Abstract
Aims: To determine whether multivessel (MV) percutaneous coronary intervention (PCI) performed in one procedure improves outcomes when compared to single-vessel (SV) PCI for the culprit lesion(s) in patients with non-ST-segment elevation acute coronary syndromes (NSTE-ACS).
Methods and results: We utilised the Acute Catheterisation and Urgent Intervention Triage StrategY (ACUITY) study database to analyse the outcomes of 2,255 patients with MV disease who underwent SV PCI compared to 609 patients who underwent MV PCI in the setting of NSTE-ACS. The primary endpoint was the one-year rate of major adverse cardiac events (MACE): death from any cause, myocardial infarction (MI), or ischaemia-driven revascularisation. At one year, patients undergoing MV PCI compared to SV PCI had similar rates of MACE (24.1% vs. 21.7%, respectively, p=0.11). However, death/MI was significantly higher in the MV PCI group (15.7% vs. 12.6%, p=0.05), primarily driven by higher rates of periprocedural non-Q-wave MI. Rates of death, ischaemia-driven revascularisation, stent thrombosis, acute renal failure and major bleeding were similar in both groups. By multivariable analysis with propensity score adjustment, MV PCI was not an independent predictor of one-year MACE (HR=1.22; 95% confidence interval [CI]: 0.96, 1.55; p=0.12) or death/MI (HR=1.28; 95% CI: 0.95, 1.74; p=0.15).
Conclusions: In patients with NSTE-ACS and MV disease, MV PCI does not appear to provide a clear clinical benefit over SV PCI. Randomised clinical trials specifically addressing these two strategies in this population, with attention to quality of life and symptom relief, are warranted.
Introduction
In 2009, 1.1 million patients were discharged from hospitals in the USA with a diagnosis of acute coronary syndrome (ACS)1. The majority of these patients were categorised as either unstable angina (UA) or non-ST-elevation myocardial infarction, collectively known as non-ST-elevation acute coronary syndrome (NSTE-ACS). In patients with moderate or high-risk NSTE-ACS, an early invasive strategy is currently a class IA American College of Cardiology Foundation (ACCF) and American Heart Association (AHA) recommendation2, and has been proven in numerous studies to improve prognosis compared to a conservative strategy3-5. However, controversy exists over the extent of revascularisation desired for those patients with multivessel (MV) disease, a group which represents as many as half of the NSTE-ACS population5.
We therefore sought to investigate the outcomes of single, culprit vessel only (SV) percutaneous coronary intervention (PCI) compared to multivessel (MV) PCI in patients with NSTE-ACS and MV disease from the Acute Catheterisation and Urgent Intervention Triage StrategY (ACUITY) trial, and to establish whether MV PCI is an independent predictor of one-year outcomes in this population.
Methods
STUDY PROTOCOL
The ACUITY trial design and results have been published previously6,7. Briefly, the ACUITY trial was a multicentre, prospective, randomised trial of 13,819 patients with moderate or high-risk NSTE-ACS who were managed with an early invasive strategy. Patients were randomly assigned before coronary angiography in a 1:1:1 fashion to receive one of three antithrombotic regimes: heparin (unfractionated or low molecular weight) plus a glycoprotein IIb/IIIa inhibitor (GPI), bivalirudin plus GPI, or bivalirudin monotherapy with provisional GPI. Angiography was performed in all patients within 72 hours of randomisation and, depending on coronary anatomy, the patients were treated with PCI, coronary artery bypass graft (CABG) surgery, or medical therapy alone at the discretion of the treating physician. In patients undergoing PCI, the choice of either bare metal or drug-eluting stent (DES) was left to operator discretion. Dual antiplatelet therapy with aspirin and clopidogrel was strongly recommended for at least one year.
We identified all patients with MV disease (≥50% stenosis by quantitative coronary angiography in a major epicardial artery or its branches) who underwent PCI. We excluded 105 patients who had staged (planned) procedures within eight weeks post index SV PCI, which was defined as intervention on only the culprit lesion(s) in one vessel during the index procedure. MV PCI was defined as intervention in ≥2 epicardial coronary vessels or their branches during the index procedure. Since we did not have data about the location of culprit lesion(s), we assumed that vessels intervened upon included the vessel with the culprit lesion.
