Abstract
Aims: Within a clinical trial population, direct thrombin inhibition using bivalirudin in patients undergoing primary percutaneous coronary intervention (PCI) for ST-elevation myocardial infarction (STEMI) is associated with a reduction in mortality and major bleeding compared to heparin/glycoprotein IIb/IIIa receptor inhibition (GPI), but a higher incidence of acute stent thrombosis (ST), particularly in the absence of pre-procedural heparin. The safety and efficacy of bivalirudin in an all-comer, real-world primary PCI setting is unknown.
Methods and results: 968 consecutive STEMI patients (mean age 63 years, 72% male, 42% anterior STEMI, 3.7% cardiogenic shock) undergoing primary PCI with bivalirudin as the recommended anticoagulation, and with heparin/GPI (abciximab) as an alternative, were prospectively followed. Bivalirudin was administered as a bolus, high-dose procedural infusion and, unlike the HORIZONS-AMI trial, as a low-dose infusion for four hours post-PCI. Additional heparin was not routinely given. Mortality, major adverse cardiovascular events (MACE), major bleeding and ST were assessed at 30 days. Initial antithrombotic therapy was bivalirudin in 885 patients (91%), of whom 123 (13.9%) received additional antithrombin therapy, and 114 (11.8%) “bail-out” GPI. Outcomes for bivalirudin-treated patients were; mortality 5.2%, MACE 7.5%, major bleeding 3.8%. The incidence of acute ST was 1.0%, including in the absence of additional heparin (1.2%). Most cases of acute ST (7/9) occurred in the first four hours post-PCI. There was no significant difference in outcomes between patients treated with bivalirudin versus heparin/GPI.
Conclusions: Routine use of bivalirudin in a real-world primary PCI population was associated with good 30-day outcomes. Acute stent thrombosis was infrequent, even without additional heparin. A continued low-dose infusion of bivalirudin did not appear to offer protection against very early stent thrombosis.
Introduction
Glycoprotein IIb/IIIa receptor inhibitors (GPIs), given in conjunction with unfractionated heparin, have until recently been routinely used as adjunctive pharmacology to reduce ischaemic complications in patients undergoing primary percutaneous coronary intervention (PCI) for acute ST-elevation myocardial infarction (STEMI)1-3. However, GPIs increase the risk of haemorrhagic complications, which are independently associated with adverse short-term and long-term outcomes, including increased mortality2,4-7.
In the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) trial, use of the direct thrombin inhibitor bivalirudin was associated with a significant reduction in bleeding, similar rates of major adverse cardiovascular events (MACE), and significantly lower mortality at 30 days, one, two, and three years compared to patients treated with GPI+heparin8,9.
However, concerns remain about the use of bivalirudin for primary PCI in routine clinical practice. Firstly, the incidence of acute stent thrombosis in the HORIZONS-AMI trial was significantly higher with bivalirudin compared with GPI. This may have been due purely to less potent inhibition of platelets with bivalirudin compared to GPI. However, suboptimal dosing of oral antiplatelet therapy, and discontinuation of bivalirudin at the end of the PCI procedure before oral antiplatelet agents have achieved maximal effect may also have been contributory factors10. Secondly, the efficacy of bivalirudin as a sole agent remains uncertain. Two thirds of the patients assigned to “bivalirudin alone” in the HORIZONS-AMI trial received unfractionated heparin (UFH) prior to randomisation, and acute stent thrombosis tended to occur more frequently in patients who did not receive heparin8. Furthermore, a report from the Swedish Angiographic and Angioplasty Registry demonstrated higher 30-day mortality rates in the absence of additional heparin11.
Finally, the HORIZONS-AMI trial population was inevitably highly selected with few patients in the greatest risk groups, including cardiogenic shock and out-of-hospital cardiac arrest. Consequently, the incidence of adverse outcomes, in particular all-cause and cardiac mortality, was markedly lower than is conventionally observed in routine clinical practice.
