Abstract
Aims: Coronary artery disease is often diffuse and patients with non-ST-segment acute coronary syndromes (NSTE-ACS) demonstrate multivessel coronary disease. The purpose of this study was to clarify whether interventions on stable chronic non-culprit lesions in patients with NSTE-ACS can prevent future adverse events.
Methods and results: We performed a retrospective cohort study of 990 consecutive patients who underwent either single-vessel PCI (SVPCI: n=379) or multivessel PCI (MVPCI: n=611) in a setting of NSTE-ACS. Cox proportional hazards regression analysis was performed to compensate for differences in baseline characteristics between the groups. To minimise the impact of confounding factors, we performed propensity matching (SVPCI: n=230, MVPCI: n=230). Patients who had MVPCI had a lower rate of prior interventional treatment or myocardial infarction, and more complex lesions than patients with SVPCI. At three years, all-cause mortality was significantly lower in the MVPCI group than the SVPCI group (13.0% vs. 18.3%, p=0.02, adjusted HR 0.55, 95% CI: 0.38-0.80), while the rates of target vessel revascularisation and a composite of all-cause death or myocardial infarction were not different between the groups. In the propensity-matched cohort, all-cause death remained significantly lower in the MVPCI group (adjusted HR 0.41, 95% CI: 0.22-0.75) compared to the SVPCI group.
Conclusions: In this retrospective study, multivessel PCI reduced all-cause mortality in a setting of NSTE-ACS compared to single-vessel PCI. Further investigations to confirm these results are warranted.
Abbreviations
HR: hazard ratio
MACE: major adverse cardiac events
MI: myocardial infarction
MV: multivessel
NSTE-ACS: non-ST-elevation acute coronary syndromes
PCI: percutaneous coronary intervention
SV: single-vessel
TVR: target vessel revascularisation
Introduction
Coronary artery disease is often diffuse and 40-60% of patients with non-ST-elevation acute coronary syndromes (NSTE-ACS) demonstrate multivessel coronary disease1-4. Patients referred for percutaneous coronary intervention (PCI) following NSTE-ACS conventionally undergo treatment of the culprit lesion only, rather than multivessel treatment2,5,6. Recent data from a large US registry, however, suggested that multivessel PCI for NSTE-ACS is as successful as single-vessel PCI in terms of in-hospital outcome6.
In the setting of ACS, multiple segments of coronary arteries can exhibit plaque disruption or instability, presumably related to widespread inflammation in the entire coronary artery tree7, as indicated by the elevation of biomarkers such as neutrophil myeloperoxidase activity8. Furthermore, a significant percentage of patients with established coronary artery disease suffer from long-term adverse cardiovascular events which are unrelated to the successfully treated culprit lesion9. It is still not clear whether interventions on non-culprit lesions in patients with NSTE-ACS can prevent future major adverse cardiac events.
Thus, we hypothesised that, among patients with multivessel coronary artery disease presenting with NSTE-ACS, there would be a reduction in short-term and long-term adverse events by performing multivessel PCI instead of single-vessel PCI. To test this hypothesis, we designed a retrospective study using data from the RESEARCH and T-SEARCH registries to compare clinical outcomes between patients with multivessel coronary artery disease who underwent multivessel PCI and patients with multivessel coronary artery disease who underwent single-vessel PCI for treatment of the culprit lesion only.
Methods
STUDY DESIGN AND PATIENT POPULATION
This is a retrospective sub-analysis of the all-comer RESEARCH and T-SEARCH registries. Between January 1, 2000, and December 31, 2005, all patients undergoing PCI were enrolled. Initially, all patients were treated with bare metal stents (BMS) but, on 16th April 2002, our institution adopted the use of sirolimus-eluting stents (SES: CYPHER®; Cordis, Warren, NJ, USA) as the default strategy for all coronary interventions, as part of the RESEARCH registry10. On 16th February 2003, SES were replaced by paclitaxel-eluting stents (PES: TAXUS® Express2TM; Boston Scientific, Natick, MA, USA) as the default stent, as part of the T-SEARCH registry11. Retrospectively, the patients with multivessel disease, presenting with unstable angina or NSTEMI, according to the Braunwald classification, were selected and included in the current analysis12,13. Multivessel disease was defined as having significant stenoses (equal to or greater than 50%) in at least two major epicardial coronary vessels by visual angiographic assessment. Patients were excluded if they had a history of coronary artery bypass graft surgery (CABG), or if they subsequently underwent planned staged PCI within one month after the initial procedure. Patients were divided into a multivessel PCI group (MVPCI) or a culprit-only single-vessel PCI group (SVPCI) according to the strategy in the initial procedure. The culprit lesion was identified by the operator after reviewing the coronary angiogram and electrocardiogram. When the patient received PCI in multiple lesions of the same vessel, it was classified as single-vessel treatment.
