
Abstract
Aims: The aim of the study was to investigate whether bivalirudin versus unfractionated heparin (UFH) reduces infarct size (IS) for primary percutaneous coronary intervention (PPCI) in large acute myocardial infarction (AMI).
Methods and results: This multicentre open-label trial randomised 78 patients undergoing PPCI for large AMI to bivalirudin or UFH. The primary endpoint was IS, assessed by cardiac magnetic resonance (CMR) five days after PPCI. Secondary endpoints included index of microcirculatory resistance (IMR), CMR-assessed microvascular obstruction (MVO) and ejection fraction, and biomarkers for thrombin activity and cell injury. No difference was observed in mean IS at five days (25.0±19.7 g for bivalirudin vs. 27.1±20.7 g for UFH; p=0.75). Early MVO was numerically lower with bivalirudin (5.3±5.8 g vs. 7.7±6.3 g; p=0.17), with no significant difference in ejection fraction at 90 days (54.6±12.0% vs. 49.1±12.1%; p=0.11). In the biomarkers, thrombin-antithrombin complexes were reduced by 4.8 ug/L over the first day for bivalirudin versus an increase of 1.9 ug/L in the heparin arm (p=0.0003). Acute IMR was lower (43.5±21.6 vs. 68.7±35.8 mmHg×s, respectively; p=0.014). In a planned interim analysis, an approximate 11% reduction in IS was observed with bivalirudin; the trial was discontinued for futility.
Conclusions: This study did not achieve its primary endpoint of significant infarct size reduction in PPCI by prolonged bivalirudin infusion compared to UFH, even though complete thrombin inhibition was achieved in the acute phase, with a lower myocardial microcirculation resistance at the end of the procedure.
Abbreviations
AMI: acute myocardial infarction
APPROACH: Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease
BARC: Bleeding Academic Research Consortium
BIVAL: Bivalirudin Infusion for Ventricular InfArction Limitation
CMR: cardiac magnetic resonance
DTPA: diethylenetriamine penta-acetic acid
EDV: end-diastolic volume
IMR: index of microcirculatory resistance
IQR: interquartile range
IS: infarct size
ITT: intention-to-treat
LVEF: left ventricular ejection fraction
MPO: myeloperoxidase
MSI: myocardial salvage index
MVO: microvascular obstruction
PPCI: primary percutaneous coronary intervention
SD: standard deviation
STEMI: ST-segment elevation myocardial infarction
TAT: thrombin−antithrombin complexes
TIMI: Thrombolysis In Myocardial Infarction
UFH: unfractionated heparin
Introduction
Primary percutaneous coronary intervention (PPCI) for ST-elevation myocardial infarction (STEMI) reduces infarct size (IS) and improves survival. Even with rapid restoration of epicardial flow, final IS is considerable, with a significant contribution from reperfusion injury and microvascular obstruction (MVO) due to oedema and capillary obstruction by microthrombi. Final IS depends on several factors including the occlusion location, duration of occlusion, the burst of thrombin triggered by plaque rupture and coronary intervention involving thrombus manipulation1.
Beyond its role in coagulation, thrombin is proinflammatory and is implicated in reperfusion injury. On a cellular level, thrombin activates platelets, endothelial cells and leucocytes; consequently, it exerts a potent proinflammatory action, with a thrombogenic and deleterious effect on the microcirculation especially downstream from the culprit lesion2. The formation of monocyte-platelet aggregates increases within minutes after PCI, reaching a peak within 2-4 hours2. Thrombin is formed and released from the occluding thrombus and by the intra-arterial manipulations of vessel reopening during and after completion of PPCI2. Bivalirudin directly inhibits both clot-bound and unbound thrombin, whereas heparin is less effective against clot-bound thrombin2. Thrombin inhibition by a prolonged 4-hour bivalirudin infusion after PPCI may therefore reduce inflammation and reperfusion injury.
The primary objective of the Bivalirudin Infusion for Ventricular InfArction Limitation (BIVAL) study was to investigate whether treatment with bivalirudin infusion for four hours versus unfractionated heparin (UFH) for PPCI in large STEMI reduces reperfusion injury and improves myocardial healing, resulting in a smaller final IS. For this we used cardiac magnetic resonance (CMR) imaging, the gold standard for measuring IS3, which can also provide a reliable quantitative measurement of global left ventricular parameters and identify prolonged MVO. In a subgroup, we measured invasively the index of microcirculatory resistance (IMR) at the end of the procedure, which predicts clinical outcomes4. Finally, we assessed the direct and indirect effects of bivalirudin and UFH on coagulation, platelets, and leucocytes to explore the effect of thrombin inhibition on IS.