The purpose of this study was to evaluate the outcome of an all-comer population of patients undergoing primary PCI in a real-world setting with adjunctive bivalirudin, supplemented by a four-hour post-procedural infusion, but without routine use of upfront unfractionated heparin.
Methods
Consecutive, unselected patients undergoing primary PCI for acute STEMI (presentation within 12 hours after the onset of symptoms with ≥1 mm ST-segment elevation in two or more contiguous leads, new left bundle branch block, or true posterior myocardial infarction) in a single tertiary cardiac centre between January 2009 and January 2010 were enrolled. The study was approved by the hospital ethics committee, and written informed consent was obtained from all subjects. The cardiac centre provides a 24/7 primary PCI service to a population of three million, encompassing patients from nine surrounding district general hospitals. Patients are admitted either directly via the ambulance service following pre-hospital ECG diagnosis, or via the district hospital emergency department.
Aspirin 300 mg was given at presentation and 75 mg daily thereafter, continued indefinitely. On arrival to hospital a loading dose of clopidogrel (600 mg) or prasugrel (60 mg) was administered prior to emergency cardiac catheterisation, followed by a daily maintenance dose (clopidogrel 75 mg; prasugrel 10 mg) for 12 months. Bivalirudin alone was recommended as procedural anticoagulation. In line with the HORIZONS-AMI trial, “bail-out” GPI in addition to bivalirudin was considered for cases of refractory intra-coronary thrombus and/or slow-flow/no-reflow. Bivalirudin was administered before guidewire insertion as an initial bolus of 0.75 mg/kg IV, followed by an infusion of 1.75 mg/kg/hr during the procedure, reducing to 0.25 mg/kg/hr for four hours post-procedure. No dosing adjustment was made for renal insufficiency. The use of unfractionated heparin (UFH) in addition to bivalirudin, or in combination with planned GPI as an alternative to bivalirudin, was at the discretion of the individual PCI operator. Primary PCI was undertaken using standard techniques. Access was radial or femoral. Aspiration thrombectomy was used at the discretion of the operator, and mechanical thrombectomy (ThromCat®; St Jude Medical, Minneapolis, MN, USA) was available for refractory thrombus. Intra-aortic balloon pump support was employed routinely in cases of cardiogenic shock. Following PPCI all patients were transferred to the Coronary Care Unit for at least 24 hours, and kept in hospital for a minimum of three days. Femoral artery sheaths were removed four hours following PCI and haemostasis achieved using manual compression, closure devices were not routinely used. Standard secondary preventative therapy (beta-blockers, angiotensin converting enzyme inhibitors/angiotensin receptor blockers and statins) was administered unless contraindicated.
Follow-up
Hospital notes were reviewed at the time of discharge to determine in-hospital outcomes. Blood results were abstracted from institutional results servers. Details of repeat coronary angiography and revascularisation were checked by cross-reference with our cathlab database, and all blood transfusions identified from hospital blood bank records. The hospital radiology database was interrogated to identify all relevant vascular radiology investigations and interventions indicating access site complications. Telephone follow-up at thirty days was conducted for all survivors with a specific enquiry as to the occurrence of bleeding events or MACE. Thirty-day mortality data were obtained via the Office of National Statistics.
Definitions
OUTCOMES ASSESSED AT 30 DAYS WERE
i) major adverse cardiovascular events (MACE),
ii) non-CABG related major bleeding and iii) stent thrombosis. MACE included death, reinfarction, unplanned target vessel revascularisation, and stroke. Major bleeding was determined according to the same definition used in the HORIZONS-AMI trial: intracranial or intraocular haemorrhage, access site bleeding with a ≥5 cm haematoma or that required intervention, a decrease in the haemoglobin level of ≥4 g/dL without an overt bleeding source or ≥3 g/dL with an overt bleeding source, re-operation for bleeding, or blood transfusion. Death from cardiac causes was defined as death due to acute myocardial infarction, cardiac perforation or pericardial tamponade, arrhythmia or conduction abnormality, procedural complications, or any death for which a cardiac cause could not be ruled out. Death from non-cardiac causes included bleeding-related death. Stent thrombosis was defined as the definite or probable occurrence of a stent-related thrombotic event according to the Academic Research Consortium classification12. Reinfarction was defined as recurrent chest pain lasting ≥30 minutes, or new ECG changes consistent with MI, and elevation in troponin to at least three times the upper limit of normal reference range.
