Abstract
Aims: Current guidelines recommend immediate multivessel percutaneous coronary intervention (PCI) in patients with cardiogenic shock, despite the lack of randomised trials. We sought to investigate the use and impact on outcome of multivessel PCI in current practice in cardiogenic shock in Germany.
Methods and results: Between January 2008 and December 2011 a total of 735 consecutive patients with acute myocardial infarction, cardiogenic shock and multivessel coronary artery disease underwent immediate PCI in 41 hospitals in Germany. Of these, 173 (23.5%) patients were treated with immediate multivessel PCI. The acute success of PCI with respect to TIMI 3 flow did not differ between the groups (82.5% versus 79.6%). In-hospital mortality with multivessel PCI and culprit lesion PCI was 46.8% and 35.8%, respectively. In multivariate analysis multivessel PCI was associated with an increased mortality (odds ratio 1.5; 95% confidence interval 1.15-1.84).
Conclusions: In current clinical practice in Germany multivessel PCI is used only in one quarter of patients with cardiogenic shock treated with primary PCI. We observed an adverse effect of immediate multivessel PCI. Therefore, a randomised trial is needed to determine the definitive role of multivessel PCI in cardiogenic shock.
Introduction
Despite the use of early revascularisation therapy, cardiogenic shock is the major cause of death in patients admitted with acute myocardial infarction and is associated with a mortality of approximately 40-50%1-3. About 75% of patients with cardiogenic shock have multivessel disease2,3. These patients have a higher mortality compared to patients with single-vessel disease2,3. Current European Society of Cardiology (ESC) guidelines recommend percutaneous coronary intervention (PCI) of all high-grade lesions in patients with acute myocardial infarction complicated by cardiogenic shock, while in haemodynamically stable patients culprit-lesion only PCI should be preferred4,5. In contrast, German and American guidelines are neutral regarding their recommendation for or against immediate multivessel (MV) PCI6,7. However, so far, reports from registries comparing culprit-lesion only versus MV-PCI in cardiogenic shock are conflicting8-13. Therefore, we evaluated a large contemporary database with respect to the use and outcome of MV-PCI in cardiogenic shock.
Methods
THE ALKK-PCI REGISTRY
The ALKK (Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte) -PCI registry is a prospective registry which was initiated in 1992 to monitor quality control and it contains all the consecutive procedures of the participating hospitals on an intention-to-treat basis14,15. Data presented were obtained by standardised questionnaires in the 41 participating hospitals, including information about medical history (prior coronary interventions, congestive heart failure, diabetes mellitus, renal insufficiency), indication for the procedure, adjunctive antithrombotic therapy, the procedure itself (target vessel, success rate, stent used, etc.) and complications until hospital discharge. All data were analysed centrally at the Karl Ludwig Neuhaus Datenzentrum at the Institut für Herzinfarktforschung, Ludwigshafen, Germany.
PATIENT SELECTION
All patients receiving PCI were included on an intention-to-treat basis. We analysed the data of consecutive patients with ST-elevation myocardial infarction (STEMI) and non-ST-elevation myocardial infarction (NSTEMI). For this analysis we selected patients with cardiogenic shock and significant stenoses (>50%) of two or three major vessels before the start of the intervention. We excluded patients with prior coronary artery bypass surgery and patients with significant left main disease. The decision to perform MV-PCI or culprit PCI was left entirely to the discretion of the operator.
DEFINITIONS
NSTEMI was diagnosed in the presence of the following two criteria: persistent angina pectoris for ≥20 minutes, and an elevation of troponin T or I. Raised levels were considered to be those exceeding the upper normal level at the local laboratory at each participating site.
STEMI was diagnosed in the presence of the following two criteria: persistent angina pectoris ≥20 minutes, and ST-segment elevation of 1 mm in ≥2 standard leads or ≥2 mm in ≥2 contiguous precordial leads, or the presence of a left bundle branch block. It was later confirmed by the elevation of enzymes (creatine kinase and its MB isoenzyme, aspartate aminotransferase, lactic dehydrogenase) to at least twice the normal value.
Cardiogenic shock was diagnosed in patients with systolic blood pressure <90 mmHg, heart rate >100 beats per minute and clinical signs of end-organ hypoperfusion, such as cold, clammy skin, oliguria, altered mental status or elevated serum lactate.
STATISTICAL METHODS
All analyses were performed using the SAS© statistical package, Version 9.1 (SAS Institute Inc., Cary, NC, USA). Data are presented as absolute numbers or percentages. Whenever possible, percentages were used to describe patient populations. The frequencies of categorical variables in four age groups were compared by the Pearson-Fisher χ2 test and by calculating odds ratios (OR) and 95% confidence intervals (CI). Continuous variables were compared by Mann-Whitney-Wilcoxon test; p-values <0.05 were considered significant. All p-values are results of two-tailed tests.