Methods
BIVAL was a multicentre, open-label, prospective, randomised controlled trial conducted at four sites in the Netherlands and France in patients undergoing PPCI for a large myocardial infarction.
Adults with STEMI who presented >20 minutes and <12 hours after symptom onset were eligible if they fulfilled the following angiographic criteria: Thrombolysis In Myocardial Infarction (TIMI) 0 or 1 flow in the infarct-related artery (IRA); angiographic score ≥21 (sizeable infarction, based on initial angiogram) according to the APPROACH score5; and eligible for PPCI. Patients with a history of Q-wave myocardial infarction or who had received antithrombotic therapy other than UFH at first medical contact were excluded. Use of glycoprotein IIb/IIIa inhibitors was permitted only as bail-out therapy for the treatment of no-reflow phenomenon or giant thrombus, defined as >2 times the diameter of the vessel. The full study criteria are detailed online (https://clinicaltrials.gov: NCT02565147).
All patients provided written informed consent. The sponsor (The Medicines Company, Parsippany, NJ, USA) was responsible for funding, protocol development in collaboration with the executive committee and the clinical coordinating centre, on-site monitoring and safety surveillance, statistical analyses, and data management. The institutional review board at each site approved the study protocol and activities.
STUDY MEDICATIONS
All patients received, as soon as logistically possible, aspirin at an initial dose of 150-325 mg orally (or 250-500 mg intravenously) and a loading dose of a P2Y12 inhibitor. Administration of UFH at first medical contact or before the angiogram was allowed as per usual practice.
Randomisation (1:1) was carried out using an interactive voice/web response system. All patients randomised to the bivalirudin arm received a bolus of 0.75 mg/kg and an infusion of 1.75 mg/kg/hr for the duration of the procedure and for four hours after completion of PPCI to maintain consistent thrombin inhibition, as (during STEMI) thrombin exerts activity beyond the restoration of flow in the IRA. Patients randomised to UFH were administered a dose as per standard institutional practice. In cases where activated clotting time was used to inform UFH dosing, a target value of ≥250 seconds was recommended. Randomisation was stratified by duration of symptom onset to randomisation (<6 hrs versus ≥6 hrs) and site.
PRIMARY AND SECONDARY OUTCOMES
The primary outcome was the difference in IS in the bivalirudin versus UFH arm, assessed by CMR performed five days (defined as 5 days±72 hours from randomisation) after PPCI. Key secondary outcomes were the difference between bivalirudin and UFH in other CMR-derived variables of myocardial recovery five days after PPCI, namely LVEF and MVO. LVEF and IS were also assessed by CMR at 90 days. Other secondary outcomes were markers of thrombin activity and cell injury, including thrombin-antithrombin complexes (TAT), platelet microparticles, and myeloperoxidase (MPO), measured by blood sample collection at baseline and three time points after PPCI.
STUDY MEASUREMENTS
In brief, CMR imaging was performed on 1.5-Tesla clinical scanners6. Repeated breath holds and gating by electrocardiogram were applied to minimise the influence of cardiac and respiratory motion on data collection. Cine magnetic resonance imaging was performed using steady-state free-precession techniques by a combination of short-axis and three long-axis views (4-chamber, 2-chamber and 3-chamber).
Early MVO was assessed on first-pass perfusion imaging and was performed at rest during 30-50 consecutive heartbeats immediately after injection of gadolinium diethylenetriamine penta-acetic acid (DTPA) (0.1 mmol/kg at 3 mL/s into an antecubital vein followed by 15 mL of saline at 3 mL/s)7,8. Delayed enhancement imaging was performed with a gated breath hold T1-sensitive recovery gradient-echo sequence with a minimum of 10 minutes after infusion of gadolinium DTPA (total of 0.2 mmol/kg i.v.)3. The inversion time was selected to null out the myocardial muscle signal. Typical inversion times ranged from 200 to 300 ms.
Left ventricular function and late gadolinium enhancement were analysed in an independent core lab (CERC) on a workstation using dedicated software. CAAS MRV, 3.4 (Pie Medical Imaging, 2011, Maastricht, the Netherlands) was used for the evaluation of left ventricular function IS and MVO.
