Abstract
BACKGROUND: In patients presenting with acute coronary syndrome (ACS), the number of diseased vessels may affect the efficacy of a complete revascularisation strategy.
AIMS: The authors sought to evaluate the safety and efficacy of immediate complete revascularisation (ICR) and staged complete revascularisation (SCR) in patients presenting with ACS stratified by the number of diseased vessels.
METHODS: In this prespecified analysis of the BIOVASC trial, ICR was compared with SCR in patients with two-vessel disease (2VD) or three-vessel disease (3VD). The primary endpoint was a composite of all-cause mortality, myocardial infarction (MI), any unplanned ischaemia-driven revascularisation or cerebrovascular events at 1 year after the index procedure. Comparisons were performed using Cox regression.
RESULTS: A total of 1,525 patients were enrolled in the BIOVASC trial, of whom 1,177 presented with 2VD and 265 with 3VD. In the 2VD group, 613 patients were assigned to ICR and 564 to SCR. In the 3VD group, 117 patients were assigned to ICR and 148 to SCR. ICR and SCR led to similar results in both the 2VD (hazard ratio [HR] 0.76, 95% confidence interval [CI]: 0.50-1.13; p=0.18) and 3VD groups (HR 0.79, 95% CI: 0.39-1.59; p=0.51) (pinteraction=0.91) in terms of the primary endpoint. ICR was associated with a lower rate of MI in patients with 3VD (HR 0.21, 95% CI: 0.046-0.93; p=0.04) (pinteraction=0.30).
CONCLUSIONS: ICR might be an option in patients presenting with extensive 3VD and might be associated with a lower rate of myocardial infarction compared with SCR.
Multivessel coronary artery disease occurs in up to half of patients presenting with acute coronary syndromes (ACS)12, and the number of diseased vessels might have a prognostic impact23. Complete revascularisation in patients with ACS and multivessel disease (MVD) improves clinical outcomes compared with a culprit-Âonly treatment strategy45678, but the optimal timing for non-culprit lesion intervention remains unclear. The recent BIOVASC trial showed that immediate complete revascularisation (ICR) is safe and leads to a lower risk of myocardial infarction (MI) and ischaemia-driven revascularisation, as well as having ancillary advantages like an overall shorter hospital stay, when compared with staged complete revascularisation (SCR)9. However, ICR in the acute setting could be particularly challenging in patients with extensive three-vessel disease (3VD), burdened by long procedural time, and high contrast and radiation use. ICR might therefore be more appealing in patients with limited coronary disease, such as those with significant lesions in only two vessels, where there would be a high likelihood of procedural success without excessive use of radiation or contrast. Given this background and the lack of data on the adoption of ICR in ACS patients with 3VD, we evaluated the safety and efficacy of ICR and SCR in the BIOVASC trial, specifically stratifying for the number of diseased vessels.
Methods
STUDY DESIGN AND PARTICIPANTS
The BIOVASC trial was a randomised, open-label, multicentre, investigator-initiated, non-inferiority trial comparing ICR with SCR in patients presenting with ACS and MVD. Details of the trial design have been previously reported10. Briefly, 1,525 patients presenting with ACS and MVD were randomly assigned to either ICR or SCR in a 1:1 ratio. A significant non-culprit lesion was defined as having at least 70% stenosis in a vessel ≥2.5 mm in diameter by visual estimation or by positive physiology testing. Invasive coronary imaging or physiology assessment was per the operator’s discretion. The exclusion criteria included the absence of a clear culprit lesion, previous coronary artery bypass grafting, cardiogenic shock, and the presence of a chronic total occlusion. This is a BIOVASC prespecified subanalysis comparing the clinical outcomes of ICR with SCR in patients with either two-vessel disease (2VD) or 3VD10.
OUTCOMES
The definitions of all efficacy and safety outcomes have been previously published in detail10. The primary endpoint was a composite of all-cause mortality, MI, any unplanned ischaemia-driven revascularisation or cerebrovascular events at 1 year after the index procedure. Secondary endpoints included the individual components of the composite primary endpoint and a composite of cardiovascular death or MI.