STUDY ENDPOINTS
The primary endpoint was major adverse cardiac events (MACE) at one year, defined as the composite of death from any cause, myocardial infarction (MI), or unplanned ischaemia-driven revascularisation. Unplanned ischaemia-driven revascularisation was defined as revascularisation with either symptoms or signs of cardiac ischaemia, or a positive functional study (“stress test”) or a target lesion with diameter stenosis >70% by quantitative coronary angiography or operator assessment of >80% in the absence of core laboratory analysis.
Secondary endpoints included: major bleeding (defined as intracranial bleeding; intraocular bleeding; access-site haemorrhage requiring intervention; ≥5 cm diameter haematoma; reduction in haemoglobin concentration of ≥4 g/dL without an overt source of bleeding; reduction in haemoglobin concentration of ≥3 g/dL with an overt source of bleeding; reoperation for bleeding; use of any blood product (transfusion), post-procedure contrast-induced nephropathy (CIN, defined as an increase of 25% or 0.5 mg/dL in serum creatinine at 48 hours after PCI compared to baseline), and stent thrombosis, which was adjudicated according to the definitions of the Academic Research Consortium classification8. Periprocedural MI was defined as MI occurring within the first 48 hours after index PCI. In patients in whom CPK-MB (or CPK) levels were falling or had normalised, diagnosis was based on a CPK-MB (or CPK) ≥3 times the upper limit of normal (ULN) that is also increased at least 50% over the most recent pre-PCI levels, or new, significant (≥0.04 second) Q-waves in ≥2 contiguous electrocardiographic leads with CPK-MB (or CPK) >ULN. In patients in whom peak CPK-MB (or CPK) had not yet been reached before PCI, diagnosis was based on recurrent chest pain ≥30 minutes, or new electrocardiographic changes consistent with a second MI and a further elevation in the next CPK-MB (or CPK) level measured eight to 12 hours after PCI by at least 50% above the previous level, or new, significant (≥0.04 second) Q-waves in ≥2 contiguous electrocardiographic leads.
STATISTICAL METHODS
Demographic and baseline characteristics, angiographic variables and treatment patterns were compared between those patients receiving SV versus MV PCI. Continuous variables were described as mean±SD or medians (with interquartile ranges) and compared using the Student’s t-test or the Wilcoxon rank-sum test, respectively. Categorical variables were described as frequencies and compared using χ2 tests or Fisher’s exact test. In-hospital, 30-day and one-year outcomes were determined using Kaplan-Meier methodology and compared using the log-rank test. A p-value of <0.05 was considered statistically significant.
We developed a multivariable logistic regression model to describe the propensity to undergo MV vs. SV PCI. The model included the following variables: age, gender, weight, diabetes, hypertension, hyperlipidaemia, smoking status, previous MI, previous PCI, previous CABG, renal insufficiency, baseline cardiac biomarker elevation, baseline troponin elevation, ST-segment deviation ≥1 mm, Thrombolysis In Myocardial Infarction (TIMI) risk score, extent of disease prior to PCI (2- vs. 3-vessel CAD), jeopardy score9 and left ventricular ejection fraction. Subsequently, Cox multivariable regression analysis was performed to identify independent predictors of MACE and of death/MI at one year. The Cox multivariable model included all the variables from the propensity model in addition to SV PCI vs. MV PCI and propensity score. The model’s discriminatory value was characterised by the c-statistic10,11. Statistical analysis was performed using SAS, version 9.2 (SAS Institute, Cary, NC, USA).
Results
PATIENTS AND PROCEDURES
Of the entire ACUITY population, 7,789 patients had PCI and 2,864 patients (36%) had MV coronary artery disease (CAD) and underwent PCI; 2,255 (78.7%) underwent SV PCI and 609 (21.3%) patients underwent MV PCI. The groups were well matched with respect to baseline characteristics (Table 1), except for higher rates of previous PCI and previous CABG in the SV PCI group. TIMI risk score was not significantly different between the groups. Ejection fraction was similar between the groups. Baseline procedural medications were similar in both groups (data not shown).
