Abstract
Background: The long-term prognostic impact of a composite of periprocedural major adverse events (PMAE) following revascularisation for patients with complex coronary artery disease (CAD) has not yet been established.
Aims: This study aimed to assess the impact on 10-year mortality of non-fatal PMAE following percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Other objectives were to evaluate 1) whether PMAE affect mortality predicted by the SYNTAX score II 2020 (SSII-2020) and 2) whether optimal medical therapy (OMT) positively affects the prognosis of patients with non-fatal PMAE.
Methods: The association between 10-year mortality and non-fatal PMAE occurring within 30 days of PCI or CABG in patients with three-vessel disease and/or left main disease enrolled in the SYNTAXES study was investigated.
Results: The main findings are that non-fatal PMAE occurred less frequently following PCI than CABG (11.2% vs 28.2%; p<0.001) and that non-fatal PMAE were an independent predictor of all-cause mortality in the first year post-procedure, but not at 5 or 10 years, in both treatment modalities. PMAE substantially alter the individual predictions of 10-year mortality by the SSII-2020. In patients with non-fatal PMAE, OMT may provide survival benefits during the first year post-procedure as well as in the long term.
Conclusions: In patients with complex CAD, non-fatal PMAE were more common following CABG than PCI, but their prognostic impact was similar, being significant in the first year and then diminishing out to 10 years. Patients with non-fatal PMAE may therefore require more careful follow-up and additional preventive treatment in the first year post-procedure.
Introduction
In patients with complex coronary artery disease (CAD), such as left main coronary artery disease (LMCAD) or three-vessel disease (3VD), the decision by the Heart Team between either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) is aided using an objective risk model such as the SYNTAX score II 2020 (SSII-2020), which facilitates an individual assessment of the short- and long-term risk benefits with each treatment modality1234567.
Prospective data from a multicentre study showed that postoperative events after major surgery were an important predictor of long-term mortality8. A retrospective analysis of data from 833,344 patients in the US Nationwide Database who underwent PCI showed that unplanned readmissions within 30 days of PCI were associated with increased in-hospital mortality and healthcare costs, with more than half of readmissions related to baseline comorbidities or non-cardiac causes9.
The impact on mortality of a single adverse post-procedural event such as a stroke101112, stent thrombosis/graft occlusion1314, myocardial infarction (MI)1516 or infection17 is documented in a few studies amongst patients with complex CAD undergoing PCI or CABG. However, the long-term prognostic impact of a composite of periprocedural major adverse events (PMAE) following revascularisation has not yet been established. To investigate this, we assessed the impact of non-fatal PMAE on 10-year mortality amongst patients with complex CAD enrolled in the SYNTAXES study, the long-term extended follow-up study of the SYNergy Between PCI With TAXUS and Cardiac Surgery (SYNTAX) Study (ClinicalTrials.gov: NCT00114972).
Methods
Study design and data source
The SYNTAX Study design, protocol and results up to 5 years have been previously reported1819. The design and extended vital status of the SYNTAX Study up to 10 years (SYNTAXES; ClinicalTrials.gov: NCT03417050) have also been reported elsewhere20. Briefly, the SYNTAX Study was a multicentre (85 European or American sites), multinational, open-label, randomised controlled trial (N=1,800: PCI: n=903; CABG: n=897) with parallel nested registries (PCI registry: n=198; CABG registry: n=1,077, of which n=644 were followed up for 5 years) designed to assess clinical outcomes following treatment of de novo 3VD and/or LMCAD with PCI using the TAXUS Express Stent (Boston Scientific) or CABG.
Information on vital status at 10-year follow-up was complete in 841 (93%) and 848 (95%) of patients in the PCI and CABG groups, respectively. Clinical and angiographic characteristics were well matched between groups as previously reported20. The median follow-up time was 11.2 years (interquartile range [IQR] 7.7-12.1) overall and 11.9 years (IQR 11.2-12.3) in survivors20. The SYNTAX and SYNTAXES studies were approved by the ethics committees at each investigating centre, and all patients provided their written informed consent prior to participation in the SYNTAX Study.
Definitions
Non-fatal PMAE were defined as the occurrence of periprocedural or spontaneous MI, ischaemic or haemorrhagic stroke, repeat percutaneous or surgical revascularisation, major infection, stent thrombosis/graft occlusion, bleeding, major arrhythmia, heart failure, acute respiratory failure, acute renal failure, or wound dehiscence within 30 days post-procedure (Supplementary Appendix 1, Supplementary Table 1)21. Major adverse cardiac or cerebrovascular events (MACCE) were a composite of all-cause death, MI, stroke, and repeat revascularisation, with definitions of these individual components previously reported1722, and all events were adjudicated by an independent clinical events committee comprised of cardiologists, cardiac surgeons, and a neurologist20. Infection-related events, defined as the occurrence of deep incisional surgical site infection at the primary chest incision or at a secondary incision site (e.g., saphenous harvest and groin cannulation site), mediastinitis, infectious myocarditis or pericarditis, endocarditis, cardiac device infection, pneumonia, empyema, clostridium difficile colitis, or blood-stream infection, were adjudicated post hoc by an independent committee according to the Centers for Disease Control and Protection/National Healthcare Safety Network (CDC/NHSN) criteria23. For other events, the event terms (e.g., bleeding, major arrhythmia, heart failure, acute respiratory failure, acute renal failure, and wound dehiscence) were searched for in the electronic case report form for serious adverse events (Supplementary Appendix 1, Supplementary Table 2) and adjudicated by the consensus of 2 cardiologists (N. Kotoku, K. Ninomiya) and 1 cardiovascular surgeon (A. Soo).