Mortality was classified as cardiovascular or non-cardiovascular. Any undetermined death was considered cardiovascular. The Third Universal Definition was used to define11 myocardial infarction, with a modification in which the ACS setting is taken into account, similarly to the COMPLETE trial4. An independent clinical events committee adjudicated all potential endpoints.
STATISTICAL ANALYSIS
Categorical data were presented as counts and percentage, and tested by chi-square tests or Fisher’s exact tests if there was an expected cell value <5. Continuous data were presented as mean and standard deviation if a Gaussian distribution was present – the Shapiro-Wilk test was used to evaluate normality – and tested by unpaired t-tests. If a Gaussian distribution was not present, continuous data were presented as median and quartiles [25th percentile-75th percentile] and compared using Wilcoxon rank-sum tests.
For primary and secondary endpoints, a time-to-event analysis was performed applying the Kaplan-Meier method. Event-free patients were censored at the date on which they were last known to be alive. Differences in cumulative event-free survival between 2VD and 3VD were evaluated by the log-rank test. Cox proportional hazard regression models were applied to further study the relation between the extent of vessel disease and the study endpoints.
Multiplicative testing was used for the interaction of the number of diseased vessels on the treatment effect by using Cox proportional hazards models with an interaction term comprising the number of diseased vessels and the treatment allocation. The statistical significance of multiplicative interaction was tested on the null hypothesis that the exponentiated beta of the interaction term equals 1. We report hazard ratios (HR) which are presented with 95% confidence intervals (CI). Assessment of the log-minus-log plot led to no suspicion of a violated proportional hazards assumption for the primary or secondary endpoints. We added a sensitivity analysis using an intention-to-treat analysis of the primary and secondary endpoints, including the 83 patients with single-vessel disease. All analyses were performed using R version 4.2.1 (R Foundation for Statistical Computing; packages used: data.table, dplyr, ggplot2, ggpubr, graphics, survival, lubridate, stats, survminer, tidycmprsk). For all tests, a two-sided p-value<0.05 was considered statistically significant.
Results
From 26 June 2018 to 21 October 2021, a total of 1,525 patients were enrolled in the BIOVASC trial. A total of 1,177 patients presented with 2VD and 265 with 3VD. In the 2VD group, 613 patients were assigned to ICR and 564 patients to SCR. In the 3VD group, 117 patients were assigned to ICR and 148 patients to SCR. Baseline and procedural characteristics for the overall population are reported in Table 1 and Table 2, respectively. Patients randomised to SCR had a longer total hospital stay in both the 2VD and 3VD groups. Overall, there was higher total contrast use and radiation dose in the 3VD group compared with the 2VD group, but an ICR strategy was associated with a significant reduction of those parameters compared with an SCR strategy in both groups (Table 2, Supplementary Figure 1, Supplementary Figure 2). Contrast use in patients assigned to ICR was not significantly different between the 2VD and 3VD groups (200 mL [interquartile rang {IQR}150-260 mL] vs 200 mL [IQR 163-280 mL]; p=0.17).
Table 1. Baseline characteristics of patients undergoing immediate versus staged complete revascularisation in two- versus three-vessel disease.