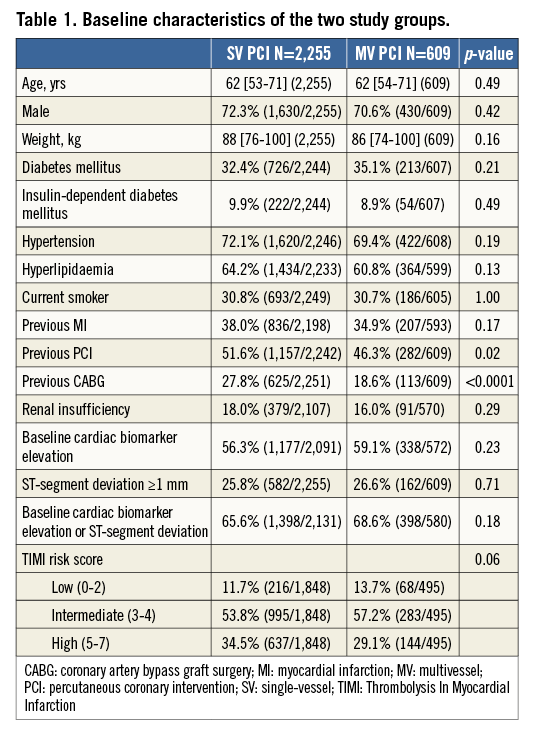
The angiographic and procedural characteristics of the two groups are shown in Table 2. More advanced CAD leading to more complex PCI, more often utilising DES, was more prevalent in the MV PCI group. The incidence of final TIMI 3 flow in all vessels treated was higher with MV PCI. Thrombus was more commonly noted in the SV PCI group.
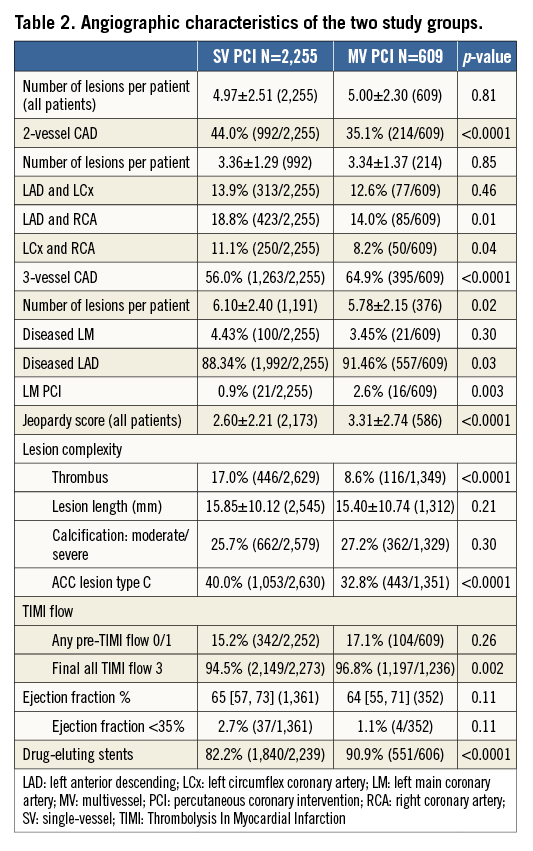
CLINICAL OUTCOMES
At hospital discharge (Table 3), MACE was significantly higher in the MV PCI group compared to the SV PCI group (9.9% vs. 7.0%, respectively, p=0.02). This was predominantly driven by higher rates of periprocedural non-Q-wave MI (7.9% vs. 5.0%, respectively, p=0.006). The CIN rate was numerically but not statistically higher in the MV vs. the SV PCI group (12% vs. 9.1%, respectively, p=0.05). There was no significant difference in rates of major bleeding or stent thrombosis between the two groups.