Left ventricular end-diastolic and end-systolic volumes were short axis-based and calculated using a combination of long- and short-axis images and are expressed in millilitres6. Epicardial and endocardial contours were automatically detected, and manual corrections applied if needed, with consensus between the two readers.
IMR was measured in three of the four preselected sites using a previously described thermodilution technique9,10. IMR was defined as distal coronary pressure multiplied by the mean hyperaemic transit time (expressed in mmHg×seconds [s]). The results are presented as mean±standard deviation (SD) for both treatment arms. In addition, a cut-off point of 32 (associated with better outcomes9) was selected as the threshold to categorise IMR.
Biomarker blood sample collection was carried out before study drug administration, at the end of PPCI, at the end of 4-hour infusion, and at 12-24 hours after PPCI on citrate and on ethylenediaminetetraacetic acid (EDTA). The following variables were measured: TAT and change in TAT (which expresses the net effect of thrombin generation and its inhibition), myeloperoxidase, and microparticles. Care was maximum to respect the preanalytical requirements strictly. Within two hours of blood collection, platelet-free plasma aliquots were prepared by double centrifugation. Plasma aliquots were frozen to –80°C and shipped to the core laboratory (<3 months) for measurement of biomarkers. TAT and myeloperoxidase were measured by an ELISA method, commercialised by Siemens Healthcare Diagnostics, Duisburg, Germany, and ALPCO Diagnostics, Salem, NH, USA, respectively. Platelet microparticles were measured on a flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) using annexin V (Beckman Coulter, Brea, CA, USA) and two antibodies (anti-CD41a and anti-CD42b from Becton) and calibration microbeads (Megamix®; Biocytex, Marseille, France). Anti-Xa activity was measured in all patients at baseline, and at subsequent time points in heparin-treated patients only, by an amidolytic method with chromogenic substrate (Stachrom® Heparin; Diagnostica Stago, Asnières sur Seine, France); direct thrombin inhibition was measured in bivalirudin-treated patients using a modified thrombin time Hemoclot® (HYPHEN Biomed, Neuville-sur-Oise, France). Both coagulation tests were performed on a coagulation analyser (STA-R; Diagnostica Stago). All biomarker analyses were carried out by a centralised, independent core laboratory.
Net adverse clinical events, defined as death, reinfarction, ischaemia-driven revascularisation, and Bleeding Academic Research Consortium (BARC) ≥3 bleeding were assessed up to five days or at discharge from hospital, whichever came first. Patients were followed to day 90.
STATISTICAL ANALYSIS
Continuous variables are summarised as mean±SD or as median (interquartile range). Comparisons were made with a Wilcoxon rank-sum test. Categorical variables are presented as frequencies and percentages and were compared with the chi-square test.
The intention-to-treat (ITT) population was defined as all enrolled subjects. The per-protocol population was defined as enrolled subjects who underwent successful PPCI (i.e., final TIMI flow 2 or 3), had CMR data at five days, and were without major protocol deviations. Primary and secondary endpoint analyses are based on the per-protocol population.
Assuming a 23% reduction in IS with bivalirudin (24±17 g) versus UFH (31±17 g), and a two-sided type I error rate of 0.05, 190 subjects (95 per arm) would achieve >80% power. Therefore, approximately 200 subjects were planned to be enrolled in the trial to achieve 190 evaluable subjects. A planned interim analysis was performed after enrolment of approximately one third of randomised patients. If the difference in IS between treatments was ≥18% at this point the trial would continue and if <18% the study would stop for futility. Statistical analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
The interim analysis showed an approximate 11% reduction in IS with bivalirudin, and the trial was discontinued for expected futility. At that point, 78 patients had been enrolled (ITT population), 69 of whom completed the study; 64 patients met the inclusion criteria for the per-protocol population (Figure 1).