| Characteristics | Two-vessel disease | Three-vessel disease | ||||
|---|---|---|---|---|---|---|
| Immediate revascularisation (N=613) | Staged revascularisation (N=564) | p-value | Immediate revascularisation (N=117) | Staged revascularisation (N=148) | p-value | |
| Age, years | 66 (57.0-72.9) | 65 (58.2-72.5) | 0.98 | 58 (66-72) | 59 (66-73) | 0.50 |
| Sex, male | 482 (78.6) | 434 (77.0) | 0.49 | 89 (76.1) | 117 (79.1) | 0.56 |
| BMI, kg/m² | 27.3 (24.5-30.0) | 27.3 (24.8-29.8) | 0.93 | 25 (27-31) | 25 (27-20) | 0.51 |
| Presentation | 0.51 | 0.83 | ||||
| STEMI | 246 (40.1) | 221 (39.2) | 53 (45.3) | 68 (45.9) | ||
| NSTEMI | 318 (51.9) | 287 (50.9) | 58 (49.6) | 70 (47.3) | ||
| UA | 49 (8.0) | 56 (9.9) | 6 (5.1) | 10 (6.8) | ||
| Medical history | ||||||
| Previous PCI | 73 (11.9) | 92 (16.3) | 0.029 | 6 (5.1) | 23 (15.5) | 0.007 |
| History of MI | 60/612 (9.8) | 71/564 (12.6) | 0.13 | 7 (6.0) | 15 (10.1) | 0.22 |
| Peripheral artery disease | 32/612 (5.2) | 26/564 (4.6) | 0.62 | 3 (2.6) | 6 (4.1) | 0.74 |
| Valve disease | 22/611 (3.6) | 13/562 (2.3) | 0.23 | 2 (1.7) | 2 (1.4) | >0.99 |
Chronic obstructive pulmonary disease |
40/612 (6.5) | 34/564 (6.0) | 0.72 | 9 (7.7) | 11 (7.4) | 0.94 |
| Atrial fibrillation or flutter | 27 (4.4) | 17 (3.0) | 0.21 | 5 (4.3) | 3 (2.0) | 0.29 |
| Renal insufficiency | 31 (5.1) | 26 (4.6) | 0.72 | 9 (7.7) | 7 (4.7) | 0.31 |
| History of stroke | 31/612 (5.1) | 18/564 (3.2) | 0.11 | 6 (5.1) | 7 (4.7) | 0.88 |
| Hypertension | 342 (55.8) | 287 (52.7) | 0.28 | 71 (60.7) | 71 (48.0) | 0.039 |
| Diabetes | 130 (21.2) | 120 (21.3) | 0.98 | 25 (21.4) | 35 (23.6) | 0.66 |
| Hypercholesterolaemia | 331/611 (54.2) | 304/563 (54.0) | 0.95 | 44 (37.6) | 74 (50.0) | 0.044 |
| Family history of CVD | 188/612 (30.7) | 174//559 (31.1) | 0.88 | 36 (31.0) | 50 (34.2) | 0.58 |
| Smoking behaviour | 0.82 | 0.52 | ||||
| Never | 291/610 (47.7) | 277/563 (49.2) | 54 (46.2) | 73 (50.0) | ||
| Current | 200/610 (32.8) | 175/563 (31.1) | 47 (40.2) | 49 (33.6) | ||
| Former | 119/610 (19.5) | 111/563 (19.7) | 16 (13.7) | 24 (16.4) | ||
| Data are median (IQR), n (%), or n/N (%). BMI: body mass index; CVD: cardiovascular disease; IQR: interquartile range; MI: myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction; UA: unstable angina | ||||||
Table 2. Procedural characteristics of immediate versus staged complete revascularisation in two- versus three-vessel disease.
| Characteristics | Two-vessel disease | Three-vessel disease | |||||
|---|---|---|---|---|---|---|---|
| Immediate revascularisation (N=613) | Staged revascularisation (N=564) | p-value | Immediate revascularisation (N=117) | Staged revascularisation (N=148) | p-value | pinteraction** | |
| Systolic blood pressure, mmHg | 125 (110-140) | 125 (111-141) | 0.57 | 126 (110-139) | 120 (110-139) | 0.33 | |
| Diastolic blood pressure, mmHg | 72 (63-81) | 71 (62-80) | 0.93 | 74 (66-82) | 70 (62-79) | 0.043 | |
| Radial access | 591/612 | 545/564 | 0.95 | 115 (98.3) | 143 (96.6) | 0.47 | |
| Location of culprit lesion* | 0.44 | 0.95 | |||||
Left main coronary artery |
1/607 (0.2) | 3/564 (0.5) | 2/116 (1.7) | 2/148 (1.4) | |||
Left anterior descending artery |
240/607 (39.5) | 206/564 (36.5) | 34/116 (29.3) | 47/148 (31.8) | |||
| Circumflex artery | 168/607 (27.7) | 153/564 (27.1) | 28/116 (24.1) | 62/148 (41.9) | |||
Right coronary artery |
198/607(32.6) | 202/564 (35.8) | 52/116 (44.8) | 62/148 (41.9) | |||
| Lesion complexity§ | 0.95 | 0.64 | |||||
| Type A | 133/1,193 (11.1) | 112/1,081 (10.4) | 40/343 (11.7) | 41/411 (10.0) | |||
| Type B1 | 358/1,193 (30.0) | 327/1,081 (30.2) | 76/343 (22.2) | 80/411 (19.5) | |||
| Type B2 | 285/1,193 (23.9) | 259/1,081 (24.0) | 61/343 (17.8) | 77/411 (18.7) | |||
| Type C | 417/1,193 (35.0) | 383/1,081 (35.4) | 166/343 (48.4) | 213/411 (51.8) | |||