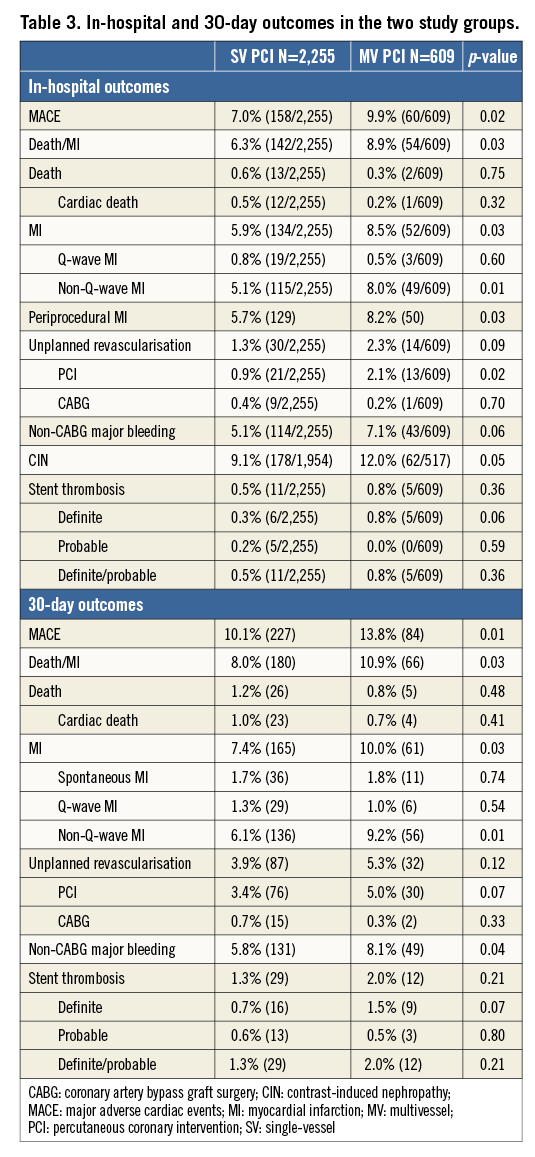
At 30 days (Table 3), MACE remained significantly higher in the MV PCI group, 13.8% vs. 10.1% for the SV PCI group (p=0.008). The higher rate of MACE in the MV PCI group continued to be driven predominantly by higher rates of non-Q-wave MI, 9.2% vs. 6.1% (p=0.005), the majority of which were periprocedural MI. The rate of definite/probable stent thrombosis was low and comparable.
At one year (Table 4, Figure 1), the MACE rate was similar between the two groups, while the composite of death or MI was significantly higher in the MV PCI group, 15.7% compared to 12.6% in the SV PCI group (p=0.05) (Figure 2). All-cause death and cardiac death rates were similar in both groups, 2.9% vs. 3.2% (p=0.54) and 1.6% vs. 2.0% (p=0.44) in the MV and SV PCI groups, respectively. The rate of MI was significantly higher in the MV PCI group as compared to SV PCI (p=0.04). Periprocedural MI accounted for 63% of the overall MI rate in the MV PCI group and 55% in the SV PCI group. Between 30 days and one year, similar proportions of patients had MI in both groups: 3.4% vs. 3.3%, p=0.78, respectively. The overall rate of spontaneous (non-procedural) MI was 5.2% and 5.0%, respectively, p=0.67. Unplanned revascularisation was similar in both groups: 15.2% for the MV PCI group compared to 14.4% for the SV PCI group (p=0.38). The incidence of definite/probable stent thrombosis was similar for the MV and SV PCI groups.
