue factor pathway inhibitor (TFPI) and (b) soluble CD40 ligand (sCD40L) in blood samples taken from patients receiving bivalirudin, bivalirudin plus GPIIb/IIIa (IIb/IIIa) or unfractionated heparin and IIb/IIIa immediately prior to anticoagulation (Pre), 10 minutes after the guidewire crossing the lesion (and administration of IIb/IIIa when given) (10 min), two hours after anticoagulation (2 hr) and the next day (24 hr). The statistical significance refers to the difference in levels between bivalirudin or bivalirudin plus GPIIb/IIIa and unfractionated heparin plus GPIIb/IIIa groups at each stage. Mann Whitney Test was performed, ns=not significant, * P <0.05, ** P <0.01, *** P <0.001, **** P <0.0001. One sCD40L value of 1625 pg/mL in the bivalirudin group at 24 hours was excluded from the graph but not the statistical analysis.
Comparing the bivalirudin ± GPIIb/IIIa with UFH+GPIIb/IIIa groups, F1+2 levels 10 minutes after the wire had crossed the lesion had not changed significantly from baseline in the bivalirudin group while in the UFH group there was a median reduction of 19.3% (P<0.01). At baseline and 10 minutes respectively, F1+2 levels were 283 (180-376) and 264 (152-389) pmol/L with bivalirudin and 249 (169-365) and 201 (115-298) pmol/L with UFH. F1+2 levels in either group were no different to baseline two hours after anticoagulant administration or the next day.
Discussion
This study describes differences of bivalirudin compared to UFH after administration. Compared to UFH, bivalirudin did not activate platelets and decreased platelet-monocyte aggregates and monocyte expression of tissue factor. However, bivalirudin did not release TFPI during PCI while UFH administration was associated with a definite increase in levels of this physiological inhibitor. TFPI inhibits tissue factor which is an initiator of thrombosis.
Effect of anticoagulation regime on platelets and monocytes
UFH or LMWH are considered as acceptable alternatives for PCI according to ACC/AHA/SCAI guidelines11 as there is no significant advantage of one agent over the other. For consistency we chose UFH which has been widely studied and commonly used.
Unlike bivalirudin, UFH administration was associated with an immediate activation of platelets as shown by the increase in P-selectin expression and fibrinogen binding. In addition, PMA were reduced from baseline immediately after administration of bivalirudin whilst in the UFH group there was no change. We postulate this is due to an associated increase in monocyte PSGL-1 expression with UFH increasing adhesion to platelets via P-selectin.
PMA formation induces tissue factor expression on monocytes12 and in this study the reduction of PMA with bivalirudin administration was associated with decreased expression of tissue factor on the monocytes, possibly conferring an improved anticoagulant effect to bivalirudin.
Effect of anticoagulants on the coagulation cascade
On damage to the endothelium by inflation of the angioplasty balloon, tissue factor is induced in the consequent neointima13 to complex with factor VIIa and potentially initiate thrombotic complications of the procedure such as acute stent thrombosis. The only inhibitor of Factor VIIa is the endogenous Kunitz-type protease inhibitor TFPI14. By inhibiting the tissue factor-VIIa complex and factor Xa, fibrin formation is also inhibited. UFH administration releases TFPI from the vascular endothelium into the blood to provide a significant antithrombotic effect15. A small amount of TFPI is found in the alpha granules of platelets and is released on platelet activation14. These mechanisms may have been involved in our study which showed a marked increase in TFPI levels from baseline 10 minutes and two hours after the UFH was administered. It is known that the endogenous TFPI stores are depleted with time during UFH infusion15 and our study demonstrated the process in the UFH group. It has been reported that the direct thrombin inhibitor argatroban does not induce a release of TFPI16 and our study has shown that bivalirudin also fails to release TFPI irrespective of GPIIa/IIIa.