Endpoints and time intervals
To assess the impact of non-fatal PMAE on long-term mortality, the primary endpoint was all-cause mortality between 30 days and 10 years. Secondary endpoints included all-cause mortality between 30 days and 1 year and, separately, between 1 year and 10 years as landmark analyses. An exploratory analysis was also performed to identify the benefit of optimal medical therapy (OMT) on mortality after a non-fatal PMAE. We also investigated whether non-fatal PMAE affect mortality predicted by the SSII-2020.
Statistical analysis
To assess the impact of a non-fatal PMAE on long-term mortality, patients who died within 30 days of the procedure (fatal periprocedural events) were excluded. After exclusion of the patients with fatal periprocedural events, the timeline was defined as “time since index procedure.” Therefore, the main analysis of this study was done according to the per-protocol principle after the exclusion of patients who did not have a procedural date (i.e., patients who did not receive PCI or CABG, including those receiving medical therapy only or who died before the procedure). As a sensitivity analysis, we also performed intention-to-treat (ITT) analyses, including death ≤30 days post-procedure, according to the definition of PMAE.
Patients with missing follow-up data were included in the analysis and censored at the time they were lost to follow-up. Patients were stratified according to the occurrence of ≥1 non-fatal PMAE within 30 days of the procedure. The Kaplan-Meier method was used to estimate cumulative event rates, and the log-rank test was performed to examine the differences between groups. Day 0 was defined as the day of the procedure. Landmark analyses were performed separately from 30 days to 1 year and from 1 year to 10 years. Hazard ratios (HR) with 95% confidence intervals (CI) for all-cause mortality from 30 days to 1 year, at 5 years and at 10 years were determined based on Cox proportional hazards regression. Cox regression analysis was performed to address the difference between patients with and without a non-fatal PMAE by adjusting the baseline variables (Supplementary Appendix 2).
The statistical analysis methods employed to evaluate i) the discriminative ability and calibration of the SSII-2020 for outcome risk, and ii) the agreement between the predicted and observed benefit of treatment with PCI or CABG5 are described in Supplementary Appendix 3. For the SSII-2020, ITT analyses were performed, and death within 30 days post-procedure was included in the definition of PMAE.
Continuous variables were described as mean±standard deviation (SD) and were compared using the Student’s t-test or Mann-Whitney U test as appropriate. Categorical variables are expressed as proportions and were compared using the χ2 test or Fisher’s exact test. A 2-sided p-value ≤0.05 was considered statistically significant. All statistical analyses were conducted with SPSS version 27 (IBM) and R version 3.5.1 (R Foundation for Statistical Computing).
Results
Patients and PMAE
From a total of 1,800 patients randomised in the SYNTAX Study between March 2005 and April 2007, 1,739 patients received their allocated treatment (PCI arm n=885; CABG arm n=854) (Supplementary Figure 1).
Within 30 days of the procedure, there were 20 deaths following PCI and 10 after CABG (2.3% vs 1.2%; HR 1.94, 95% CI: 0.91-4.15; p=0.086), of which 83.3% were cardiovascular deaths (90% following PCI; 70% following CABG). As described in the methods, to assess the impact of a non-fatal PMAE on long-term mortality, the 30 patients who died within 30 days of their procedure (fatal periprocedural events) were excluded.
Non-fatal PMAE within 30 days of PCI and CABG occurred in 97 and 238 patients, respectively (11.2% vs 28.2%; p<0.001) (Table 1, Supplementary Figure 2).
As shown in Table 1, within 30 days of the procedure, patients treated with PCI had fewer strokes, major infections, major arrhythmias, acute respiratory failure, and acute renal failure compared to those treated with CABG.
Demographic characteristics and comorbidities of patients with, versus without, a non-fatal PMAE following PCI or CABG are shown in Table 2. In both arms, patients with a non-fatal PMAE were older, more frequently had chronic kidney disease (CKD) and had a higher EuroSCORE than those without a PMAE. Ten-year mortality calculated by the SSII-2020 was higher in patients with a non-fatal PMAE irrespective of treatment modality.
Table 1. Non-fatal periprocedural major adverse events within 30 days after PCI and CABG.
| PCI (n=865) | CABG (n=844) | p-value | |
|---|---|---|---|
| Non-fatal periprocedural major adverse events | 97 (11.2%) | 238 (28.2%) | <0.001 |
| MI | 22 (2.5%) | 16 (1.9%) | 0.414 |
| Within 7 days post-procedure | 15 (1.7%) | 12 (1.4%) | 0.699 |
| Between 7 days and 30 days post-procedure | 7 (0.8%) | 4 (0.5%) | 0.548 |
| Stroke | 1 (0.1%) | 9 (1.1%) | 0.011 |
| Repeat revascularisation | 20 (2.3%) | 11 (1.3%) | 0.147 |
| Major infection | 15 (1.7%) | 127 (15.0%) | <0.001 |
| Stent thrombosis/graft occlusion | 17 (2.0%) | 7 (0.8%) | 0.063 |
| Bleeding | 21 (2.4%) | 21 (2.5%) | 1.000 |
| Major arrhythmia | 21 (2.4%) | 73 (8.6%) | <0.001 |
| Heart failure | 10 (1.2%) | 11 (1.3%) | 0.829 |
| Acute respiratory failure | 0 (0.0%) | 12 (1.4%) | <0.001 |
| Acute renal failure | 8 (0.9%) | 19 (2.3%) | 0.032 |
| Wound dehiscence | 1 (0.1%) | 12 (1.4%) | 0.002 |
| CABG: coronary artery bypass grafting; MI: myocardial infarction; PCI: percutaneous coronary intervention | |||
Table 2. Baseline characteristics of patients with and without a non-fatal PMAE within 30 days after PCI and CABG.