A multivariate regression analysis for independent predictors of in-hospital mortality was performed including all parameters with a p-value of <0.1 in the univariate analysis, and age, gender, triple-vessel disease, diabetes and renal insufficiency. These values were calculated from the available cases.
Results
Between January 2008 and December 2011 a total of 34,145 consecutive patients with acute coronary syndromes undergoing PCI in 41 hospitals in Germany were enrolled into the registry. Of these, 12,678 had STEMI and 21,467 NSTEMI, and cardiogenic shock was observed in 811 (6.4%) and 216 (1.1%), respectively. From these 1,027, a total of 735 (71.5%) patients had multivessel disease without significant left main stenosis, and 173 (23.5%) patients were treated with immediate MV-PCI.
The baseline variables of patients treated with MV-PCI versus those with culprit-lesion only PCI are presented in Table 1. The angiographic features and procedural details of the patients are shown in Table 2. The TIMI flow grades of the treated vessels before PCI are displayed in Figure 1. There was no difference in TIMI grade 3 flow after PCI between the two groups, 82.5% versus 79.7% (p=0.50). Thrombus aspiration was used in 10.6% versus 9.5% of patients with MV-PCI and culprit PCI (p=0.4). Patients were treated with an intensive antithrombotic regimen (Table 3) in both groups. During PCI, inotropes were given in 81.8% of patients with MV-PCI and 97.2% with culprit-lesion PCI (p=0.01).
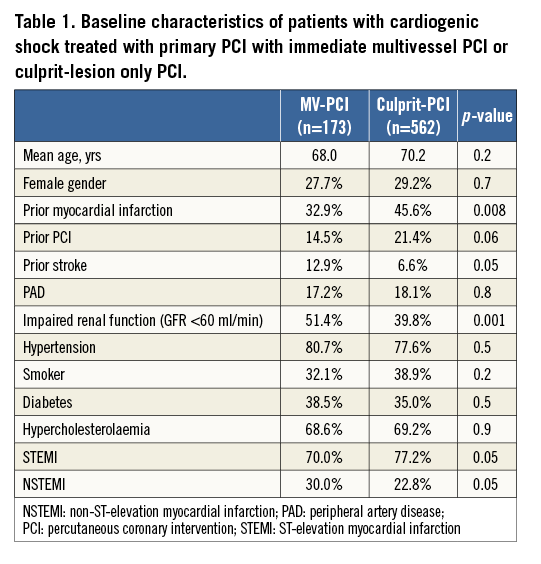
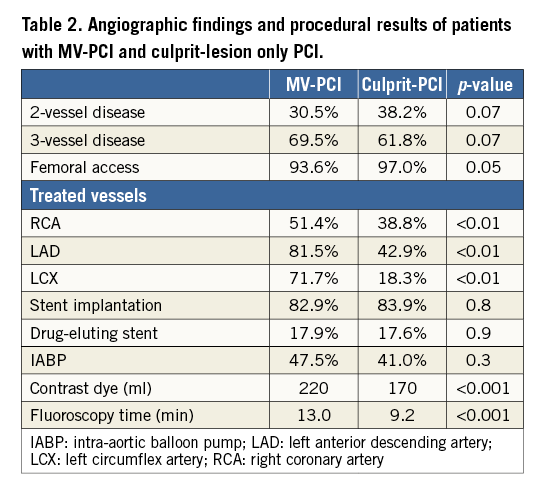
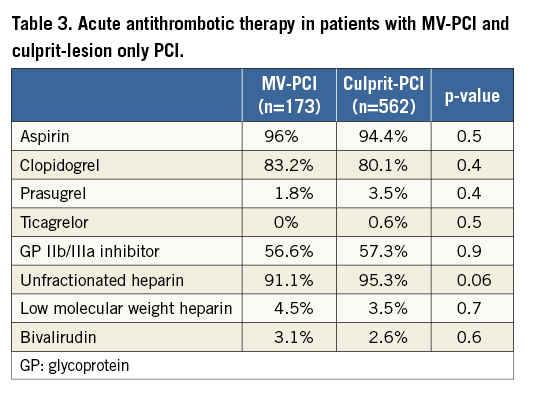
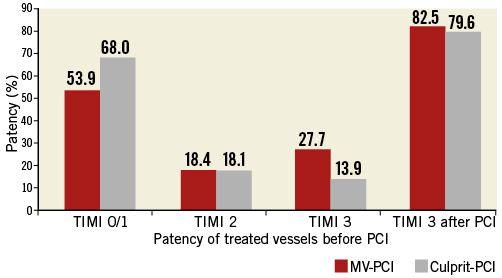
Figure 1. Flow in the treated vessels. TIMI flow grades of the treated vessels before PCI and incidence of TIMI 3 flow after PCI.