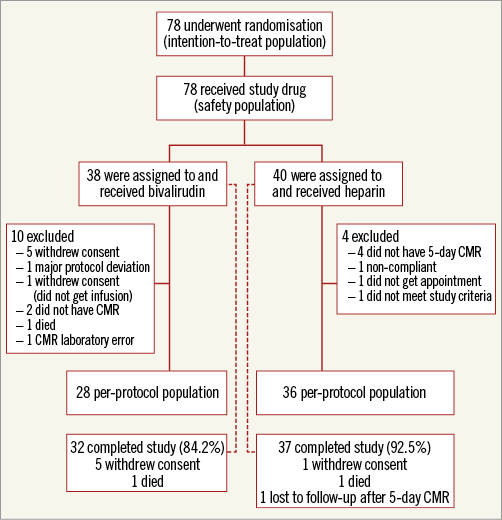
Figure 1. Consort diagram.
Most of the patients (83%) were screened within six hours of symptom onset, and 56% within three hours. Sixty-two of 64 patients received UFH before randomisation (at first medical contact or before the diagnostic angiogram). Mean age was 62.8±11.8 years and 81% were men (Table 1). Aspirin was given to 98% of patients and a P2Y12 inhibitor loading dose in 97%.
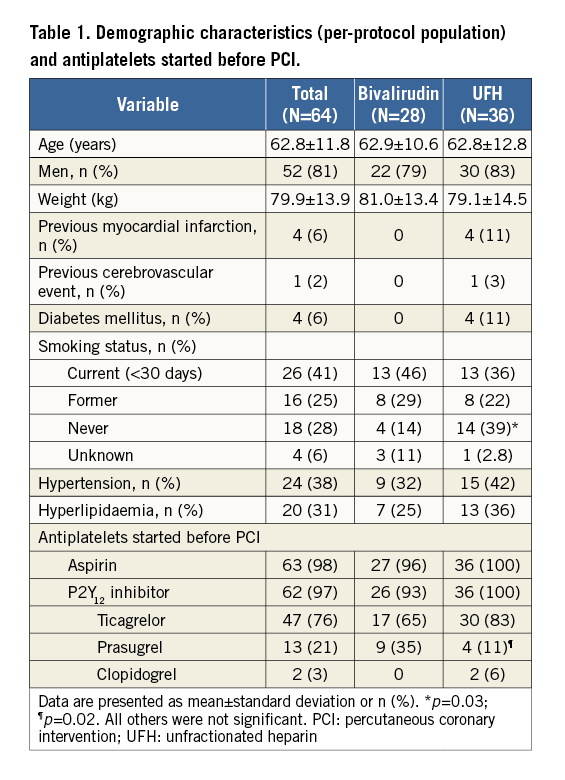
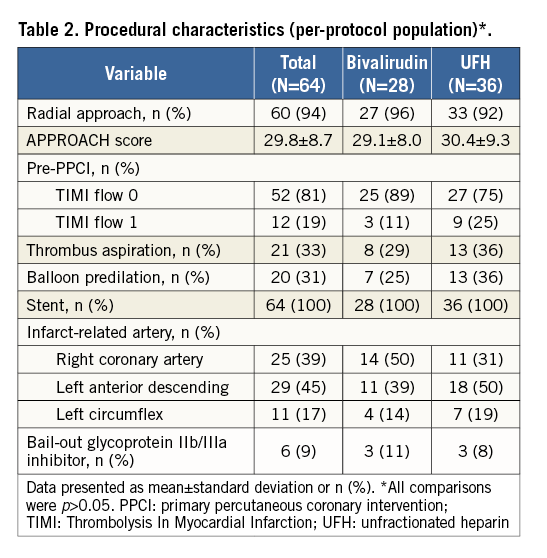
Radial access was used in 94% of patients (Table 2). At presentation, 81% of patients had TIMI 0 flow. The culprit artery was the left anterior descending in 45% of the patients. Thrombus aspiration was performed in 33%; 9% of patients received bail-out glycoprotein IIb/IIIa inhibitor treatment. Mean APPROACH score was 29.8±8.7. Total infusion duration for bivalirudin was 4.09 hours and the total dose was 648 mg, given as a bolus and followed by an infusion. Post randomisation UFH was administered at 58.5 U/kg. No between-group differences were observed for the angiographic findings (Table 3).