All procedures were performed according to standard clinical guidelines at the time14. Angiographic success was defined as a residual stenosis ≤30% by visual analysis in the presence of Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow. All patients were pretreated with 300 mg of clopidogrel. At least one month of clopidogrel treatment (75 mg/day) was recommended for patients treated with BMS. Clopidogrel was prescribed for at least three months for patients with drug-eluting stents (DES). Lifelong aspirin therapy was recommended in all patients.
ENDPOINT DEFINITIONS AND CLINICAL FOLLOW-UP
The primary endpoint was a composite of all-cause mortality or myocardial infarction (MI). MI included reinfarction (defined as recurrence of symptoms together with ST-elevation or new left bundle branch block and an increase in cardiac enzymes following stable or decreasing values) or spontaneous MI (diagnosed by a rise in creatine kinase-MB fraction of three times the upper limit of normal together with symptoms and either new ST-elevation or left bundle branch block). Secondary endpoints included all-cause mortality, MI, target vessel revascularisation (TVR), definite stent thrombosis, and major adverse cardiac events (MACE, defined as all-cause death or non-fatal MI or TVR). MI was diagnosed by a rise of creatine kinase-MB greater than three times the normal upper limit15. TVR was defined as a repeat revascularisation of a lesion in the same epicardial vessel treated in the index procedure16. Definite stent thrombosis was defined as TIMI grade 0 or 1 flow or the presence of a flow-limiting thrombus, accompanied by acute symptoms, irrespective of whether there had been an intervening reintervention17. The timing of ST was categorised as early (within 30 days after implantation), late (between 30 days and one year) or very late (more than one year)18.
FOLLOW-UP DATA
Survival data for all patients were obtained from municipal civil registries on a yearly basis. A questionnaire was subsequently sent to all living patients with specific enquiries on rehospitalisation and MACE. Most repeat revascularisations (either percutaneous or surgical) are normally performed at our institution and recorded prospectively in our database, as ours is the principal regional cardiac referral centre. For patients who suffered an adverse event at another centre, medical records or discharge letters from the other institutions were systematically reviewed. General practitioners and referring physicians were contacted for additional information if necessary.
Statistical analysis
Continuous variables are presented as mean±standard deviation, whereas categorical variables are expressed as percentages. Comparisons among groups were performed by the independent t-test for continuous variables and Pearson’s chi-square test for categorical variables. All statistical tests were two-tailed and a p-value of <0.05 was considered statistically significant. The incidence of events over time was studied with the use of the Kaplan-Meier method, whilst log-rank tests were applied to evaluate differences between the treatment groups. Patients lost to follow-up were considered at risk until the date of last contact, at which point they were censored. To elucidate the treatment effect, separate Cox regression models were built to adjust multiple potential confounders in the baseline characteristics. Multivessel treatment was forced into forward stepwise models using all the variables listed in Table 1 with entry and stay criteria of 0.05 and 0.10, respectively. The results are presented as adjusted hazard ratios (HR) with 95% confidence intervals (CI).
To minimise further the impact of confounding, we made a logistic regression model to generate a propensity score for individuals who had undergone multivessel PCI using the following preprocedural variables: age, sex, hypertension, hypercholesterolaemia, diabetes, family history of coronary artery disease, current smoking, old myocardial infarction, previous history of PCI, in-stent restenosis, chronic total occlusion, use of DES, lesion type B2 or C, left anterior descending (LAD) lesion, bifurcation lesion, left main lesion, glycoprotein IIb/IIIa antagonist use, and the number of diseased vessels19. According to the generated propensity score, each patient from the multivessel PCI group was matched with a patient who had undergone single-vessel PCI. Statistical analysis was performed with SPSS 16 for Windows (SPSS Inc., Chicago, IL, USA).