STATISTICAL ANALYSIS
Categorical variables were compared by means of the chi-square test or Fisher’s exact test. Continuous variables were compared by means of the t-test and Wilcoxon rank-sum test. Logistic regression models were used to identify the predictors for certain outcomes, such as major bleeding and cardiac death. Odds ratios and relative risks were expressed with their 95% confidence intervals.
Results
Nine hundred and sixty eight patients underwent PPCI and were included in the study.
Thirty-day mortality data were available in 100% of cases, and complete follow-up data in 99.7% (965/968). Initial antithrombotic therapy was bivalirudin in 885 patients (91.4%), GPI+heparin in 82 (8.5%), and heparin only in one (0.1%). Of the bivalirudin treated patients, 123 (13.9%) received additional antithrombin therapy (heparin or fondaparinux) prior to administration of bivalirudin, and 114 (12.9%) were given “bail-out” GPI after initial treatment with bivalirudin. No patient received bivalirudin plus up-front GPI.
Baseline characteristics of the study population are shown in Table 1. Median age was 63.4 (53.4-73.4) years, 71.9% of the patients were male and 13.2% had pre-existing diabetes. Almost all (99.9%) patients received aspirin and high-dose thienopyridine (clopidogrel 600 mg 95.4%, prasugrel 60 mg 4.5%) prior to PCI. Patients treated with bivalirudin were significantly older than those receiving upfront GPI+heparin, whereas a history of prior MI or prior PCI, and the incidence of cardiogenic shock were significantly greater in patients treated with GPI+heparin (10.6% vs. 3.1%; p=0.003). Patients receiving “bail-out” GPI in addition to bivalirudin were a high-risk cohort, with an increased incidence of insulin-treated diabetes (7.9% vs. 2.6%; p=0.008), prior MI (20.2 vs. 10.3%, p=0.04), and prior PCI (15.8 vs. 5.3%, p<0.001) than those treated with bivalirudin alone. Males (76.4% vs. 70.9%; p=0.010) and patients presenting with anterior STEMI (51.2% vs. 39.5%; p=0.018) were more likely to have received additional antithrombin therapy prior to administration of bivalirudin.
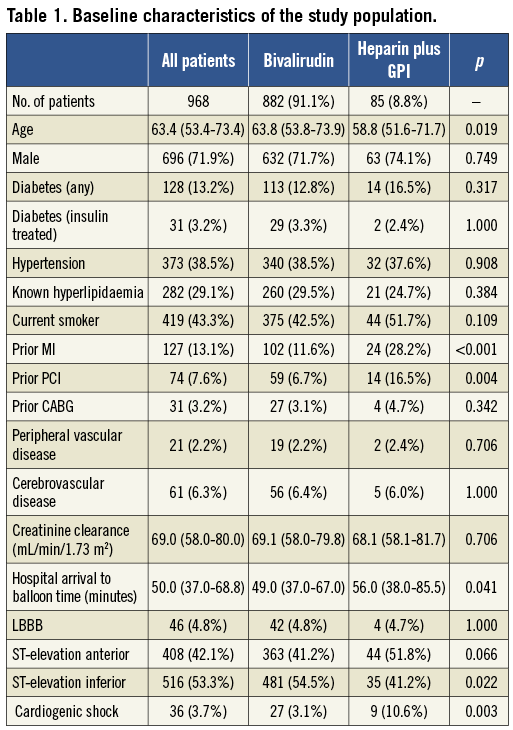
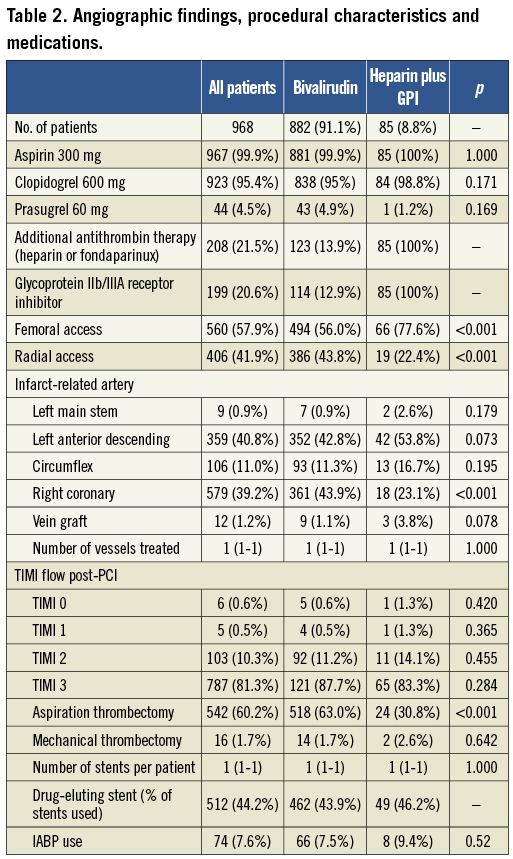