| Off-hours procedure|| | 164 (26.8) | 161 (28.5) | 0.49 | 39 (33.3) | 48 (32.4) | 0.88 | 0.66 |
| Complete revascularisation¶ | 596/613 (97.2) | 543/563 (96.4) | 0.44 | 111 (94.9) | 135 (91.2) | 0.25 | 0.60 |
| FFR/iFR | 77 (12.6) | 115 (20.4) | <0.001 | 11 (9.4) | 24 (16.2) | 0.10 | 0.91 |
| IVUS/OCT | 35 (5.7) | 75 (13.3) | <0.001 | 10 (8.6) | 34 (23.0) | 0.001 | 0.60 |
| Total hospital stay, days | 3 (2-5) | 4 (3-6) | <0.001 | 3 (2-5) | 5 (3-7) | <0.001 | 0.74 |
| Time to staged procedure, days | NA | 16 (4-28) | NA | NA | 13 (3-23) | NA | NA |
| Total no. of stents used per patient | 3 (2-3) | 3 (2-4) | 0.03 | 4 (3-6) | 4 (4-5) | 0.85 | 0.42 |
| Total length of stents, mm | 57 (44-77) | 30 (22-44) | <0.001 | 100 (79-135) | 104 (79-132) | 0.74 | 0.63 |
| Index procedure duration, minutes | 62 (46-82) | 47 (35-61) | <0.001 | 79 (61-95) | 45 (35-64) | <0.001 | <0.001 |
| Total procedure duration, minutes | 62 (46-82) | 86 (63-115) | <0.001 | 79 (61-95) | 116 (90-147) | <0.001 | 0.026 |
| Index procedure contrast use, mL | 200 (150-260) | 140 (102-185) | <0.001 | 200 (163-280) | 129 (100-180) | <0.001 | 0.09 |
| Total procedure contrast use, mL | 200 (150-260) | 250 (196-320) | <0.001 | 200 (163-280) | 300 (230-390) | <0.001 | 0.002 |
| Index procedure total DAP, cGy·cm2 | 4,640 (2,499-10,043) | 2,819 (1,480-6,031) | <0.001 | 5,096 (2,998-10,369) | 2,552 (1,486-4,895) | <0.001 | 0.08 |
| Total procedure total DAP, cGy·cm2 | 4,640 (2,499-10,043) | 5,778 (3,328-12,681) | 0.002 | 5,096 (2,998-10,369) | 6,760 (4,026-14,067) | 0.023 | 0.013 |
| P2Y12 inhibitor at discharge‡ | 0.54 | 0.65 | 0.23 | ||||
| Ticagrelor | 423/612 (69.1) | 400/563 (71.0) | 83/115 (72.2) | 100/148 (67.6) | |||
| Prasugrel | 82/612 (13.4) | 78/563 (13.9) | 19/115 (16.5) | 26/148 (17.6) | |||
| Clopidogrel | 107/612 (17.5) | 85/563 (15.1) | 13/115 (11.3) | 22/148 (14.9) | |||
| Data are median (IQR), n (%), or n/N (%).*In seven patients, the culprit was reported as unclear. **pinteraction denotes the interaction between two- and three-vessel disease. §The total number of vessels with significant lesions (with a vessel diameter ≥2.5 mm) was 3,179. However, the lesion complexity was not reported for 151 lesions (4.7%). ||On-hours procedures were defined as those performed from Monday to Friday between 8 AM and 6 PM. A procedure outside this interval was considered off-hours. ¶A patient was considered completely revascularised if all the significant lesions with a vessel diameter ≥2.5 mm were treated and if they were assessed as having a final Thrombolysis in Myocardial Infarction grade 3. One patient withdrew consent before the staged procedure; therefore, completeness of revascularisation could not be ascertained. ‡Three patients died before discharge, so no medications were prescribed, and one patient was discharged with single antiplatelet therapy and anticoagulation (aspirin and warfarin). DAP: dose-area product; FFR: fractional flow reserve; iFR: instantaneous wave-free ratio; IVUS: intravascular ultrasound; NA: not applicable; OCT: optical coherence tomography | |||||||
PRIMARY AND SECONDARY OUTCOMES
The cumulative incidence of the primary endpoint at 1-year follow-up in the 2VD group was 7.0% in ICR patients and 8.9% in the SCR patients. In the 3VD group, the primary endpoint occurred in 11.2% of patients treated with ICR and in 13.6% of the cases treated with SCR. The primary endpoint was not significantly different between the 2VD (HR 0.76, 95% CI: 0.50-1.13; p=0.18) and 3VD groups (HR 0.79, 95% CI: 0.39-1.59; p=0.51) (pinteraction=0.91) (Table 3). No significant interactions were detected in terms of all-cause mortality, cardiovascular mortality, cerebrovascular events, stent thrombosis or unplanned ischaemia-driven revascularisation between ICR and SCR patients in the 2VD and 3VD groups. The additional intention-to-treat analysis did not affect the results (Supplementary Table 1).