Results
BASELINE AND PROCEDURAL CHARACTERISTICS
Amongst 1,312 patients with multivessel disease who underwent PCI in a setting of NSTE-ACS, 990 patients were finally included in the analysis (MVPCI: n=611, SVPCI: n=379) after excluding 269 patients with CABG and 53 patients undergoing staged PCI (Figure 1). The baseline and procedural characteristics of the patients stratified by multivessel or culprit-only coronary intervention are shown in Table 1. Patients with multivessel treatment had a lower rate of previous history of interventional treatment or myocardial infarction, and more complex lesions, reflected by higher rates of left main disease and lesion type B2 or C. Patients undergoing multivessel PCI had significantly longer fluoroscopy times (33.9±26.6 vs. 23.8±21.4 minutes, p<0.0001), and higher amounts of contrast (352±149 vs. 260±111 ml, p<0.0001) compared with those who underwent single-vessel only PCI.
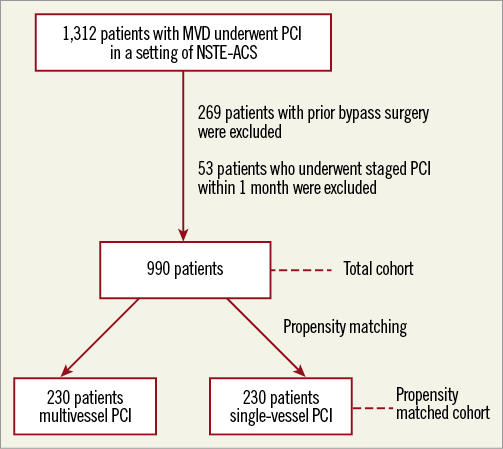
Figure 1. Flow chart of patient selection and matching. MVD: multivessel disease; NSTE-ACS: non-ST-elevation acute coronary syndromes; PCI: percutaneous coronary intervention
CLINICAL OUTCOMES
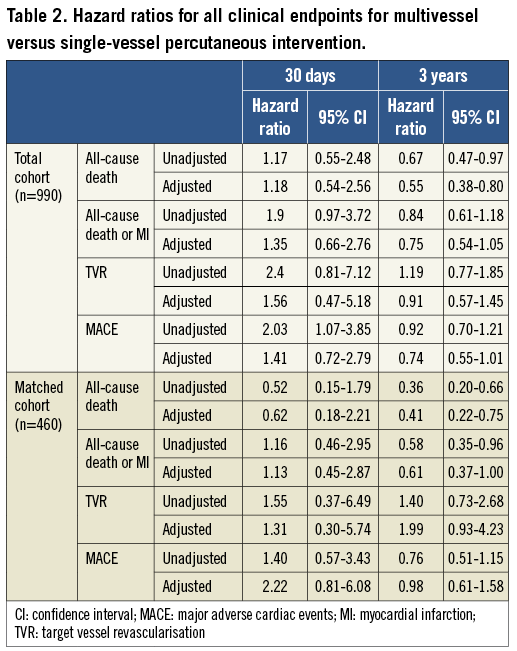
Clinical follow-up was available in 96% of patients after a median duration of 1,447 days. Cumulative rates of clinical endpoints are presented in Figure 2. At three years, the composite of death or non-fatal myocardial infarction as well as the rates of TVR, MACE and definite stent thrombosis were not different between the two treatment groups. As a secondary endpoint, all-cause mortality was significantly lower in the multivessel treatment group than the single-vessel treatment group (MVPCI 13.0% vs. SVPCI 18.3%, p=0.02).