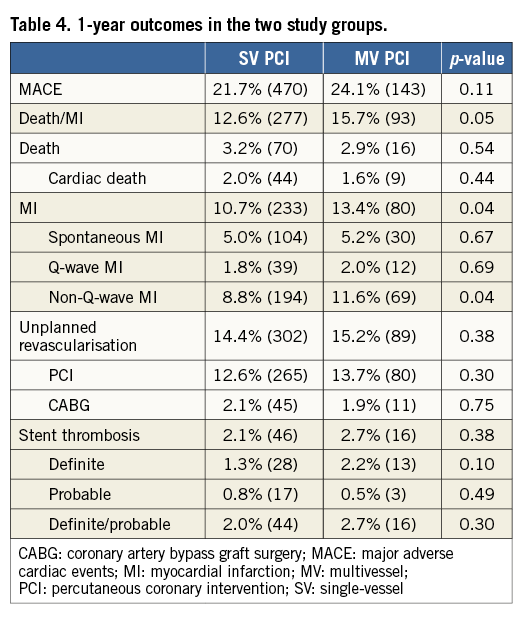
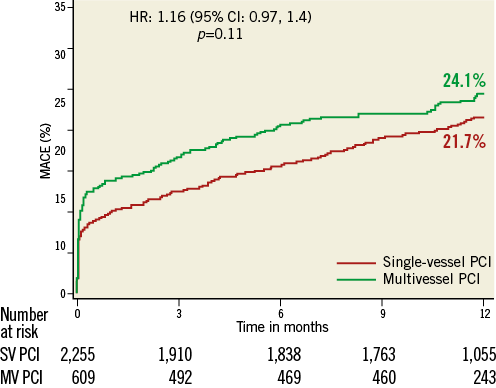
Figure 1. One-year rate of major adverse cardiac events (MACE) for patients undergoing single-vessel vs. multivessel percutaneous coronary intervention.
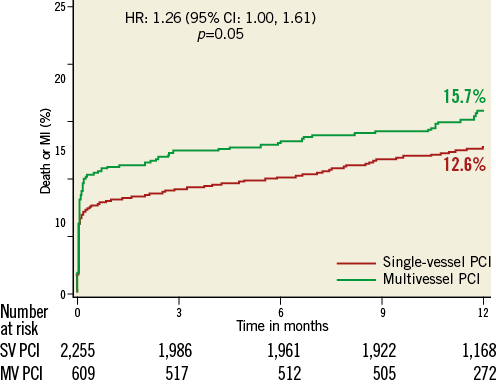
Figure 2. One-year rate of death or myocardial infarction (MI) for patients undergoing single-vessel vs. multivessel percutaneous coronary intervention.
PROPENSITY SCORE ADJUSTMENT AND MULTIVARIABLE ANALYSIS
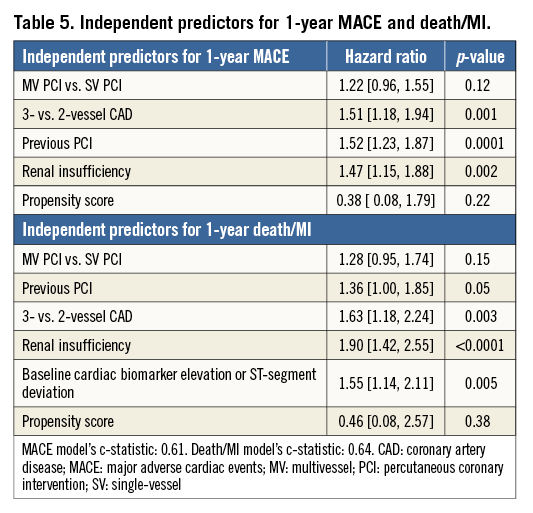
The mean propensity score (0=SV PCI, 1=MV PCI) for MV PCI was 0.26±0.09, compared with 0.22±0.07 for the SV PCI group (p<0.0001). The c-statistic for the model was 0.62, indicating only moderate discriminatory ability. The principal predictors for performance of MV PCI were higher jeopardy score (p<0.0001), diabetes mellitus (p=0.01), absence of previous CABG (p=0.02), absence of renal insufficiency (p=0.02), and presence of three-vessel CAD (p<0.0001).
At one year, MV PCI was not an independent predictor of either MACE (384 events, HR=1.22 [0.96, 1.55], p=0.12) or the composite of death/MI (236 events, HR=1.28 [0.95, 1.74], p=0.15). The c-statistic was 0.61 and 0.64 for each endpoint, respectively.
Independent predictors for MACE at one year (Table 5), in order of importance, were previous PCI, three-vessel CAD and renal insufficiency, similar to predictors for the composite of death/MI at one year.