ICR was associated with a lower rate of myocardial infarction in the 3VD group, at 1.8%, as opposed to 8.2% in patients undergoing SCR (HR 0.21, 95% CI: 0.046-0.93; p=0.04) and a trend towards significance in the 2VD group, at 2.0%, as opposed to 3.9% in patients undergoing SCR (HR 0.50, 95% CI: 0.25-1.00; p=0.050) (Table 3, Central illustration). In total, the number of MIs in the ICR patients was similar between the 2VD (2%) and 3VD groups (1.8%), while in the SCR patients there were more MIs in the 3VD group (8.2%) compared with the 2VD group (3.9%). The types of MI are reported in Supplementary Table 2. After excluding type 4a MIs, the incidence of MI was not significantly different between SCR and ICR patients (Table 3).
Table 3. Primary and secondary outcomes in two- versus three-vessel disease.
| Outcome | Immediate complete revascularisation (N=613) | Staged complete revascularisation (N=564) | Hazard ratio (95% CI) | p-value | pinteraction | |||
|---|---|---|---|---|---|---|---|---|
| No. of events | Percentage† | No. of events | Percentage† | |||||
| Primary outcome | ||||||||
| All-cause mortality, any myocardial infarction, unplanned ischaemia-driven revascularisation or cerebrovascular event | 2VD | 42 | 7.0 | 50 | 8.9 | 0.76 (0.50-1.13) | 0.18 | 0.91 |
| 3VD | 13 | 11.2 | 20 | 13.6 | 0.79 (0.39-1.59) | 0.51 | ||
| Secondary outcomes | ||||||||
| Cardiovascular mortality or myocardial infarction | 2VD | 16 | 2.7 | 27 | 4.8 | 0.54 (0.29-1.00) | 0.049 | 0.96 |
| 3VD | 5 | 4.3 | 12 | 8.2 | 0.52 (0.18-1.47) | 0.22 | ||
| All-cause mortality | 2VD | 8 | 1.3 | 8 | 1.4 | 0.92 (0.35-2.45) | 0.87 | 0.10 |
| 3VD | 5 | 4.3 | 1 | 0.7 | 6.43 (0.75-55.09) | 0.089 | ||
| Cardiovascular mortality | 2VD | 5 | 0.8 | 6 | 1.1 | 0.77 (0.23-2.52) | 0.66 | 0.13 |
