
Coronary artery disease (CAD) is the leading cause of death in patients with chronic kidney disease (CKD)1. Patients with CKD and CAD undergoing percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) have a poor prognosis1,2. In fact, CKD attenuates the benefits of myocardial revascularisation due to the higher risk of procedural complications, reduced durability of both surgical and percutaneous revascularisation techniques and the high risk of death from non-cardiac causes1. Historically, patients with CKD have been excluded from randomised controlled trials evaluating management strategies for CAD, particularly those with advanced CKD1. Recently, in the ISCHEMIA-CKD trial, patients with advanced CKD (defined as an estimated glomerular filtration rate [eGFR] of <30 ml/min/1.73 m2 or on dialysis) and moderate or severe myocardial ischaemia on stress testing were randomised to an initial invasive strategy consisting of coronary angiography and revascularisation if feasible or an initial conservative strategy consisting of medical therapy alone3. The trial found that there were no significant differences in terms of death or myocardial infarction (MI) between the two strategies at a median follow-up time of 2.2 years. In addition, an initial invasive strategy was associated with higher risk of stroke and death or initiation of dialysis3. Also, there was no improvement in angina-related health status during follow-up with the invasive approach in these patients with advanced CKD, in contrast to patients enrolled in the main ISCHEMIA trial with preserved renal function or only mild CKD4.
Among patients with CAD, those with obstructive disease of the left main (LM) coronary artery are at substantial risk of mortality if left untreated, given the large amount of subtended myocardium at risk5. LM-CAD was an exclusion criterion of the main ISCHEMIA trial, and only eight patients randomised to an invasive strategy in ISCHEMIA-CKD had LM disease3. Revascularisation strategies for LM-CAD have now been evaluated in multiple randomised trials comparing PCI versus CABG; however, few patients with severe CKD were recruited in these trials2. The comparative effectiveness of PCI versus CABG in patients with LM-CAD and renal dysfunction thus remains inconclusive.
In this issue of EuroIntervention, Kim et al6 evaluate the comparative effectiveness of PCI versus CABG according to renal function in the international, multicentre IRIS-MAIN registry.
This registry included patients who were diagnosed with LM-CAD (defined as a stenosis >50% on angiography) at 65 centres in the Asia-Pacific region. The median follow-up time was ~3.5 years. The primary outcome of interest was major adverse cardiovascular and cerebrovascular events (MACCE), defined as the composite of death from any cause, MI, stroke, or any revascularisation. MI included both periprocedural and spontaneous MI, where periprocedural MI was defined as a creatine kinase–myocardial band (CK-MB) elevation >5× the upper reference limit with objective evidence of ischaemia occurring within 48 hours from the procedure. A total of 4,894 patients treated between January 2003 and September 2017 were included in this analysis, of whom 3,824 (78.1%) had an eGFR ≥60 mL/min, 838 (17.1%) had an eGFR of 30 to 60 mL/min, and 232 (4.7%) had an eGFR <30 mL/min or were on dialysis. Of these, 2,825 (57.7%) underwent PCI, 1,453 (29.7%) underwent CABG and 616 (12.6%) were managed with medical therapy. Of note, among those who underwent PCI, 2,158 (76.9%) were treated with new-generation drug-eluting stents (DES) and intravascular ultrasound imaging was used in 75% of cases. Consistent with prior studies, patients with CKD had higher rates of adverse events during follow-up irrespective of their management strategy, with progressively increasing event rates with worsening baseline renal insufficiency. In multivariable-adjusted models there were no significant differences in MACCE between PCI and CABG, an effect that was consistent across strata of renal function; however, among patients with severe renal dysfunction or on dialysis there was a signal for higher risk of MACCE in patients treated with PCI. In terms of secondary endpoints, PCI was associated with lower risk of major bleeding but higher risk of repeat revascularisation compared with CABG, consistently across strata of kidney function. All-cause mortality was lower with PCI compared with CABG among patients with normal renal function but similar among those with CKD, with significant statistical interaction. Finally, there were no significant differences in terms of MI and stroke between groups, irrespective of renal function.
The major strength of the present analysis is the inclusion of a large all-comers sample, including a significant proportion of patients with renal dysfunction, a patient population that has been underrepresented in randomised clinical trials. However, major limitations should be noted. First, the anatomical complexity of the patient population was not characterised and information on the SYNTAX score was not available. Second, key renal outcomes such as acute renal failure and new requirement for dialysis were not collected. Also, data on renal function during follow-up were not available. Third, the authors do not differentiate between periprocedural MI and spontaneous MI. Finally, as with any non-randomised study, these findings are subject to bias by unmeasured confounders.