Discussion
Our study investigated outcomes of MV vs. SV PCI in patients with NSTE-ACS and MV disease. To our knowledge, this is the largest study to examine the long-term safety and efficacy of these two different approaches in an NSTE-ACS cohort enrolled in a randomised clinical trial. The principal findings were as follows. 1) MV disease is common in patients with NSTE-ACS, occurring in one third of the ACUITY PCI patients. In about three fourths of cases operators preferred SV over MV PCI. 2) Unadjusted rates of MACE in the MV PCI group were higher than in the SV PCI group at hospital discharge and at 30 days, predominantly due to periprocedural MI, but no statistically significant difference was observed at one year. 3) MV PCI was not an independent predictor of MACE or death/MI at one year, while more extensive disease was predictive, highlighting the importance of diffuse CAD for outcome.
MV CAD encountered during the course of NSTE-ACS PCI poses a therapeutic dilemma. Although it is a class 1A ACCF/AHA recommendation2 that moderate to high-risk ACS patients undergo an early invasive strategy because it leads to superior outcomes compared to a conservative approach3-5, there is ambiguity regarding the extent of revascularisation to be performed. On the one hand, one may argue that the better outcomes of more complete revascularisation observed in patients with elective MV PCI12-14 can be extrapolated to patients receiving MV PCI in the setting of NSTE-ACS. Another potential incentive for proceeding with MV PCI is eliminating the need for staged procedures, and thus preventing exposure to additional risks and costs. On the other hand, the increasing complexity of the procedure during MV PCI might lead to overexposure to radiation and to heightened risk of developing contrast-induced nephropathy and bleeding15. Even more importantly, treating non-culprit lesions poses the risk of jeopardising additional myocardium, beyond that affected by the acute coronary syndrome lesion16.
We demonstrated in our study that the rate of MACE was statistically similar between the two groups at one year, and that MV PCI was not an independent predictor of MACE. While we observed similar rates of all-cause and cardiovascular death in the two groups, a finding consistent with previous studies in the NSTE-ACS population17-21, our study showed higher rates of MI in the MV PCI population. This was driven predominantly by the rates of periprocedural non-Q-wave MI. This finding was also reported by Brener et al19 in their study involving 105,866 patients with NSTE-ACS and MV disease in the National Cardiovascular Data Registry (NCDR), of whom 72,048 underwent SV PCI and 33,818 underwent MV PCI. In that study, though the rate of death was similar between the groups, the rate of periprocedural MI was significantly higher in the MV PCI group at discharge (p<0.0001). The added risk that periprocedural cardiac biomarker elevation potentially poses has long been a hotly debated issue16,22. However, more recent data23 have demonstrated that mild elevation in CK-MB, <10x ULN, has no significant prognostic impact, while spontaneous MIs considerably affected long-term survival24. These findings have been reflected in the new universal definition of periprocedural MI, which uses a significantly higher cut-off for cardiac biomarker elevation for making the diagnosis and requires additional clinical or angiographic confirmatory findings25. The lack of excess mortality in the MV PCI cohort in our study supports the lack of adverse impact of most periprocedural MI on survival, at least up to one year. Indeed, the detection of high rates of periprocedural MI in our study and in Brener et al, but not in similar studies17-21, may merely reflect the strict scrutiny applied in randomised clinical trials and national registries.
Several studies17-21 looking at outcomes for MV PCI in the NSTE-ACS population have demonstrated lower rates of repeat revascularisation compared to the SV PCI group during long-term follow-up. The study by Palmer et al21 demonstrated lower rates of recurrent angina and long-term use of antianginal medications in the MV PCI group. Several of these studies17,18 had the advantage of longer-term follow-up (>2 years), which was lacking in our study. In the Fractional flow reserve versus Angiography for Multivessel Evaluation (FAME) trial, all 1,005 patients had MV CAD as in our study, and 328 had ACS. One third of the lesions (37%) thought to be candidates for revascularisation by angiography had normal flow reserve, highlighting the importance of choosing the correct lesions for revascularisation. This selective approach was associated with a significant reduction in MACE (13.2% vs. 18.4%, p=0.02) overall and in patients with ACS26.