There were more events during PCI in the MV-PCI group (Figure 2). During the index hospital stay, additional PCIs were performed in 1.7% and 6.3% and coronary artery bypass graft surgery in 1.6% and 2.9% in the MV-PCI and culprit-lesion PCI groups, respectively. The in-hospital events are shown in Figure 3. There was a significant difference in mortality (46.8% versus 35.8%, p<0.01), while there were no differences in reinfarction, stroke and bleeding complications. The need for dialysis was higher in the multivessel PCI group (p=0.01). Independent predictors of in-hospital mortality derived from multivariable analysis are shown in Figure 4 and confirm the significantly higher mortality in the multivessel PCI group from the univariate analysis.
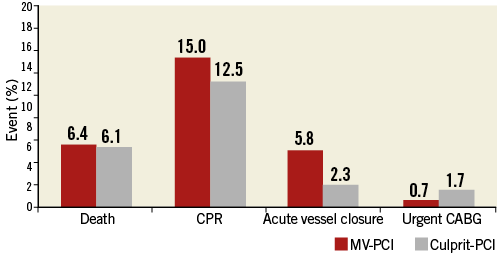
Figure 2. Adverse events during PCI. Events occurring in the catheterisation laboratory. CABG: coronary artery bypass grafting; CPR: cardiopulmonary resuscitation
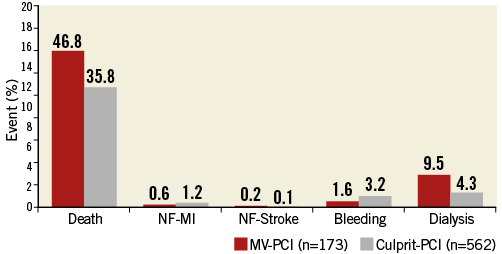
Figure 3. Adverse events until discharge. In-hospital events in patients treated with MV-PCI or culprit-lesion PCI. MI: myocardial reinfarction; NF: non-fatal
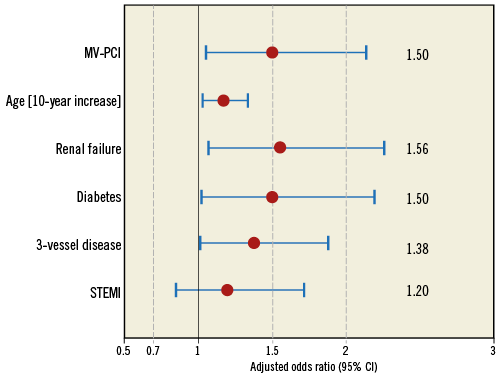
Figure 4. Independent predictors of mortality. Multivariate analysis with independent predictors of in-hospital mortality.
Discussion
In contrast to current ESC guideline recommendations, our current registry of cardiogenic shock patients with multivessel coronary artery disease presenting with acute myocardial infarction revealed that MV-PCI is only performed in approximately one quarter of the patients. Furthermore, and even more intriguingly, MV-PCI as compared to culprit-lesion only PCI resulted in increased mortality according to the results of this prospective registry.
Nevertheless, these guideline recommendations in cardiogenic shock are not based on strong clinical evidence because it is not clear whether MV-PCI might be beneficial in cardiogenic shock. There are no randomised data evaluating the benefit of an MV-PCI approach in cardiogenic shock and the recommendations are mainly based on pathophysiological considerations. Theoretically, treatment of non-culprit lesions could help limit the infarct size and preserve left ventricular function, which are major prognostic factors in patients with acute myocardial infarction. It may also be hypothesised that multivessel PCI may reduce the subsequent adverse events after primary PCI by preventing the incidence of both early and late recurrent ischaemia in the non-infarct-related lesions, which in turn could obviate the need for recurrent procedures, reducing overall ischaemic burden and attenuating the incidence of unpredictable subsequent cardiac events. Complete revascularisation at the time of infarction may also reduce overall hospital stay and the total cost of care. On the other hand, major concerns exist regarding the risks of prolonged interventional procedures with higher amounts of contrast dye and the hypothetical risk of stent thrombosis in non-culprit lesions when stent implantation has taken place in the thrombogenic milieu of acute myocardial infarction. Successful primary PCI of the infarct-related artery and a complicated or unsuccessful PCI to the non-infarct-related artery would be potentially hazardous, especially in the setting of cardiogenic shock. Contrast load must also be taken into account, which might lead to a volume load of the left ventricle with subsequent ischaemia. In addition, multivessel PCI is likely to cause an increased risk of contrast-induced nephropathy, which is associated with adverse clinical outcome. Furthermore, unnecessary PCI might increase the need for subsequent revascularisation procedures due to restenosis, acute stent thrombosis, or it might induce periprocedural myocardial infarction by distal embolisation, side branch occlusion, acute vessel occlusion, or other inherited technical problems.