Angiographic and procedural characteristics are shown in Table 2. Median “call-to-balloon” time (duration from activation of emergency services by patient to thrombus aspiration or initial balloon inflation) was 127 (105-159) minutes, while “door-to-balloon” time (duration from arrival at PCI centre to thrombus aspiration or initial balloon inflation) was 50 (37-69) minutes. PCI was via the radial artery in 41.9% of cases, and thrombectomy (aspiration or mechanical) performed in 61.9%. Thirty-six (3.7%) patients presented with cardiogenic shock while 74 (7.6%) required intra-aortic balloon counterpulsation immediately prior to or during PCI. Patients receiving planned GPI were more likely to undergo transfemoral PCI (77.6% vs. 56.0%; p<0.001) and less likely to have aspiration thrombectomy performed (30.8% vs. 63.0%; p<0.001), probably reflecting differences in practice in operators who continued to favour the use of GPI+heparin. “Bail-out” GPI use was more common in left main stem PCI (3.0% vs. 0.6%; p-value=0.044) or in cases with reduced TIMI flow (<3) following PCI (25.8 vs. 10.4%; p<0.001). There were no important procedural differences between patients receiving bivalirudin plus heparin compared to bivalirudin alone.
Outcomes
MORTALITY
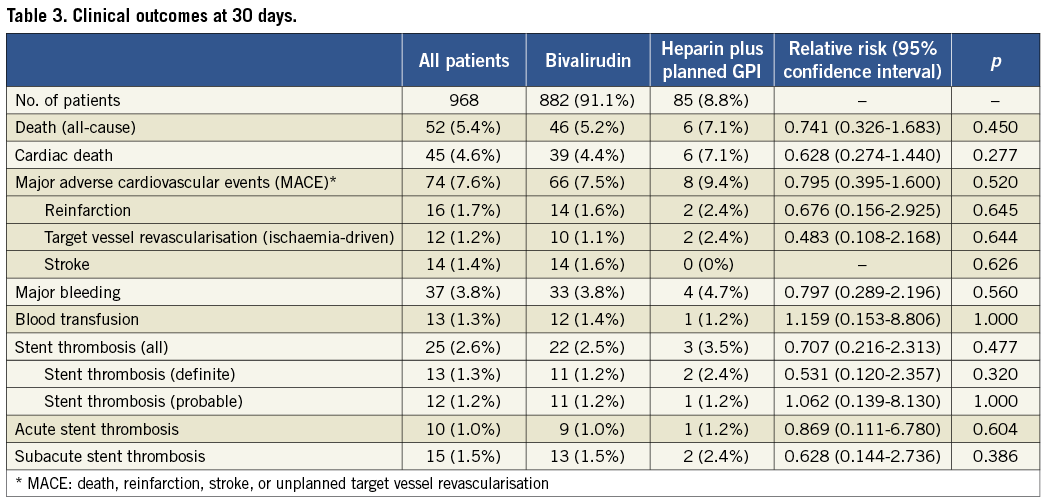
Thirty-day outcome data are shown in Table 3. Overall mortality rate was 5.4% at 30 days with no difference between patients treated with bivalirudin compared to GPI+heparin (5.2% bivalirudin vs. 7.1% GPI+heparin; p=0.45). The incidence of cardiac death at 30 days was 4.6% (4.4% vs. 7.1%; p=0.28). Independent predictors of 30-day mortality were age >65 years (OR 3.0; 95% CI: 1.1-8.3), cerebrovascular disease (OR 4.1; 95% CI: 1.4-12.1), cardiogenic shock (OR 12.8; 95% CI: 4.5-36.4), reduced eGFR (<60 mL/min/1.73 m2) (OR 0.24; 95% CI: 0.10-0.60) and transfemoral PCI (OR 2.9; 95% CI: 1.1-8.2). The overall incidence of MACE at 30 days was 7.6% (7.5% bivalirudin vs. 9.4% GPI+heparin; p=0.520).
BLEEDING