| After PCI | After CABG | |||||||
|---|---|---|---|---|---|---|---|---|
| With non-fatalPMAE (n=97) | Without non-fatal PMAE (n=768) | p-value | With non-fatal PMAE (n=238) | Without non-fatal PMAE (n=606) | p-value | |||
| Age (years) | 68.7±9.3 | 64.7±9.6 | <0.001 | 66.4±9.8 | 64.2±9.8 | 0.004 | ||
| Male | 70 (72.2%) | 596 (77.6%) | 0.249 | 187 (78.6%) | 486 (80.2%) | 0.634 | ||
| BMI (kg/m2) | 27.6±5.1 | 28.2±4.7 | 0.279 | 28.2±4.5 | 27.7±4.4 | 0.113 | ||
| Dyslipidaemia | 73 (76.0%) | 604 (79.2%) | 0.508 | 195 (81.9%) | 454 (75.9%) | 0.066 | ||
| Hypertension | 75 (77.3%) | 523 (68.1%) | 0.080 | 146 (61.3%) | 386 (63.7%) | 0.527 | ||
| Medically treated diabetes | 32 (33.0%) | 185 (24.1%) | 0.063 | 62 (26.1%) | 134 (22.1%) | 0.239 | ||
| CKD | 31 (33.3%) | 127 (17.4%) | <0.001 | 53 (25.4%) | 93 (16.8%) | 0.010 | ||
| COPD | 9 (9.3%) | 59 (7.7%) | 0.550 | 22 (9.2%) | 54 (8.9%) | 0.894 | ||
| Current smoking | 16 (16.5%) | 144 (18.8%) | 0.678 | 49 (20.7%) | 138 (22.9%) | 0.520 | ||
| Previous MI | 36 (37.5%) | 237 (31.2%) | 0.245 | 78 (33.3%) | 201 (33.5%) | 1.000 | ||
| Previous stroke or TIA | 12 (12.4%) | 53 (6.9%) | 0.065 | 22 (9.4%) | 54 (9.0%) | 0.894 | ||
| LVEF <30% | 2 (3.6%) | 4 (0.8%) | 0.120 | 6 (4.3%) | 9 (2.3%) | 0.244 | ||
| History of congestive heart failure | 4 (4.2%) | 28 (3.7%) | 0.774 | 17 (7.4%) | 26 (4.3%) | 0.083 | ||
| Peripheral vascular disease | 12 (12.4%) | 63 (8.2%) | 0.179 | 34 (14.3%) | 51 (8.4%) | 0.015 | ||
| History of carotid artery disease | 12 (12.4%) | 59 (7.7%) | 0.117 | 19 (8.0%) | 50 (8.3%) | 1.000 | ||
| Preoperative unstable angina | 33 (34.0%) | 216 (28.1%) | 0.235 | 71 (29.7%) | 167 (27.1%) | 0.446 | ||
| SYNTAX score I | 30.0±10.7 | 28.0±11.3 | 0.101 | 30.0±11.5 | 28.8±11.2 | 0.169 | ||
| SYNTAX 2020 score II (%) | Predicted 10 year-mortality with PCI | 36.9±20.0 | 27.3±18.5 | <0.001 | 34.0±22.1 | 27.5±19.0 | <0.001 | |
| Predicted 10 year-mortality with CABG | 29.8±16.9 | 23.0±16.2 | <0.001 | 28.4±19.7 | 22.9±16.7 | <0.001 | ||
| EuroSCORE | 4.7±2.5 | 3.5±2.5 | <0.001 | 4.1±2.5 | 3.5±2.6 | 0.003 | ||
| Left main lesion | 29 (29.9%) | 310 (40.4%) | 0.048 | 93 (39.1%) | 241 (39.8%) | 0.876 | ||
| Values are mean±SD or % (n/N). BMI: body mass index; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; PMAE: periprocedural major adverse events; SYNTAX: Synergy Between PCI with Taxus and Cardiac Surgery; TIA: transient ischaemic attack | ||||||||
Impact of non-fatal PMAE on long-term mortality
Between 30 days and 10 years, 103 patients with a non-fatal PMAE and 310 patients without a PMAE died (30.7% vs 22.6%; HR 1.46, 95% CI: 1.16-1.82; p<0.001). Kaplan-Meier survival curves from 30 days to 10 years (Figure 1A) show higher mortality amongst patients with non-fatal PMAE compared to those without after CABG (29.8% vs 19.3%; log-rank p<0.001) and PCI (33.0% vs 25.1%; log-rank p=0.063). Landmark analyses show that this increased mortality with non-fatal PMAE following PCI and CABG was seen predominantly between 30 days and 1 year (6.2% vs 2.0%; log-rank p=0.010 and 5.5% vs 1.2%; log-rank p<0.001, respectively). This was no longer apparent in the following 6-7 years of follow-up, whilst a resurgence of increased mortality occurred in the final 3 years (Figure 1B, Figure 1C).
In Cox regression analysis, after adjustment for confounders, non-fatal PMAE remained an independent predictor of all-cause mortality between 30 days and 1 year irrespective of treatment modality (PCI 6.2% vs 2.0%, adjusted HR 3.42, 95% CI: 1.03-11.33; p=0.044; CABG 5.5% vs 1.2%, adjusted HR 9.99, 95% CI: 2.10-47.46; p=0.004) (Figure 2). Non-fatal PMAE, however, was not an independent predictor for all-cause mortality between 30 days and 5 years, nor between 30 days and 10 years in either treatment modality (Figure 2).