CMR RESULTS
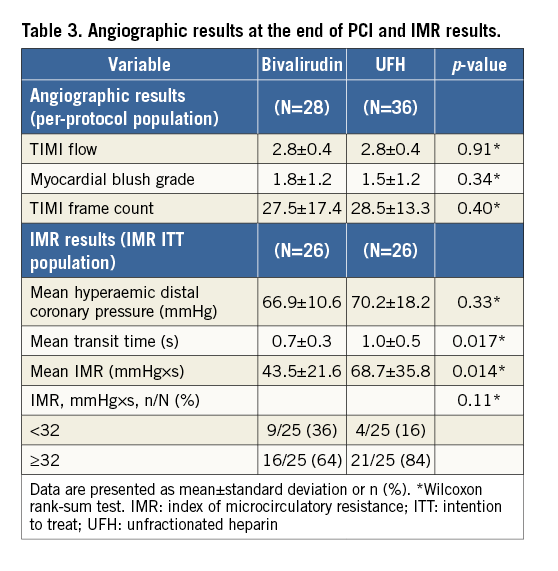
Overall mean IS at five days was 26.2±20.1 g (16.3±11.6% of left ventricular mass), early MVO was 6.6±6.1 g, and ejection fraction was 48.6±10.9%. CMR data per treatment arm are presented in Table 4. No statistically significant reduction was observed in the mean IS at five days (primary endpoint) (25.0±19.7 for bivalirudin vs. 27.1±20.7 for UFH; p=0.75) (Figure 2). Early MVO was numerically lower in the bivalirudin arm (5.3±5.8 g vs. 7.7±6.3 g, p=0.17). There was no significant difference in ejection fraction (p=0.97).
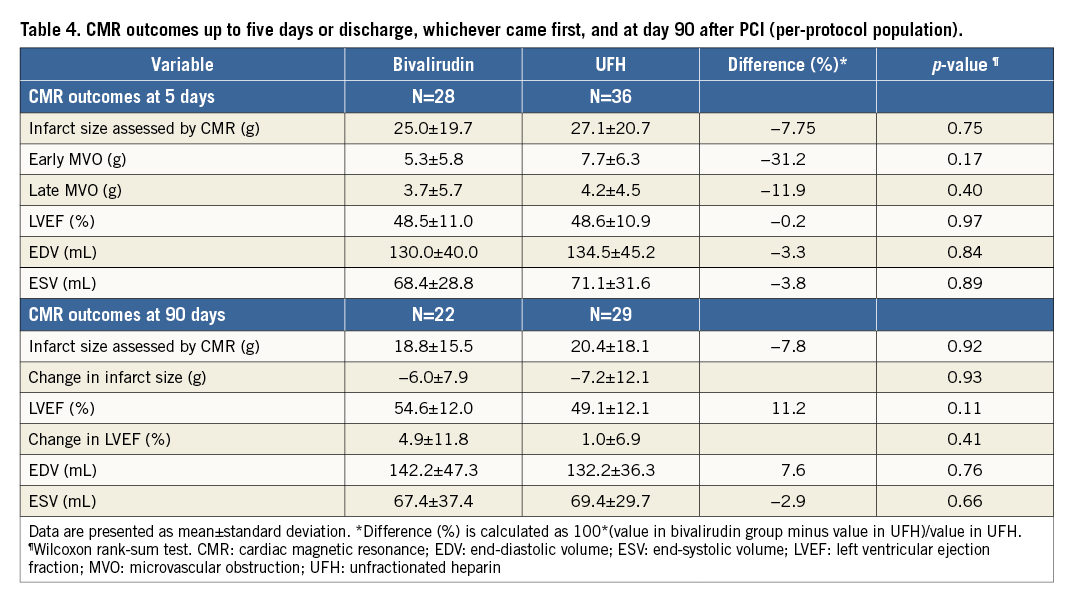
Figure 2. Mean±standard deviation. A) Infarct size at day 5. B) Left ventricular ejection fraction (LVEF) at day 90.
Data for 90-day CMR were available in 51 patients (Table 4). Overall mean ejection fraction increased to 54.6±12.0 from 49.7±10.5 (change from day 5 to day 90 with bivalirudin: 4.9±11.8%; p=0.066; change with UFH: 1.0±6.9%, p=0.43). No statistically significant difference was observed in ejection fraction at late follow-up (Figure 2). Overall, mean IS decreased independently of the treatment arm (change from day five to day 90 with bivalirudin: –6.0±7.9, p=0.0023; change with UFH: –7.2±12.1, p=0.0038).