Major bleeding occurred in 3.8% of patients, and was statistically no different in patients treated with bivalirudin upfront vs. GPI+heparin upfront (3.8% vs. 4.7%; p=0.560). Major bleeding was numerically lowest (2.7%) in bivalirudin-treated patients who did not receive either additional antithrombin therapy or “bail-out” GPI. All-cause mortality (16.2% vs. 5.0%; p=0.012) and cardiac death (16.2% vs. 4.2%; p=0.006) at 30 days were significantly higher in patients who experienced major bleeding. Major bleeding occurred in 2.7% of radial and 4.7% of femoral procedures (p=0.13), and was most common in patients requiring IABP support (10.0%). Despite being numerically less frequent in patients undergoing radial PCI with bivalirudin alone (1.9%), no statistical difference was seen compared to femoral cases with GPI+heparin (4.5%; p=0.197). Thirteen patients (1.3%) required a blood transfusion (1.4% bivalirudin vs. 1.2% GPI+heparin; p=1.000).
STENT THROMBOSIS
Among 964 patients in whom stents were successfully implanted, the overall rate of definite or probable stent thrombosis (ST) at 30 days was 2.6% (2.5% bivalirudin, 3.5% GPI+heparin; p=0.48). Acute ST occurred in 1.0% of patients (n=10), again with no difference between the bivalirudin and GPI+heparin groups (1.0% vs. 1.2%; p=0.60). Of the nine patients who suffered acute ST after treatment with bivalirudin, 7 (78%) occurred within four hours of the PCI procedure. Among the 10 patients in whom stent thrombosis developed within 24 hours, two patients died (20%). There were no cases of acute ST in patients receiving prasugrel (n=44).
EFFECT OF ADDITIONAL ANTITHROMBIN THERAPY IN BIVALIRUDIN-TREATED PATIENTS
One hundred and twenty-three patients (13.9%) received additional antithrombin therapy prior to treatment with bivalirudin. In the majority of patients this was unfractionated heparin given at the time of PCI (82%) according to operator preference, while a smaller proportion received fondaparinux (11%) or low molecular weight heparin (7%) prior to transfer to the cathlab (patients presenting initially with a diagnosis of NSTEMI, who subsequently develop ST-elevation). Additional antithrombin therapy was not associated with any difference in acute stent thrombosis (0% vs. 1.2%; p=0.621), all definite/probable stent thrombosis (0.8% vs. 2.8%; p=0.345), MACE (9.8% vs. 7.1%, p=0.350), or major bleeding (4.9% vs. 3.6%; p=0.445) at 30 days compared to bivalirudin alone.