When we stratify patients according to the number of PMAE 0, 1, or ≥2 (a Kaplan-Meier curve for all-cause mortality is shown in Supplementary Figure 3), non-fatal PMAE ≥2 was an independent predictor for all-cause 10-year mortality (42.3% vs 22.5%, adjusted HR 1.78, 95% CI: 1.11-2.86; p=0.016, compared to PMAE =0) (Supplementary Table 3).
The difference and time dependency of the HR for mortality over 1, 5, and 10 years for each individual PMAE component are shown in Supplementary Table 4 with the event details at 1, 5, and 10 years in Supplementary Table 5-Supplementary Table 7, respectively.
Sensitivity analyses of the impact of non-fatal PMAE with alternative definitions15 (Supplementary Table 8) of periprocedural MI on all-cause mortality are shown in Supplementary Figure 4-Supplementary Figure 8. All the results were consistent with the result of the main analysis, with MI within 30 days post-procedure (see definition in Supplementary Table 1).
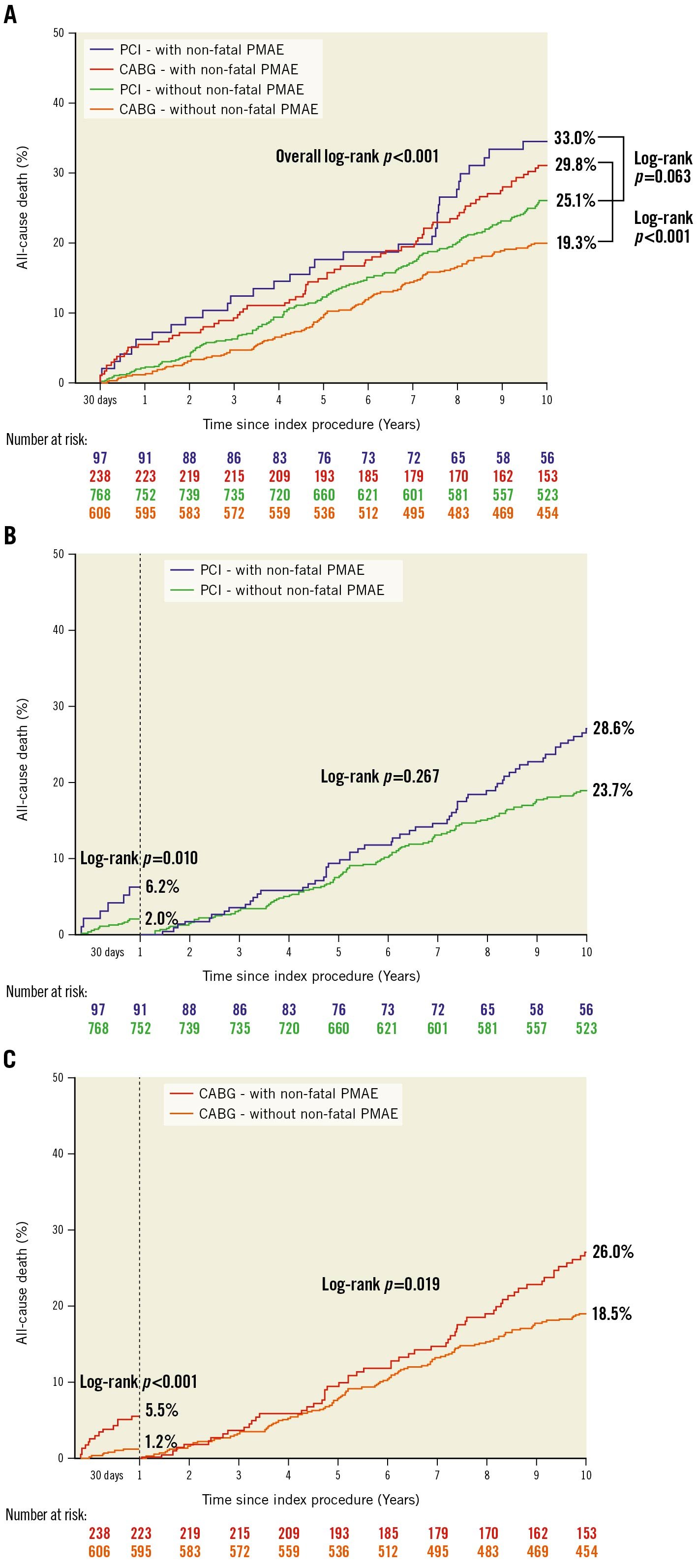
Figure 1. Kaplan-Meier curve for all-cause mortality with and without non-fatal PMAE. A) In patients after PCI and CABG, B) landmark analysis from 30 days to 1 year and from 1 year to 10 years in PCI, and C) in CABG. CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention; PMAE: periprocedural major adverse events
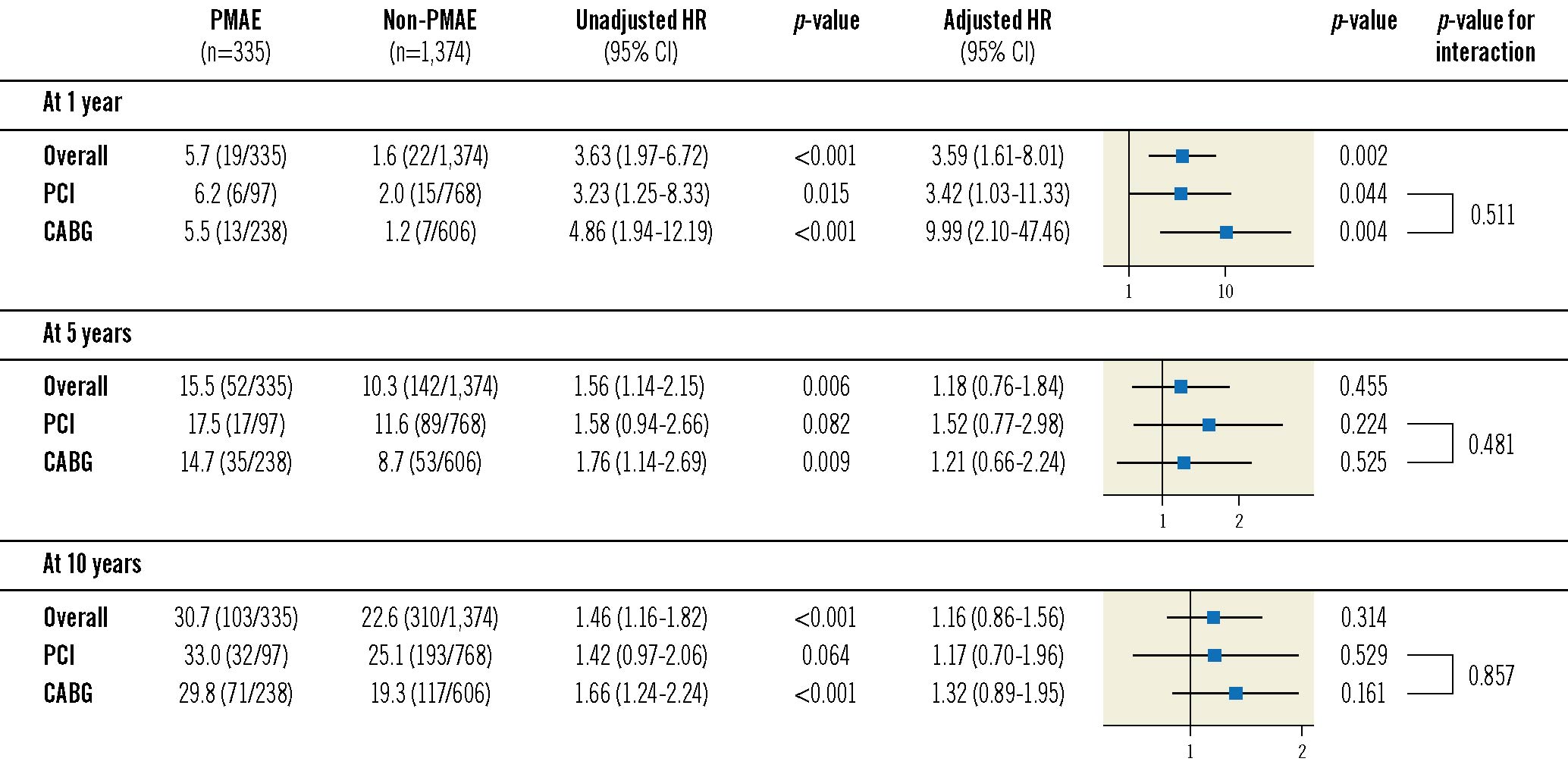
Figure 2. Impact of non-fatal PMAE on all-cause mortality expressed as hazard ratios. CABG: coronary artery bypass grafting; CI: confidence interval; HR: hazard ratio; PCI: percutaneous coronary intervention; PMAE: periprocedural major adverse events
The benefit of OMT on mortality after non-fatal PMAE