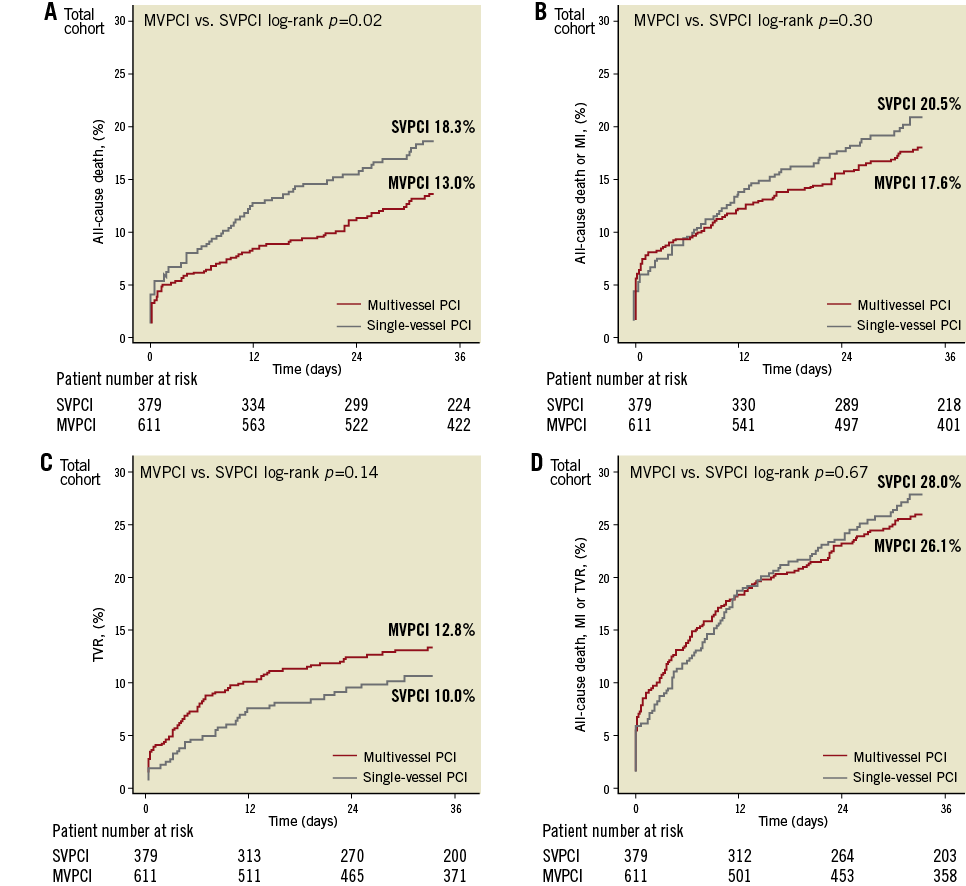
Figure 2. Kaplan-Meier survival curves for the total cohort (n=990) stratified according to the strategy of single-vessel (SVPCI) or multivessel percutaneous coronary intervention (MVPCI). A) All-cause death, B) the composite of death or myocardial infarction (MI), C) target vessel revascularisation (TVR), D) the composite of all-cause mortality, any myocardial infarction or TVR.
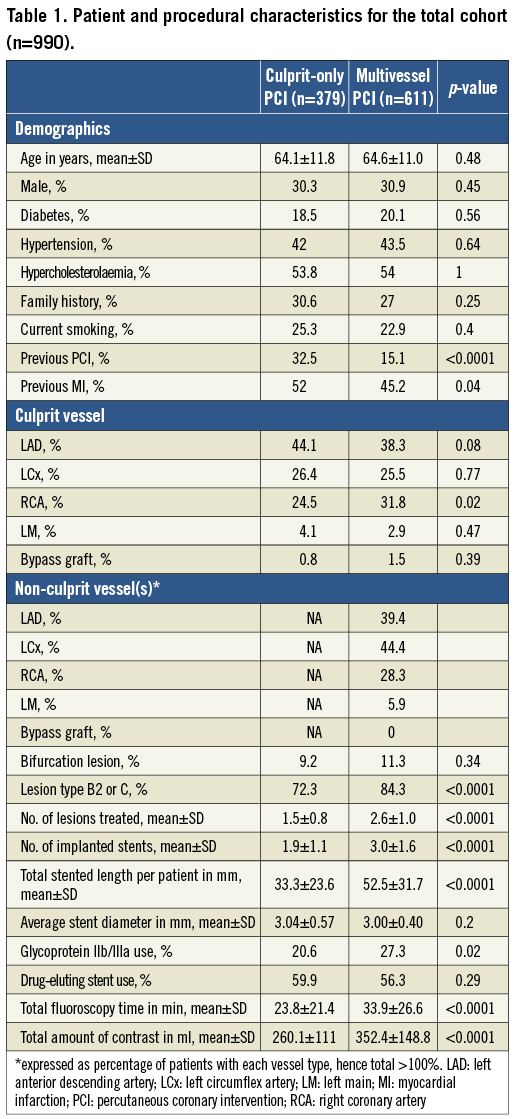
The risk of each clinical endpoint at 30 days and at three years is shown in Table 2. At 30 days, multivessel PCI was associated with an increased risk of MACE (unadjusted HR 2.03; 95% CI: 1.07-3.85), but the difference was not significant after adjustment (adjusted HR 1.41; 95% CI: 0.72-2.79). At three years, all-cause death was significantly lower in the multivessel treatment group (unadjusted HR 0.67, 95% CI: 0.47-0.97). After multivariable adjustment, the difference still remained significant (adjusted HR 0.55, 95% CI: 0.38-0.80).