| 3VD | 4 | 1.7 | 1 | 0.7 | 5.15 (0.58-46.11) | 0.14 | ||
| Any myocardial infarction | 2VD | 12 | 2.0 | 22 | 3.9 | 0.50 (0.25-1.00) | 0.0503 | 0.30 |
| 3VD | 2 | 1.8 | 12 | 8.2 | 0.21 (0.046-0.93) | 0.040 | ||
| Unplanned ischaemia-driven revascularisation | 2VD | 22 | 3.7 | 33 | 5.9 | 0.60 (0.35-1.04) | 0.067 | 0.95 |
| 3VD | 8 | 7.1 | 16 | 10.9 | 0.62 (0.27-1.46) | 0.27 | ||
| Cerebrovascular event | 2VD | 9 | 1.5 | 9 | 1.6 | 0.92 (0.37-2.32) | 0.86 | 0.94 |
| 3VD | 2 | 2.0 | 3 | 1.8 | 0.85 (0.14-5.10) | 0.86 | ||
| Probable or definite stent thrombosis | 2VD | 5 | 0.8 | 5 | 0.9 | 0.92 (0.27-3.19) | 0.90 | 0.56 |
| 3VD | 1 | 0.9 | 3 | 1.8 | 0.43 (0.04-4.10) | 0.46 | ||
| Target vessel revascularisation | 2VD | 18 | 3.0 | 30 | 5.4 | 0.54 (0.30-0.97) | 0.041 | 0.90 |
| 3VD | 7 | 6.0 | 15 | 10.2 | 0.58 (0.24-1.43) | 0.24 | ||
| Target lesion revascularisation | 2VD | 14 | 2.3 | 27 | 4.9 | 0.47 (0.25-0.90) | 0.022 | 0.82 |
| 3VD | 6 | 5.3 | 14 | 9.6 | 0.54 (0.21-1.40) | 0.20 | ||
| All-cause mortality, myocardial infarction, stroke or major bleeding (BARC 3 or 5) | 2VD | 39 | 6.4 | 44 | 7.9 | 0.80 (0.52-1.23) | 0.32 | 0.42 |
| 3VD | 7 | 6.0 | 16 | 10.9 | 0.53 (0.22-1.30) | 0.17 | ||
| Major bleeding (BARC 3 or 5) | 2VD | 14 | 2.3 | 11 | 2.0 | 1.18 (0.54-2.60) | 0.68 | 0.27 |
| 3VD | 1 | 0.9 | 4 | 2.7 | 0.32 (0.035-2.83) | 0.30 | ||
| Excluding procedure-related infarction* | ||||||||
| All-cause mortality, any myocardial infarction, unplanned ischaemia-driven revascularisation or cerebrovascular event | 2VD | 41 | 6.8 | 47 | 8.4 | 0.79 (0.52-1.20) | 0.26 | 0.76 |
| 3VD | 13 | 11.2 | 18 | 12.3 | 0.89 (0.44-1.82) | 0.76 | ||
| Cardiovascular mortality or myocardial infarction | 2VD | 15 | 2.5 | 22 | 3.9 | 0.62 (0.32-1.20) | 0.16 | 0.70 |
| 3VD | 5 | 4.3 | 8 | 5.5 | 0.80 (1.25-2.44) | 0.69 | ||