EFFECT OF “BAIL-OUT” GPI
“Bail-out” GPI was used in 114 (11.8%) patients receiving bivalirudin. MACE rates at 30 days were numerically highest in this patient group, although no statistical difference was seen compared to bivalirudin alone (11.4% vs. 6.9%, p=0.197). However, a significant increase in major bleeding (9.6% vs. 2.9%; p=0.002) and blood transfusion (6.1 vs. 0.7%; p<0.001) was observed with “bail-out” GPI use compared to bivalirudin alone.
Discussion
In this prospective, observational study involving consecutive “all-comer” patients with ST-segment elevation myocardial infarction undergoing primary PCI, treatment with the direct thrombin inhibitor bivalirudin followed by a low-dose infusion continued for four hours resulted in low rates of mortality, MACE, stent thrombosis and major bleeding at 30 days. All-cause mortality rate at 30 days was 5.4%, while MACE occurred in 7.6% of patients. Although overall mortality is notably higher than the 2.6% seen in the clinical trial population of the HORIZONS-AMI study13, this represents a true “all-comer” population, including patients presenting with out-of-hospital arrest and cardiogenic shock. Thirty-day mortality in this study was highly comparable to that reported by other real-world registries, including the National Cardiovascular Data Registry (NCDR; n=285,440, 30-day mortality 4.69%)13, and the Register of Information and Knowledge about Swedish Heart Intensive Care Admissions (RIKSHIA; n=7,084, 30-day mortality 4.9%)14,15.
BLEEDING
Bleeding is common after PCI, particularly in the primary PCI setting16, and is associated with increased ischaemic events and mortality7. Clinical trials have shown a consistent reduction in major bleeding events with bivalirudin in elective, urgent and emergency PCI8,17,18. In keeping with this, the 30-day rate of major bleeding in this study was low (3.8%), and somewhat lower than the rate reported in the HORIZONS-AMI trial with bivalirudin (4.9%). This may reflect under-reporting of some bleeding events, particularly large haematoma, but could also be due to the greater use of radial access in this study19. We also found a very low incidence of the hard endpoint of blood transfusion (1.3% compared to 2.8% in HORIZONS-AMI), which is well recognised as an independent predictor of increased morbidity and mortality20. Whilst bleeding events were low with bivalirudin use, we cannot exclude an increase in bleeding with a four-hour infusion compared to procedural use alone.
We did not observe a significant difference in major bleeding between patients treated with bivalirudin compared to GPI+heparin (3.8% vs. 4.5%, p=0.56). However, numbers were very small in the GPI+heparin group. Major bleeding was numerically lowest in those patients who received bivalirudin alone (2.7%), without either prior administration of antithrombin therapy or subsequent treatment with GPI. In particular major bleeding was substantially more common in patients receiving bivalirudin plus “bail-out” GPI (9.6%). This is consistent with the findings from registry data and reports from a meta-analysis of the REPLACE-2, ACUITY and HORIZONS-AMI trials21. Since there is no evidence of benefit from the use of “bail-out” GPI in addition to bivalirudin, it may be that a higher threshold for its use would be appropriate.
STENT THROMBOSIS
Definite or probable stent thrombosis occurred in 2.6% of patients at 30 days. The incidence of acute stent thrombosis (1.0%) was comparable to that seen in the HORIZONS-AMI trial with bivalirudin (1.3%), and notably higher than in the GPI arm of the trial (0.3%). As in HORIZONS-AMI, most acute stent thrombosis events occurred very early (within four hours). These findings suggest that the use of a post-procedural four-hour infusion of low-dose bivalirudin in this study did not protect against very early stent thrombosis. We used a very low dose post-procedural infusion (0.25 mg/kg/hr) based on the dose used in the ACUITY trial before PCI and suggested as an option post-PCI in the HORIZONS-AMI trial. Further investigation is warranted to determine if a higher dose post-procedural infusion and/or more rapid and potent platelet inhibition with newer P2Y12 receptor inhibitors would reduce the risk of acute stent thrombosis without adversely affecting MACE or bleeding rates.
Interestingly, we did not see any cases of stent thrombosis in the relatively small number of patients receiving prasugrel, supporting the hypothesis that stent thrombosis may predominantly be a platelet-mediated phenomenon.