INDEX OF MICROCIRCULATORY RESISTANCE
IMR was measured in 52 patients. Mean transit time was significantly lower in the bivalirudin versus UFH arm (p=0.016) (Table 4). With similar distal coronary artery pressures, this resulted in a lower IMR for bivalirudin (43.5±21.6 vs. 68.7±35.8 mmHg×s, respectively; p=0.014). The percentage of patients with impacted flow in the microcirculation (>32 mmHg×s) was numerically lower with bivalirudin (64% vs. 84%) than with UFH.
BIOLOGICAL MARKERS
Following study drug administration, TAT (indicative of thrombin levels) decreased in the bivalirudin arm and increased slowly in the UFH group (Figure 3A, Table 5). Inhibition of MPO was initially greater in the bivalirudin arm (Figure 3B, Table 5). Significant increases from baseline in platelet microparticles CD41 and CD42 were observed in both groups, with no difference between treatment arms (Figure 3C, Figure 3D, Table 5).
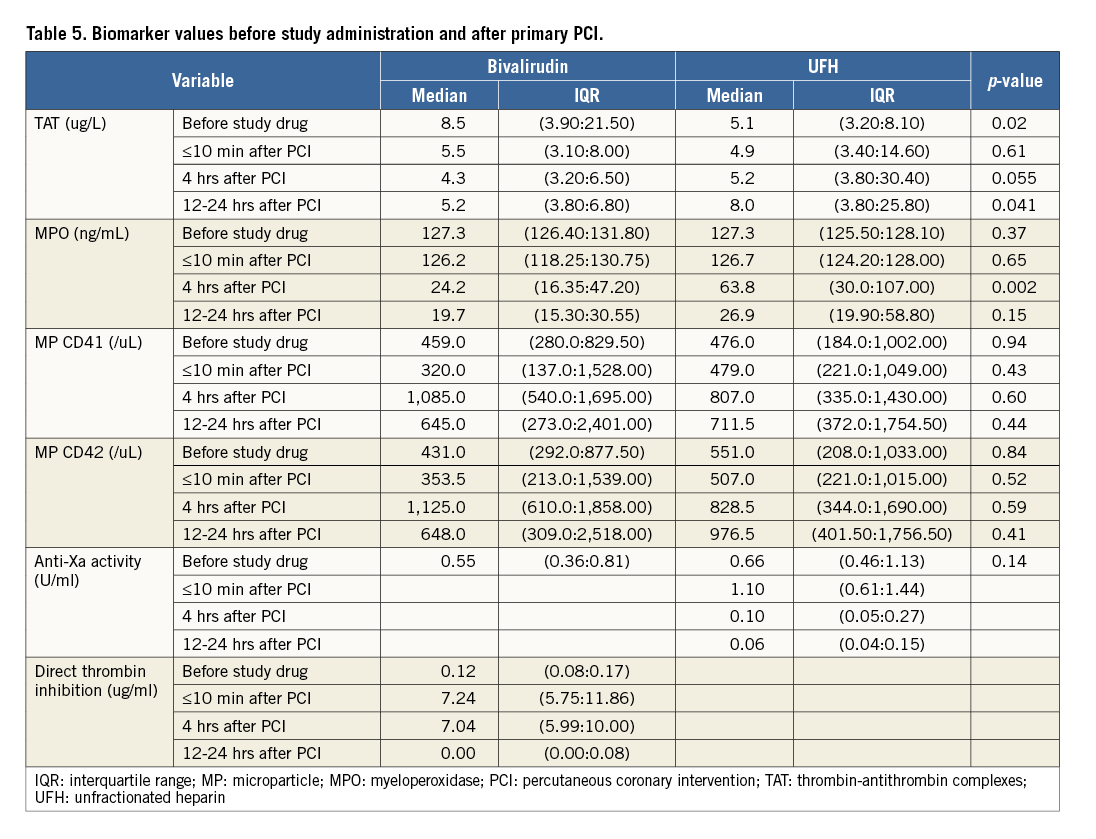
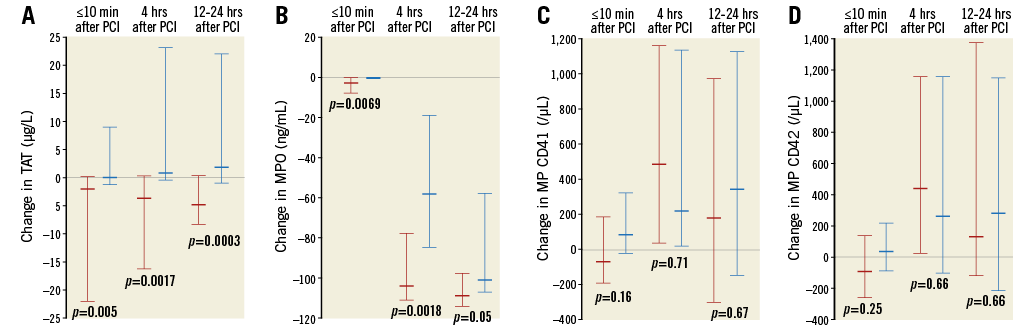
Figure 3. Median (interquartile range) changes from baseline (before study drug administration) to the end of PPCI, the end of 4-hour infusion, and at 12-24 hours after PPCI. A) TAT. B) MPO. C) MP CD41. D) MP CD42. MP: microparticle; MPO: myeloperoxidase; PCI: percutaneous coronary intervention; TAT: thrombin-antithrombin complexes
Anti-Xa values measured at admission were similar in both arms, indicating that patients in both groups were similarly pretreated with heparin. After admission, anti-Xa was followed only in the heparin group, showing the expected activity during treatment for the dose administered (Table 5). Similarly, no Hemoclot direct thrombin inhibitor activity was observed at admission in any patient; direct thrombin inhibitor activity was followed only in bivalirudin-treated patients, showing the expected activity for full thrombin inhibition throughout treatment (Table 5).
CLINICAL OUTCOMES
The rate of in-hospital net adverse clinical events (up to five days or discharge, whichever came first) was 7% (2/28) in the bivalirudin group and 8% (3/36) in the UFH group. BARC ≥3 bleeding occurred in 1/28 (4%) patients in the bivalirudin group. One patient (UFH group) died in the period up to 90-day follow-up.
Discussion
Three main observations can be made from this trial. First, the study failed to show a significant reduction in IS at five days with a 4-hour bivalirudin infusion versus standard heparin in PPCI. Second, bivalirudin completely inhibited thrombin generation within 10 minutes of completion of PPCI, whereas UFH did not prevent a small increase in thrombin generation, and this was accompanied by a larger reduction in MPO during bivalirudin infusion. Third, at the end of the procedure, microvascular resistance in the culprit vessel was lower in the bivalirudin-treated patients.