| Any myocardial infarction | 2VD | 11 | 1.8 | 17 | 3.0 | 0.59 (0.27-1.26) | 0.17 | 0.49 |
| 3VD | 2 | 1.8 | 8 | 5.5 | 0.32 (0.07-1.50) | 0.15 | ||
| All-cause mortality, myocardial infarction, stroke or major bleeding (BARC 3 or 5) | 2VD | 38 | 6.3 | 40 | 7.1 | 0.86 (0.55-1.35) | 0.52 | 0.62 |
| 3VD | 7 | 6.0 | 13 | 8.8 | 0.67 (0.27-1.68) | 0.39 | ||
| †Cumulative incidence at 365 days according to the Kaplan-Meier method. *Only applicable for type 4a myocardial infarctions related to the index or staged procedure. 2VD: two-vessel disease; 3VD: three-vessel disease; BARC: Bleeding Academic Research Consortium; CI: confidence interval | ||||||||
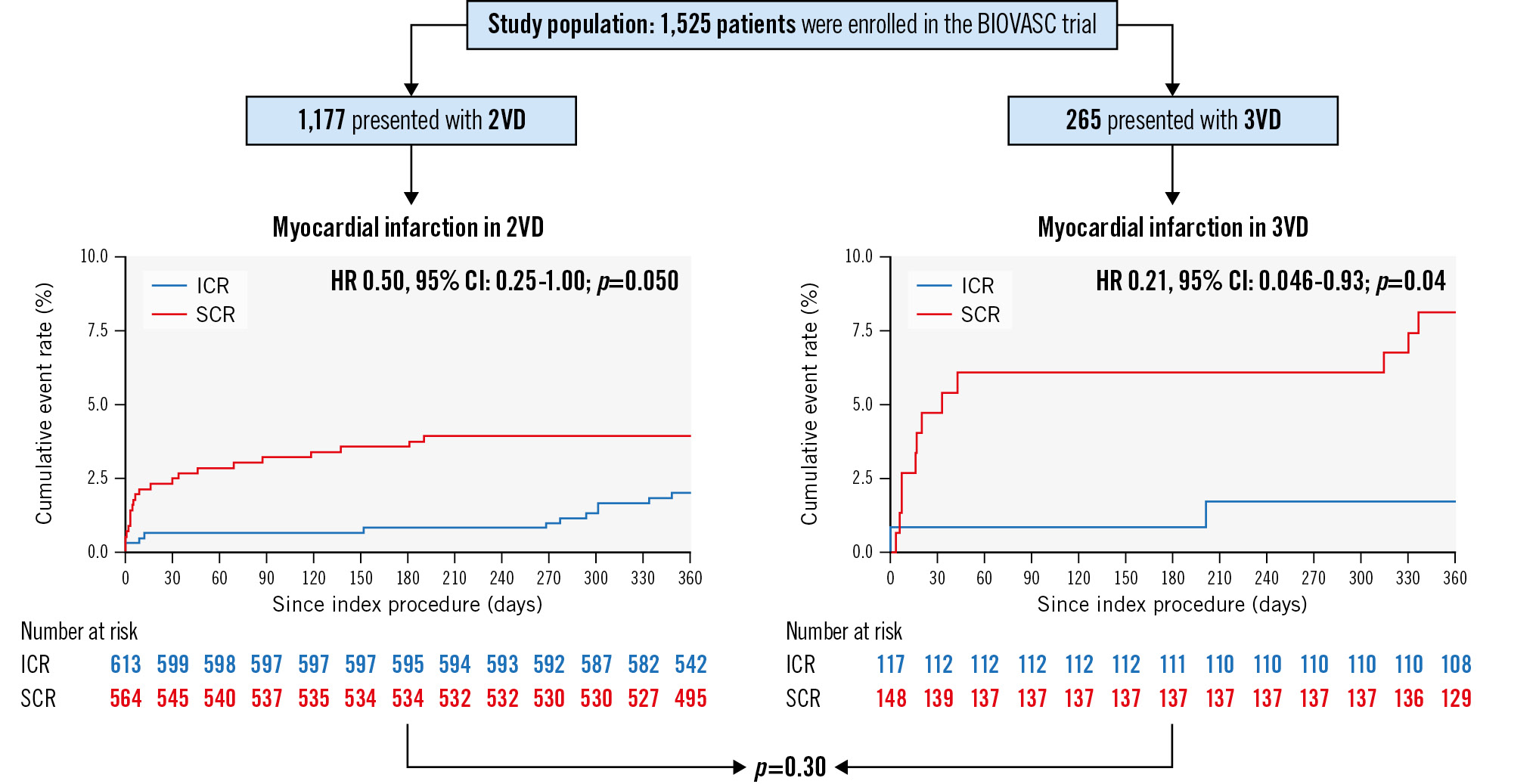
Central illustration. Clinical outcomes after immediate or staged complete revascularisation in patients presenting with two- or three-vessel disease. Myocardial infarction rates and their p interactions in patients with 2VD and in patients with 3VD. 2VD: two-vessel disease; 3VD: three-vessel disease; CI: confidence interval; HR: hazard ratio; ICR: immediate complete revascularisation; SCR: staged complete revascularisation
Discussion
The BIOVASC trial showed that ICR is non-inferior to SCR in patients with ACS and MVD in terms of the primary endpoint at 1-year follow-up. SCR was associated with more MIs and repeat revascularisation compared with ICR9. In the current subanalysis, we compared the impact of the timing of complete revascularisation between 2VD and 3VD. We did not identify significant interactions with respect to the effect of an immediate or staged strategy on the primary endpoint. ICR was associated with a lower risk of MI in patients with 3VD, and this was also a strong trend in patients with 2VD, resulting in a significant reduction in the composite of cardiovascular death and MI.