According to a previously reported substudy of the SYNTAXES trial, OMT was defined as the combination of 4 types of medications with at least 1 antiplatelet drug, statin, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker and a beta blocker included in current practice guidelines for the management of ischaemic heart disease (Supplementary Appendix 4)24. Kaplan-Meier survival curves between 1 month and 1 year in patients with and without non-fatal PMAE, classified according to the number of individual OMT agents at 1-year follow-up, show significantly lower mortality amongst patients on ≥3 types of medications compared to those on ≤2, irrespective of whether a non-fatal PMAE occurred (3.2% vs 13.1%; log-rank p<0.001) or not (1.2% vs 3.5%; log-rank p=0.006) (Figure 3A). Similarly, mortality between 5 and 10 years tended to be lower in patients on ≥3 types of medications at 5-year follow-up compared to those on ≤2 in patients with (14.3% vs 25.7%; log-rank p=0.060) as well as in those without a non-fatal PMAE (12.2% vs 18.4%; log-rank p=0.017), (Figure 3B). In Cox regression analysis, after adjustment for confounders, ≥3 types of medications at 1-month follow-up reduced all-cause mortality between 1 month and 1 year compared to those on ≤2 types of medications in patients with a non-fatal PMAE (adjusted HR 0.18, 95% CI: 0.05-0.57; p=0.004), with no significant difference in those without (adjusted HR 0.75, 95% CI: 0.18-3.10; p=0.693; p for interaction=0.027) (Supplementary Figure 9). All-cause mortality between 5 and 10 years was reduced by taking ≥3 types of medications at 5-year follow-up, irrespective of whether a non-fatal PMAE occurred (adjusted HR 0.21, 95% CI: 0.07-0.65; p=0.007) or not (adjusted HR 0.56, 95% CI: 0.34-0.91; p=0.019; p for interaction=0.473) (Supplementary Figure 10).