Plasma sCD40L results from the cleavage of CD40 ligand which has become expressed on the surface of stimulated platelets and it is involved with both inflammation and thrombosis. GPIIb/IIIa has been demonstrated to inhibit the release of sCD40L in vitro17. In our study sCD40L levels were significantly higher during and after PCI in the bivalirudin group irrespective of GPIIb/IIIa.
A previous study demonstrated that F1+2 levels decreased after PCI in patients anticoagulated with UFH and no GPIIb/IIIa18. Our study demonstrated that UFH with GPIIb/IIIa decreased thrombin generation 10 minutes after the guidewire crossed the lesion as shown by reduced F1+2 levels while bivalirudin had no such effect.
TFPI, sCD40L and F1+2 levels all indicated inferior inhibition of the coagulation cascade with bivalirudin and provisional GPIIb/IIIa compared to UFH with mandatory GPIIb/IIIa.
While our study was limited by the small numbers of patients recruited, its findings are supported by observations from the large clinical trial HORIZONS-AMI3 in which there was an increased rate of acute stent thrombosis with bivalirudin monotherapy. There was also a report of two cases of thrombosis with bivalirudin used during γ-brachytherapy19 and a thrombo-elastography study which indicates that in vitro bivalirudin is less effective than UFH in reducing clot propagation and strength20. In the ACUITY trial subgroup analysis revealed benefit for UFH and GPIIb/IIIa over bivalirudin alone in those patients who were clopidogrel naïve, the bivalirudin group having a relative risk of composite ischaemia of 1.29 (Confidence Interval: 1.03-1.63)2. In these patients with high clinical risk for thrombosis, bivalirudin monotherapy without concomitant antiplatelet therapy increased thrombotic sequalae.
The CLEAR PLATELETS-2 Study investigated PCI patients receiving clopidogrel with either a 75 mg/day maintenance dose or a 600 mg loading dose after stenting. The study showed a higher rate of periprocedural myocardial infarction in patients receiving bivalirudin and clopidogrel compared to those who additionally received eptifibatide21.
Compared to UFH, weaker inhibition of blood coagulation by bivalirudin combined with its’ shorter half life and less frequent GPIIb/IIIa use may contribute to the reduced bleeding complications observed with its use. The major outcome of previous trials of bivalirudin2,3 was net clinical benefit driven by a lower rate of major bleeding. The criticism of these has been that aggressive antiplatelet therapy with mandatory GPIIb/IIa and UFH was a departure from routine clinical practice in which GPIIb/IIa is used more selectively than is recommended by AHA/ACC guidelines 22.
Conclusion
Bivalirudin itself did not initially activate platelets as did UFH. In addition, it was associated with a decrease in PMA and tissue factor expression on monocytes. However, the study clearly showed that,irrespective of GPIIb/IIIa, bivalirudin lacks the important anticoagulant characteristic of UFH of releasing TFPI. Furthermore, patients given bivalirudin had higher levels of the prothrombotic sCD40L. There was evidence that compared to UFH plus GPIIb/IIIa, bivalirudin with provisional GPIIb/IIIa was less effective at reducing levels of F1+2. This study provides sufficient evidence to suggest a further large scale study of the inhibition of the coagulation cascade when bivalirudin and UFH with matched GPIIb/IIIa are compared.
Bivalirudin patients with high risk acute coronary syndrome and weak antiplatelet therapy or no antiplatelet pre-treatment should be considered for rapid platelet inhibition with agents such as GPIIb/IIIa inhibitors to prevent thrombotic sequelae.
Acknowledgements
We are grateful to The Prince Charles Hospital Foundation and Pathology Queensland Study Education and Research Trust Fund for their financial support and to CSL Australia for supplying the bivalirudin. We would like to thank Tony Limpus for his assistance and to the staff in the Catheter Laboratory and Recovery Ward for their cooperation. Thanks also to Chris Raffel for his advice and Harry Bartlett for his statistical analysis.