Interestingly, in a study by Kim et al27 comparing MV vs. SV PCI in nearly 2,000 patients with MV disease and NSTEMI, lower rates of cardiac death (3.5% vs. 6.4%, p=0.009) and recurrent myocardial infarction (0.6% vs. 2.1%, p=0.01) were observed in the MV PCI group at one year. Furthermore, in multivariable analysis, MV PCI was associated with lower rates of death or MI (HR 0.59, 95% CI: 0.41-0.84, p=0.004). In the recently published randomised trial of Preventive Angioplasty in Myocardial Infarction (PRAMI) trial28, Wald et al randomised 465 patients presenting with STEMI to either culprit vessel PCI or MV PCI including the culprit lesion and all other lesions with >50% stenosis. The primary outcome, a composite of death from cardiac causes, non-fatal myocardial infarction or refractory angina, was significantly lower in the MV PCI group (HR 0.35; 95% CI: 0.21-0.58; p<0.001) at a mean follow-up of 23 months. The rate of cardiac death or MI was also found to be significantly lower in the MV PCI group (HR 0.36, 95% CI: 0.18-0.73; p=0.004). Importantly, because these patients were treated for STEMI, periprocedural MI could not be adjudicated as in our study.
Finally, the incidence of the safety endpoints in our study, such as stent thrombosis and post-procedural CIN and major bleeding, which could be increased by more complex and prolonged procedures, was not statistically different between the groups, a finding consistent with similar reports and attesting to the safety of the MV PCI approach17,18,20.
Limitations
There are several limitations to this study. 1) The study is a retrospective analysis of a prospective, randomised study. Although we attempted to minimise the impact of confounding variables on the endpoints by using propensity score as a covariate in the multivariable model, we could not adjust for unmeasured variables. The c-statistic of the propensity score model indicates only moderate discrimination ability. 2) As we did not have data on the location of the culprit lesion, we assumed that vessels intervened upon included the vessel(s) with the culprit lesion. We could also not be certain that MV PCI was not performed because multiple culprit lesions were present. 3) We could not evaluate the operators’ intentions for revascularisation or how complete it was. That is to say, patients intended to have MV PCI may have had SV PCI due to unforeseen lesion complexity or intraprocedural complications. Conversely, patients intended for SV PCI may have had MV PCI because of complications affecting other vessels, intervening haemodynamic compromise, or because the operator was encouraged by the results of the culprit lesion treatment. 4) We did not have data on residual angina, quality of life or medication utilisation at one year. 5) We did not have detailed information on CK-MB elevation after PCI to identify smaller vs. larger MIs. 6) Finally, lack of follow-up beyond one year may have obscured the potential benefits of MV PCI.
Conclusion
These limitations notwithstanding, in a retrospective analysis of MV PCI in the setting of NSTE-ACS and MV disease, this strategy does not appear to provide clear clinical benefit compared to SV PCI. Individualised decisions based on angina burden and other factors may be necessary in view of the fact that there remains considerable equipoise. Incorporation of advances in PCI, including use of intracoronary imaging devices able to identify vulnerable plaques, use of fractional flow reserve, less thrombogenic stents, and a more thorough assessment of a patient’s baseline symptomatic status before an ACS event would allow a more tailored approach to patient care, and thus assignment to an appropriate revascularisation strategy. Future, adequately powered, randomised studies are warranted to clarify this important clinical issue.
| Impact on daily practice Multivessel coronary artery disease is frequent among patients with NSTE-ACS. While early revascularisation is indicated in these patients, its extent remains controversial. Our data, after propensity score adjusted analysis, suggest that MV PCI is not associated with a higher rate of adverse events than SV PCI, neither in the short term nor at one year of follow-up. Importantly, there was no indication in this data set of higher rates of bleeding, stent thrombosis or renal dysfunction in patients undergoing more extensive revascularisation. Its utility needs to be assessed prospectively while considering not only hard endpoints but also quality of life, repeat hospitalisations and utilisation of resources. |
Conflict of interest statement
G. Stone reports serving on the advisory boards for and receiving honoraria from Boston Scientific and Abbott Vascular. The other authors have no conflicts of interest to declare.