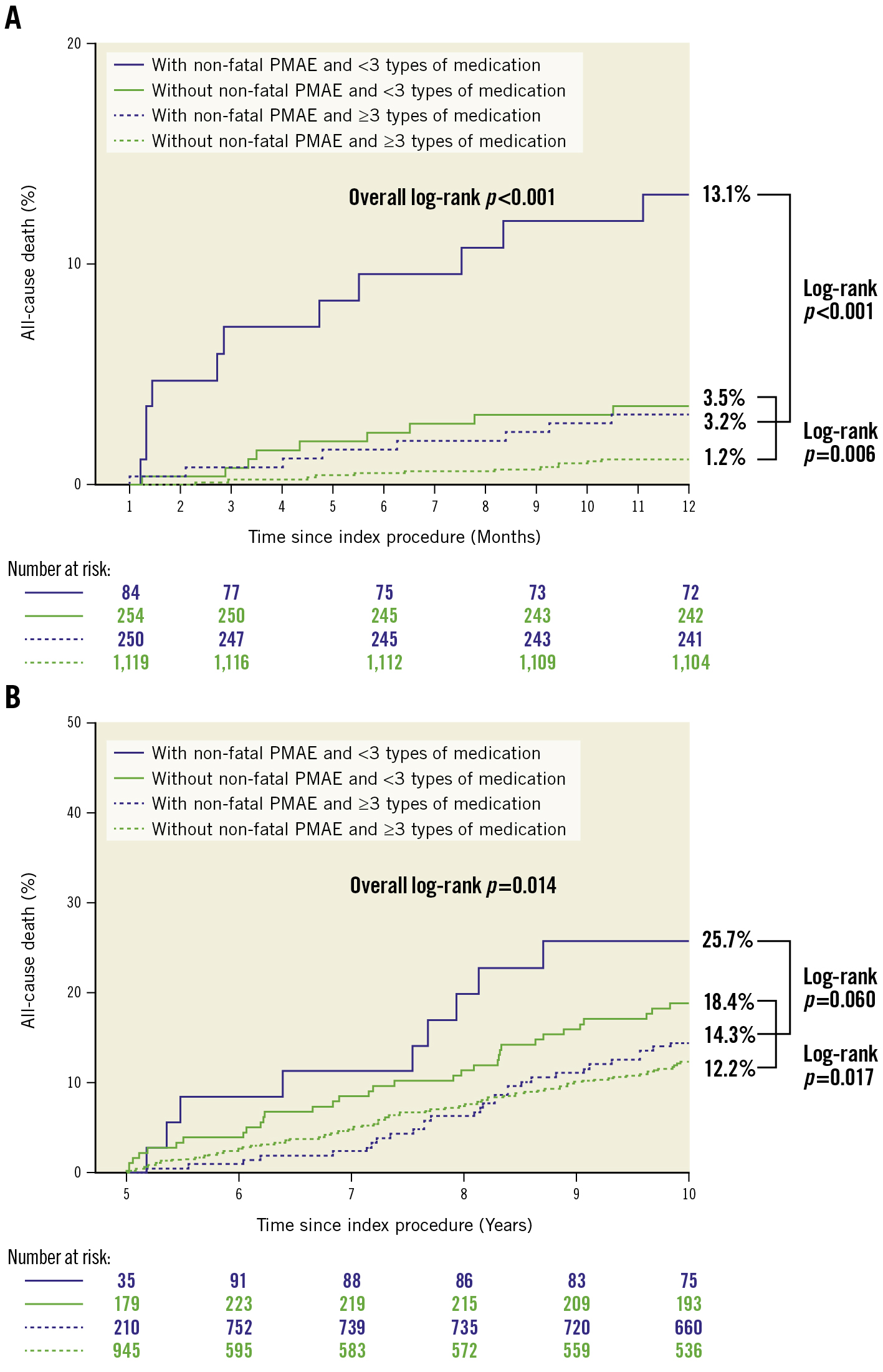
Figure 3. Kaplan-Meier curve for all-cause mortality in patients with and without non-fatal PMAE, classified according to the number of the individual OMT agents. A) Between 1 month and 1 year, classified according to the number of the individual optimal medical therapy (OMT) agents at 1-month follow-up and (B) between 5 and 10 years, classified according to the number of the individual OMT agents at 5-year follow-up. PMAE: periprocedural major adverse events
INTENTION-TO-TREAT ANALYSIS
As a sensitivity analysis, we also performed an ITT analysis, including death within 30 days post-procedure, according to the definition of PMAE. Non-fatal and fatal PMAE within 30 days of PCI and CABG occurred in 118 and 248 patients, respectively (13.1% vs 27.6%; p<0.001) (Supplementary Table 9, Supplementary Figure 11). In the ITT analysis, which included fatal PMAE, the occurrence of MI, repeat revascularisation, or stent thrombosis/graft occlusion within 30 days post-procedure was more frequent following PCI than CABG. Demographic characteristics and comorbidities of patients in the ITT analysis are shown in Supplementary Table 10. In the ITT analysis, 133 patients with a PMAE and 328 patients without a PMAE died within 10 years of randomisation (36.3% vs 22.9%; HR 1.82, 95% CI: 1.49-2.23; p<0.001). Kaplan-Meier survival curves show that mortality at 10 years was higher among patients with PMAE compared to those without in both arms (PCI arm 44.9% vs 25.0%; log-rank p<0.001; CABG arm 32.3% vs 20.3%; log-rank p=0.001) (Supplementary Figure 12A). Landmark analyses in the ITT analysis showed increased mortality between 1 and 10 years after a PMAE following CABG (25.9% vs 19.3%; log-rank p=0.039) but not PCI (36.8% vs 23.9%; log-rank p=0.200) (Supplementary Figure 12B, Supplementary Figure 12C). In the Cox regression analysis of the ITT, including fatal PMAE and after adjustment of confounders, PMAE remained an independent predictor of all-cause mortality at 10 years in the PCI arm (44.9% vs 25.0%; adjusted HR 1.96, 95% CI: 1.29-2.97; p=0.001), whereas in the CABG arm, PMAE was an independent predictor for 1-year mortality but no longer an independent predictor for 10-year mortality (32.3% vs 20.3%; adjusted HR 1.39, 95% CI: 0.95-2.02; p=0.084) (Supplementary Figure 13). However, a landmark analysis between 1 and 10 years in the Cox regression analysis shows that PMAE was not an independent predictor of long-term death in either treatment arm after eliminating the prognostic impact of short-term mortality (PCI arm: 29.3% vs 23.6%, adjusted HR 0.93, 95% CI: 0.52-1.66; p=0.806; CABG arm: 25.9% vs 19.3%, adjusted HR 1.04, 95% CI: 0.68-1.59; p=0.835).