Despite the ESC guideline recommendations and possibly owing to the limited evidence supporting these recommendations, multivessel PCI is currently performed in only 1/3 to 1/4 of cardiogenic shock patients with multivessel disease, as in our analysis. This has also been shown in several other registries8-13 as well as in the recent randomised IABP-SHOCK II trial.
So far, reports from registries comparing culprit-lesion only versus MV-PCI in patients with acute myocardial infarction complicated by cardiogenic shock do not support immediate multivessel PCI. In the SHOCK trial, more complete revascularisation by immediate multivessel PCI was associated with an unfavourable outcome compared to culprit-lesion only PCI10. In a single-centre observational study one-year mortality did not significantly differ between culprit-lesion PCI (n=124) and immediate multivessel PCI (n=37)11. Data from the multicentre National Cardiovascular Data Registry also revealed an increased mortality for multivessel PCI. Among patients with cardiogenic shock (n=3,087), those receiving multivessel PCI had greater in-hospital mortality (36.5% versus 27.8%; adjusted OR 1.54, 95% CI: 1.22 to 1.95)12. In the recently published Euro Heart Survey PCI registry, there was a trend towards higher mortality after multivariable adjustment for the MV-PCI strategy (n=82) versus culprit-lesion only PCI (n=254) in multivessel disease8. The only registry showing a beneficial effect of MV-PCI derives from a recently published series of patients with cardiogenic shock after resuscitation13. These patients represent a high-risk subgroup of myocardial infarction patients with a high mortality despite aggressive reperfusion therapy16. The positive results are therefore hypothesis-generating. Just recently, a randomised study in patients with multivessel disease and STEMI without cardiogenic shock has been published17. Here, immediate multivessel PCI was associated with an improved clinical outcome, mainly driven by a reduction in recurrent infarctions. However, the study was not powered to detect a difference in mortality. In addition, the strategy in the conservative group was not according to the current guidelines, which recommend staged PCI after fractional flow reserve or proof of the haemodynamic relevance of the stenosis in the non-culprit lesions by non-invasive stress tests. Another trial is currently underway to evaluate the value of immediate multivessel PCI in patients with STEMI without cardiogenic shock18.
A more definitive answer about the impact of MV-PCI on outcome in patients with primary PCI for cardiogenic shock will come from the CULPRIT-SHOCK trial (ClinicalTrials.gov: NCT01927549). This prospective international multicentre trial will randomise 706 cardiogenic shock complicating acute myocardial infarction with multivessel disease patients to an MV-PCI strategy or a culprit-lesion only PCI strategy with the option of a staged revascularisation of other lesions. The primary endpoint will be 30-day mortality and/or renal replacement therapy, and a number of secondary clinical endpoints, as well as haemodynamic measures, will be reported.
Limitations
As always in registries, a selection bias cannot be fully ruled out. Therefore, even after adjustment for confounding for baseline variables, we cannot state categorically that we were able to adjust for every factor which might have influenced the results, especially as the decision for or against MV-PCI was left to the discretion of the operator. Therefore, selection bias in favour of a culprit-lesion PCI might have played a role. On the other hand, the use of catecholamines was higher in the culprit-lesion group, which does not indicate a more unstable situation in these patients. Still, factors such as lesion morphology and haemodynamic improvement after PCI of the culprit lesion were not evaluated in our questionnaire but might have been important for or against the decision to proceed to multivessel PCI.
Conclusions
In conclusion, in current clinical practice in Germany MV-PCI is used in only one quarter of patients with cardiogenic shock treated with primary PCI. However, the results of this registry indicate an even higher mortality with the MV-PCI approach, challenging current guideline recommendations. Therefore, the results of a large randomised clinical trial are warranted to define the role of MV-PCI in patients with cardiogenic shock19.
| Impact on daily practice In patients with multivessel disease presenting with an acute myocardial infarction complicated by cardiogenic shock the optimal revascularisation strategy is still uncertain. In most cases culprit-lesion only PCI should be the preferred strategy. However, despite the negative results of our analysis, in select cases with prolonged haemodynamic instability after successful PCI of the culprit lesion, additional immediate intervention of other lesions can be considered. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