PROPENSITY SCORE-MATCHING COHORT
We performed propensity score matching to account for multiple confounders associated with multivessel PCI. As a result, 230 patients who underwent multivessel PCI were matched with 230 patients with single-vessel PCI. Baseline and procedural characteristics put in the logistic regression model were comparable between groups. There were no differences in pre-procedural variables between the two matched groups (see Methods). Clinical follow-up was available in 96% of these patients with a median follow-up of 1,435 days. As shown in Figure 3, the cumulative incidence of all-cause death was significantly lower in the multivessel PCI group at three years, while the rates of other clinical endpoints were not. Hazard ratios with and without multivariable adjustment are presented in Table 2. At 30 days, there were no differences between the two groups, while at three years the risk of all-cause death and the composite of death or non-fatal myocardial infarction were significantly lower in the multivessel PCI group. After multivariable adjustment, all-cause death remained significantly lower in the multivessel PCI group.
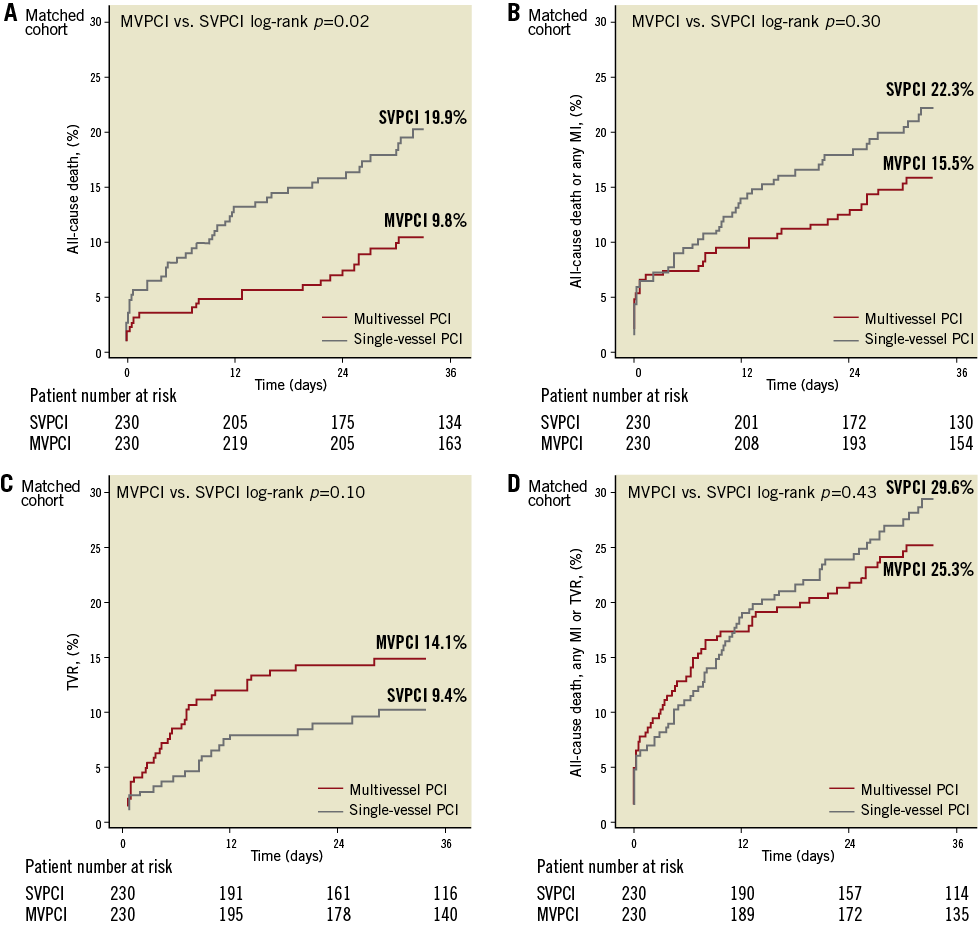
Figure 3. Kaplan-Meier curves for the matched cohort (n=460) stratified according to the strategy of single-vessel (SVPCI) or multivessel percutaneous coronary intervention (MVPCI). A) All-cause death, B) the composite of death or myocardial infarction (MI), C) target vessel revascularisation (TVR), D) the composite of all-cause mortality, any myocardial infarction or TVR.
Discussion
In this analysis of patients who underwent PCI for the treatment of unstable angina or non-ST-elevation myocardial infarction, multivessel PCI was associated with similar 30-day clinical outcomes. Although the composite of death or non-fatal myocardial infarction was not different at three years between the two groups, multivessel PCI was associated with a significantly lower rate of all-cause mortality at three years both after adjustment for differences in baseline characteristics and after propensity score matching.