Predicted and observed individual treatment benefit in survival by SSII-2020
Calibration plots for 10-year all-cause mortality (Figure 4) suggest that patients with a PMAE had a higher overall predicted mortality by the SSII-2020 than those without a PMAE, whilst also showing a tendency to underestimate the observed 10-year all-cause mortality, particularly in the third and fourth quartiles.
Absolute risk differences in predicted and observed mortality after PCI or CABG (Figure 5, Supplementary Figure 14) show that PMAE substantially alter the individual predictions of 10-year mortality by the SSII-2020.
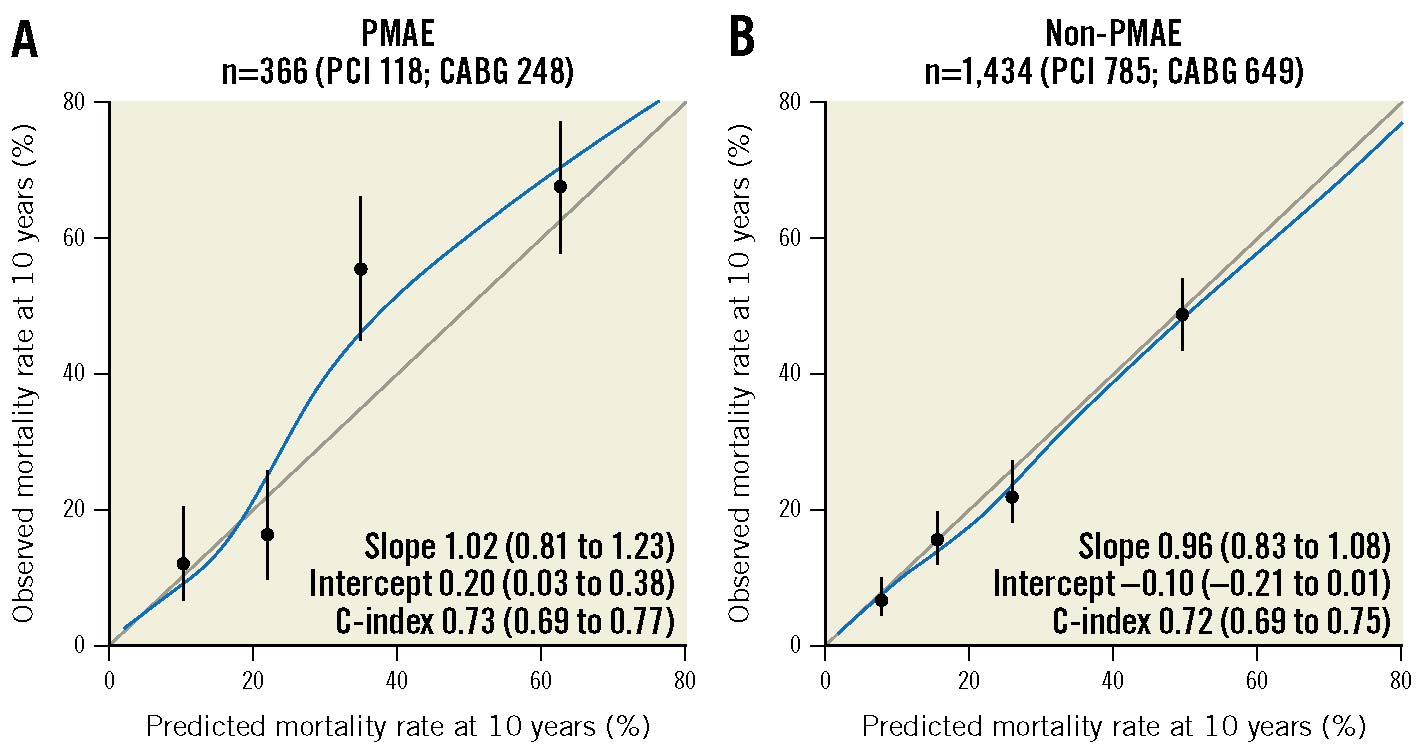
Figure 4. Calibration plots for 10-year all-cause mortality with and without PMAE. Dots correspond to quartiles of patients with mean predicted probability and mean observed mortality rate with 95% CI. The smooth calibration curves (blue line) to each plot are based on a Cox model that fitted outcomes to a restricted cubic spline of the predictions. CABG: coronary artery bypass grafting; CI: confidence interval; PCI: percutaneous coronary intervention; PMAE: periprocedural major adverse events
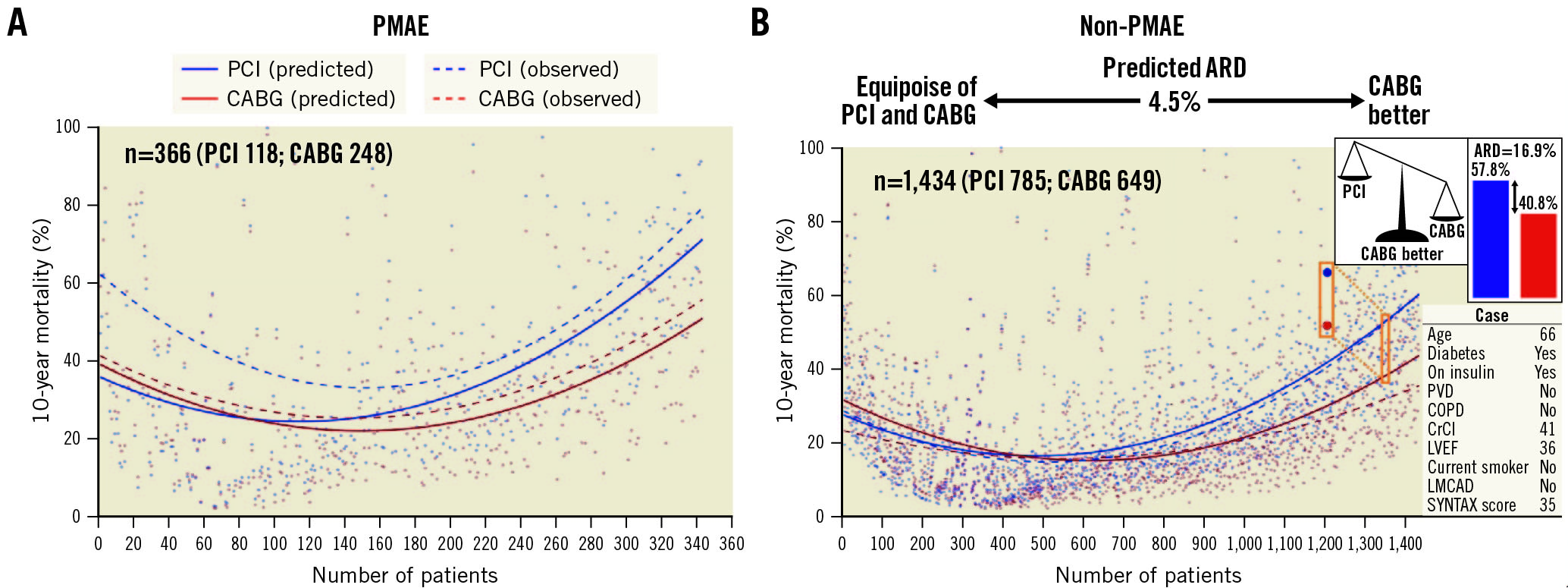
Figure 5. Absolute risk difference for 10-year all-cause mortality after PCI or CABG with and without PMAE in the intention-to-treat analysis. Individual differences in predicted mortality (individual scatterplot and solid smoothing curve) and observed mortality (dashed smoothing curve) after PCI or CABG are shown. ARD: absolute risk difference; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; CrCl: creatinine clearance; LMCAD: left main coronary artery disease; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention; PMAE: periprocedural major adverse events; PVD: peripheral vascular disease
Discussion
The major findings of this study were 1) non-fatal PMAE occur less frequently following PCI than CABG; 2) after adjusting for confounders, non-fatal PMAE were an independent predictor of all-cause mortality at 1 year, but not at 5 or 10 years, in both treatment modalities. The results of the ITT analysis were consistent with the clinical implications of the impact of non-fatal PMAE on long-term mortality from the per-protocol analysis.
A few studies comparing PCI to CABG in patients with LMCAD or 3VD have reported PMAE (Supplementary Table 11).