The treatment of myocardial infarction has evolved dramatically over the past 30 years, resulting in a major reduction in mortality. Thrombolysis was the first therapy aimed at reperfusion. Primary angioplasty increased the success rate for restoration of epicardial flow to above 95%. Still, myocardial infarction is a major cause of heart failure related to IS and ventricular remodelling. Additional therapies are needed to take the next step in the reduction of morbidity after acute myocardial infarction. Reperfusion injury and MVO are among the key targets in this process, and bivalirudin has the potential to affect both. In the present study, we failed to demonstrate a significant reduction in IS. The observed approximately 8% smaller mean IS of 25.0 g for bivalirudin versus 27.1 g for UFH was not statistically different in the 64 patients, and nowhere near the anticipated 23% reduction, resulting in the pre-specified early termination of the study. Still, the secondary endpoints that investigated the difference in biological activity of these medications and the microvascular function allow us to generate a hypothesis on the mechanism involved to explain the repeated, but not consistent, finding of a mortality reduction in STEMI patients treated with bivalirudin, which was not explained by the lower incidence of bleeding events11-13.
Bivalirudin inhibited thrombin more effectively than UFH, as measured by the significant drop in TAT within 10 minutes of injection, which persisted throughout the 4-hour infusion, without an increase observed in UFH-treated patients. As postulated, this may have contributed to the better microcirculation, as expressed by the lower IMR values, in the bivalirudin arm. Even after five days, the CMR data point in the same direction of reduced MVO in the early and late imaging series.
The importance of the coronary microcirculation in determining subject outcomes has been demonstrated previously14-16. IMR correlates with true microvascular resistance and is independent of the flow in the epicardial arteries. Furthermore, IMR provides a reproducible assessment of the microcirculation, which is independent of haemodynamic perturbations10.
Both IMR- and CMR-based microcirculatory assessments have been linked to adverse late events7, including mortality17. Although MVO is closely related to IS, a large meta-analysis showed the independent prediction of MVO versus IS on mortality8. Potential reasons for cardiac death in patients with MVO have been suggested18. Patients with, versus those without, no-reflow more often had malignant arrhythmias and early heart failure18.