Regarding short-term outcome, multivessel intervention could potentially have adverse effects secondary to increased contrast load, leading to renal dysfunction and side branch closure or distal embolisation leading to periprocedural MI. In a recent article examining 105,866 patients undergoing PCI for ACS with multivessel coronary artery disease from 402 centres in the American College of Cardiology National Cardiovascular Database Registry, Brener et al reported that in-hospital mortality was comparable between patients who underwent multivessel or single-vessel PCI (MVPCI 1.2% and SVPCI 1.3%, respectively, p=0.09; adjusted OR 1.11, 95% CI: 0.97-1.27)6. The rates of in-hospital morbidity, such as bleeding, the development of renal failure, or non-fatal cardiogenic shock were similar for both groups. In line with this study, our current analysis demonstrated that the short-term outcome was similar between single-vessel and multivessel PCI in terms of death or MI, which suggests that multivessel intervention is as safe as single-vessel intervention in the setting of NSTE-ACS.
Little is reported in the literature about the long-term safety and efficacy of non-culprit multivessel treatment in the setting of NSTE-ACS. Multivessel PCI may be associated with an increased likelihood of in-stent restenosis, and leaving significant lesions untreated may increase repeat revascularisation rates due to residual ischaemia. In a subgroup analysis of the TACTICS-TIMI 18 trial, Brener et al20 identified 290 patients with multivessel disease, of whom only 23% had MVPCI. At six months, the incidence of death (SVPCI 2.2% vs. MVPCI 3%), MI (8% vs. 6.1%), and the composite of death, MI, or rehospitalisation for ACS (23.2% vs. 21.2%) were comparable between patients receiving SVPCI and MVPCI. In 1,240 NSTE-ACS patients having multivessel disease treated by PCI exclusively with BMS, Shishehbor et al5 reported that, compared to culprit-only intervention, multivessel intervention was associated with a lower rate in the composite of death, myocardial infarction, or revascularisation (including both target vessel and non-target vessel revascularisation) after propensity score-matched analysis (HR 0.67; 95% CI: 0.51-0.88; p=0.004), which was mainly driven by a significantly lower incidence of repeat revascularisation in the multivessel intervention group (HR 0.59; 95% CI: 0.41-0.84). Recently, Hannan et al reported that, in 1,040 propensity-matched pairs of patients with ACS (but without STEMI) who received complete revascularisation (CR) either at index hospitalisation or at staged procedure within 60 days, the three-year all-cause mortality rates were not different (6.59% in those who underwent CR in the index hospitalisation, 5.92% in patients staged for CR, p=0.41)21. In contrast, our study including ACS patients with or without complete revascularisation demonstrated that multivessel PCI was associated with lower rates of all-cause death or non-fatal MI than single-vessel PCI. The TVR rate was higher in the multivessel stenting group, although data on non-target vessel revascularisation were not collected.
Limitations
The current study suffers from the inherent limitations of a non-randomised trial, despite the fact that we performed propensity score matching to compensate for these differences. We cannot adjust for hidden confounding factors and other sources of bias typical of observational studies, such as the operator’s mindset. In addition, this analysis was retrospective and hypothesis-generating, and the study was underpowered to detect the difference between two strategies in the primary endpoint.
Conclusion
In conclusion, in this single-centre study, multivessel PCI reduced long-term all-cause mortality in patients with multivessel disease in a setting of NSTE-ACS compared to single-vessel PCI. Randomised studies that examine further the safety and efficacy of multivessel PCI strategy in patients with unstable angina and NSTEMI are warranted.
Guest Editor
This paper was Guest Edited by Bernhard Meier, MD, FACC, FESC, University Hospital for Cardiology, Bern, Switzerland.
Acknowledgements
We would like to acknowledge the senior cardiologists involved in the PCI procedures: E. McFadden, MD, PhD; P.J. de Feyter, MD, PhD; P.P.T. de Jaegere, MD, PhD; S.H. Hofma, MD, PhD; E. Regar, MD, PhD; G. Sianos, MD, PhD; P.C. Smits, MD, PhD; H. Duckers, MD, PhD; M.J. van der Ent, MD, PhD; W.J. van der Giessen, MD, PhD; C.A. van Mieghem, MD.
Conflict of interest statement
The authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.
References