The reduction in MIs was evident in the 3VD population comparing ICR with SCR, despite the limited number of patients. In addition, a substantial difference in hazard ratio and absolute incidence in terms of any MI was evident when comparing 2VD and 3VD, indicating that patients with 3VD present with more MIs than patients with 2VD for those undergoing SCR, while the incidence among those undergoing ICR was similar. A statistical difference could not be established in the 2VD group, yet no significant interaction of 3VD/2VD was identified for the effect of ICR or SCR on MIs. However, it should be considered that an interaction test in a substudy of a clinical trial is likely to be underpowered1213.
Accurate culprit lesion identification in MVD might be challenging14, especially in the case of complex and extensive 3VD, potentially leading to the treatment of a non-culprit lesion during the index procedure. This was also reiterated by the FIRE trial, which showed that more complex patients, such as elderly patients, benefit from a complete and timely revascularisation15. In addition, imaging studies in ST-elevation myocardial infarction (STEMI) patients revealed the presence of one or more non-culprit lesion with unstable characteristics in up to 50% of patients undergoing primary percutaneous coronary intervention16, possibly translating into an early recurrent MI (re-MI) if those lesions remain untreated17. Specifically, in a staged procedure, residual vulnerable plaques might prompt ischaemic events in the time window between the index and the staged procedure18.
The identification of procedure-related MI during the acute phase might be challenging; therefore, in our study we performed an exploratory analysis excluding all type 4a MIs occurring at the index or at the staged procedure. After excluding type 4a MIs, no significant difference was found between ICR and SCR, and the interaction between 3VD/2VD remained similar. This could be because of the low number of events that remained after excluding the procedure-related MIs, therefore, reducing the ability to detect significant differences, yet showing a substantial difference in hazard ratio when comparing 2VD with 3VD.
The number of days in hospital, total contrast use and total radiation dose were higher in both the 3VD and 2VD groups when an SCR approach was adopted. In terms of interaction between treatment allocation and the number of diseased vessels, the ICR strategy in the 3VD population was associated with a greater reduction in hospital stay, total contrast use and total radiation dose compared with the 2VD group. The safety of an increased use of contrast during the index procedure compared with an increased overall use of contrast in the staged procedure remains to be established. One of the main determinants of contrast use and radiation exposure is the number of diseased vessels1920, implying that patients with 3VD receive more contrast and radiation than patients with 2VD, which is in line with our findings. Hence, the advantage of ICR is a subsequent decrease in contrast use and radiation.
Finally, the staged procedure is often performed days to weeks after the index procedure, resulting in a potential increase in healthcare costs2122.
Limitations
Identification of the culprit lesions was more challenging to assess by angiography, as there was limited use of imaging, which might have led to an incorrect determination of the culprit lesion. Furthermore, this trial was supposedly not powered to compare 2VD with 3VD; despite this, a numerically significant increase in the risk of MI in the 3VD group was found. Given the sample size, it is not possible to draw conclusions regarding the potential interaction of STEMI versus non ST-elevation acute coronary syndrome in 2VD and 3VD. Due to randomisation, the allocation of ICR and SCR was uneven in the 2VD and 3VD group, which might have had an effect on the results.
Conclusions
ICR is safe in patients presenting with ACS and MVD and might also be an option in patients with extensive 3VD. It could represent a novel treatment paradigm, associated with potential clinical and ancillary advantages.
Impact on daily practice
This prespecified analysis of the BIOVASC trial suggests that immediate complete revascularisation is safe in both patients with two-vessel disease and those with three-vessel disease. Therefore, immediate complete revascularisation is a feasible alternative to a staged approach for extensive and complex coronary disease and could represent a novel treatment paradigm.
Conflict of interest statement
R. Diletti has received institutional research grants from Biotronik, Medtronic, ACIST Medical Systems, and Boston Scientific. N. Van Mieghem has received institutional research grants from Biotronik, Abbott, Medtronic, Edwards Lifesciences, PulseCath, Abiomed, and Daiichi Sankyo; speaker fees from Abiomed and Amgen; and a travel grant from JenaValve. W. Den Dekker has received institutional research grants from Biotronik. J. Bennett has received institutional grants from Biotronik, Abbott, and Shockwave Medical. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