The results of the study of Kim et al confirm CKD as a strong risk factor for morbidity and mortality among patients with LM-CAD undergoing revascularisation. Overall, the study is in line with a pre-specified secondary analysis of the EXCEL trial reporting no significant differences at three years in terms of death, MI or stroke between PCI with everolimus-eluting stents and CABG in patients with LM-CAD and CKD2. In EXCEL, PCI was associated with a significantly lower risk of both acute renal failure and need for dialysis in both patients with and without CKD, which were in turn strongly associated with a higher risk of mortality over three years2. Currently, the totality of the evidence comparing revascularisation strategies for LM-CAD supports a similar survival between PCI and CABG up to five-year follow-up, with PCI being associated with a lower risk of short-term adverse events (including periprocedural MI, major bleeding, transfusions, arrhythmias and acute renal failure) and CABG being associated with lower rates of spontaneous MI and the need for repeat revascularisation7. However, clinical decisions surrounding treatment strategies among patients with renal dysfunction should also take into account factors that are unique to the CKD population. These include the risk of further renal injury and progression towards the need for dialysis and a careful perioperative optimisation of the volume status, electrolytes balance and anaemia. In addition, patients with CKD often have more severe atherosclerotic disease, with greater coronary calcification which may interfere with the optimal DES implantation as well as with the surgical anastomosis during CABG1. Moreover, renal dysfunction itself has been shown to be a strong risk factor for both DES failure and surgical graft occlusion2. A summary of the general and technical considerations in patients with CKD requiring coronary revascularisation is provided in Figure 1. The optimal care of patients with CKD and complex CAD requires a “heart-kidney team” approach in centres with expertise in implementing effective strategies for prevention of progressive kidney damage and performing safe, effective and appropriately indicated percutaneous and surgical revascularisation procedures in this high-risk patient cohort.
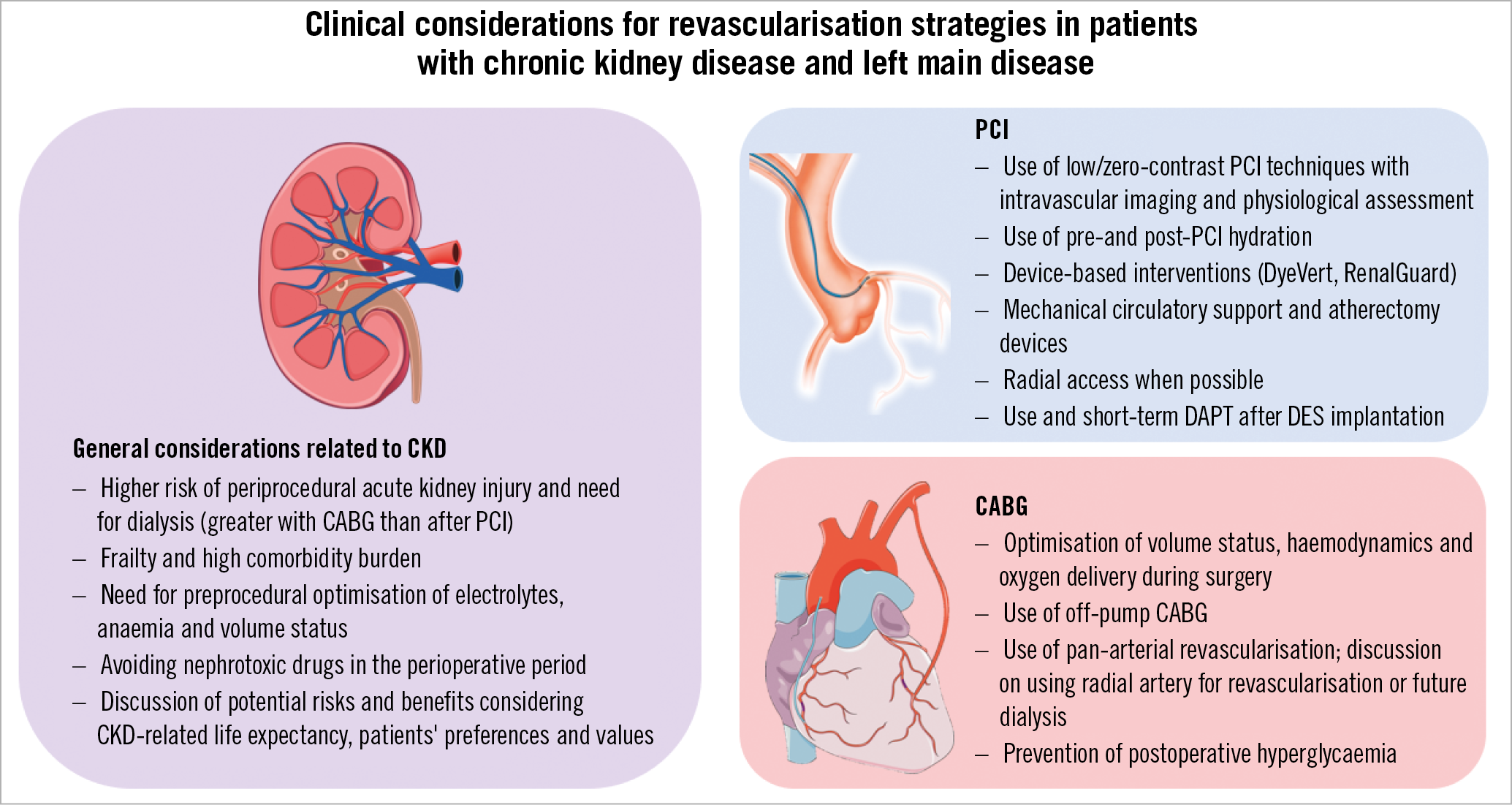
Figure 1. General and technical considerations with percutaneous and surgical revascularisation strategies in patients with left main disease and chronic kidney disease. DAPT: dual antiplatelet therapy; DES: drug-eluting stent
Conflict of interest statement
G. Giustino served as a consultant for Bristol-Myers-Squibb/Pfizer. G. Stone has received speaker honoraria from Cook and Terumo; served as a consultant for Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva and Matrizyme; and has equity/options in Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, MedFocus family of funds and Valfix.
Supplementary data
To read the full content of this article, please download the PDF.