MECHANISTIC HYPOTHESIS
Thrombin is a potent thrombogenic agent: it transforms fibrinogen into fibrin, it inhibits fibrinolysis through the thrombin activatable fibrinolysis inhibitor, and it is a potent cell activator2. When thrombin activates endothelial cells, it reduces their antithrombotic capability, causes the release of prothrombotic factors (e.g., tissue factor), and modifies cell permeability. The change in permeability results in cell oedema, which (along with microthrombosis) is one of the major factors responsible for MVO. Thrombin is initially generated from the lesion at the time of plaque rupture; then, during restoration of flow in the coronary artery and through thrombus manipulation, more thrombin is released from inside the clot and continues to be generated even after completion of PCI. Among the circulating cells, leucocytes are activated by thrombin and release their granular content, such as plasma MPO. The formation of platelet-leucocyte aggregates indicates the effect of thrombin and is implicated in MVO2.
The amount of thrombin generated varies between patients and this, in combination with the small size of this study, explains the significantly different TAT levels at baseline between the groups. After initiation of anticoagulation, TAT levels in the bivalirudin arm were immediately reduced and remained low, whereas an increase was observed in the heparin arm, indicative of the direct effect of bivalirudin on thrombin, expressing a complete inhibition of bound and unbound thrombin.
Plasma MPO values, which were similar at baseline in the two groups, decreased four hours after the procedure, with a greater reduction in the bivalirudin arm. After 24 hours, MPO levels were similarly low in both groups. This response may be because polymorphonuclear leucocytes, in addition to being activated by thrombin, have a direct role in the thrombotic process, known as NETosis19.
Thrombin inhibition (expressed through TAT measurement) and leucocyte activation (expressed through MPO measurement) were the two parameters (both thrombin-related) that demonstrated fluctuations during the acute phase of the myocardial infarction and which responded to thrombin inhibition, underlining the role of thrombin and its effect on the microcirculation. Platelets, which often attract focus both clinically but also for research reasons, were not differentially involved, as suggested by the platelet microparticle values, which were similarly dispersed throughout the study and reflect the lack of a difference in the antiplatelet treatment between the groups.
Limitations
The small sample size, due to early discontinuation of the trial, is an obvious limitation. The sample size is even smaller due to the predefined condition that the analysis would be performed in the per-protocol population, even though results are consistent for the larger ITT population. Owing to the small size and the early discontinuation, any results should be addressed with caution and should better be accepted as hypothesis-generating. Furthermore, 97% of patients received some dose of UFH at first medical contact, so any potential benefit of bivalirudin is in addition to UFH.
Conclusions
Using a 4-hour bivalirudin infusion in the PPCI setting resulted in complete thrombin inhibition in the acute phase, whereas UFH did not prevent a small increase in thrombin generation. This strategy resulted in a lower IMR but failed to reduce IS, the primary endpoint of this study. The trial was discontinued for expected futility after a planned interim analysis. The preliminary findings of the secondary endpoints provide a hypothesis for understanding the repeated, but not consistent, finding in previous studies of a mortality reduction with bivalirudin.
| Impact on daily practice The main purpose of anticoagulation at the acute phase of myocardial infarction is inhibition of thrombin. Even though this study failed to demonstrate the effect of prolonged bivalirudin as adjuvant therapy during PPCI to reduce IS versus UFH, and is unlikely to impact on guidelines or clinical practice, observations from the secondary endpoints carry the potential to explain the clinical benefit of complete and prolonged inhibition of thrombin. |
Acknowledgements
Uwe Otto (The Medicines Company) provided administrative and clinical operations support throughout the trial. Dr Sophie Rushton-Smith (MedLink Healthcare Communications Limited) provided editorial support and was funded by The Medicines Company.
Funding
The study and the editorial support for this study were funded by The Medicines Company.
Conflict of interest statement
C. Bal dit Sollier reports receiving grants to IVS (the core lab) for biomarker measurements from The Medicines Company, during the conduct of the study. J. Garot reports receiving personal fees from CERC, during the conduct of the study. L. Ding, P. Anthopoulos and I. Lechthaler are employees of The Medicines Company. E. Deliargyris is a shareholder of The Medicines Company. The other authors have no conflicts of interest to declare.