EFFECT OF PRE-PROCEDURAL HEPARIN
In the HORIZONS-AMI study, 66% of patients received additional unfractionated heparin prior to randomisation. In those patients randomised to bivalirudin, outcomes were significantly worse in the absence of pre-procedural heparin, in particular acute stent thrombosis (2.6% vs. 0.9%). The SCAAR registry also found that the combined endpoint of death or acute stent thrombosis (11.0% vs. 6.4%; p<0.01) occurred more frequently in bivalirudin-treated patients who did not receive additional heparin before or at the time of PCI. However, both these analyses were of uncontrolled data, while a mechanism to explain a benefit for heparin on top of bivalirudin is hard to identify, since heparin is a less potent inhibitor of thrombin, and may activate platelets during PCI. In this study pre-procedural heparin was not routinely given, and outcomes were comparable to the overall HORIZONS-AMI cohort, both when considering the overall population of this study, and when excluding the small group of patients who did receive additional heparin. In particular, the incidence of acute stent thrombosis observed in this study in the majority of patients who did not receive additional heparin was only 1.0%, much lower than the 2.6% rate seen in HORIZONS-AMI patients who did not receive heparin, and comparable to the overall HORIZONS-AMI cohort (1.3%). There were no cases of acute stent thrombosis in bivalirudin patients receiving additional heparin. However, the relatively small number of patients in this study who received heparin in addition to bivalirudin does not allow a valid comparison of their respective outcomes; nevertheless, we did not observe any differences in mortality, MACE, stent thrombosis, or major bleeding.
COMPARISON WITH GPI+HEPARIN
A little over 10% of patients in this study received upfront treatment with a glycoprotein IIb/IIa inhibitor+heparin instead of bivalirudin. This was due predominantly to the personal preference of some operators. We did not observe any difference in outcomes, including mortality, MACE, stent thrombosis, or major bleeding, between the bivalirudin and GPI-treated patients. However, since the numbers are small, and inevitable differences exist between the patients, both identifiable and unidentifiable, this study does not permit a valid comparison of bivalirudin versus GPI+heparin.
STUDY LIMITATIONS
This study is a prospective observational registry of consecutive patients undergoing primary PCI designed to evaluate clinical outcomes with the use of bivalirudin in a large “real-world” population. As such it is subject to the inherent limitations of a non-randomised study. It does not allow robust comparisons to be made between outcomes with bivalirudin and other treatment options. Although some patients received upfront GPI+heparin instead of bivalirudin, the small numbers involved and differences between the groups prevent any valid evaluation of outcome according to treatment. Similarly, the relatively small and uncontrolled population of patients who received additional heparin means that no real conclusions can be made regarding the effects of co-prescription of heparin. The study was underpowered to assess low-frequency endpoints, specifically stent thrombosis. We did not randomly assign patients receiving bivalirudin to a post-procedural infusion. As such, we are unable to comment on whether a post-procedural infusion reduces early stent thrombosis and other adverse events compared to a regimen of stopping bivalirudin immediately on completion of PCI. Whilst outcomes within this study are acceptable compared to historical controls, without an adequately sized active comparator group it is difficult to make definitive statements regarding the safety and efficacy of bivalirudin.
The lack of an independent data monitoring committee to adjudicate outcome events, or a core laboratory for assessment of PCI procedures and angiographic images, is also a limitation of this study.
Conclusions
The results of this study indicate that routine treatment with bivalirudin in a real-world population of patients undergoing primary PCI for acute ST-elevation myocardial infarction, with a four-hour post-procedural infusion but without routine additional heparin, is associated with good outcomes, including a favourable overall mortality and a low incidence of stent thrombosis, major adverse cardiovascular events, and major bleeding. However, a four-hour post-PCI bivalirudin infusion did not eliminate the previously described risk of acute stent thrombosis, and hence its routine use is not supported.
Conflict of interest statement
The authors have no conflicts of interest to declare.