In this study, landmark analyses showed that the increased mortality associated with having a PMAE following PCI or CABG was seen predominantly in the first year (6.2% vs 2.0%; log-rank p=0.010 and 5.5% vs 1.2%; log-rank p<0.001, respectively) and was no longer apparent in the following 6-7 years of follow-up, with a resurgence of increased mortality in the final 3 years (Figure 1B, Figure 1C). The mean ages at enrolment of patients who died in the final 3 years in the PCI arm who had a non-fatal PMAE compared to those without were 74.3±7.8 vs 68.5±8.9 (p=0.034), whilst in the CABG arm they were 73.3±7.3 vs 69.7±8.9 (p=0.109), respectively. A previous analysis from the SYNTAXES study reported that the mean survival time (within 10 years) after PCI or CABG in the elderly >70 years was 7.7 years (95% CI: 7.4-8.1) and 7.9 years (95% CI: 7.5-8.3), respectively25. Therefore, the resurgence of increased mortality in the final 3 years in patients with a PMAE may be due to the difference in age at randomisation, considering the overall difference in life expectancy.
In this study, non-fatal PMAE was an independent predictor of all-cause mortality at 1 year following PCI or CABG; however, it ceased to have any prognostic impact long term, either at 5 or at 10 years (Figure 2). Hence, the prognostic impact of a non-fatal PMAE on short-term mortality appears to subside over long-term follow-up. The impact of preprocedural factors or periprocedural events on clinical outcomes is seemingly time dependent. For example, a substudy of the SYNTAXES trial showed that the association between post-procedural infections and long-term mortality was significant at 5 years but not at 10 years17, suggesting that these infections could contribute to early- or mid-term mortality. However, once the infection had cleared it no longer affected long-term survival. In another substudy of the SYNTAXES trial, landmark analyses showed that, in diabetic patients with 3VD, all-cause death at 5 years was higher after PCI compared with CABG. Between 5 and 10 years, this risk was reversed such that PCI no longer had a significantly increased risk of all-cause death at 10 years compared with CABG6. As a caveat, however, among the small number of insulin-treated patients (n=182), all-cause death at 10 years remained numerically higher with PCI than CABG (47.9% vs 39.6%, difference 8.2%, 95% CI: −6.5 to 22.5%; p=0.227).
In this study, Cox regression analysis showed that non-fatal PMAE were not an independent predictor of long-term death. Despite its potential confounding interaction with other preprocedural and/or procedural risk factors, the presence of PMAE at 1 month may therefore serve as a trigger for physicians to consider more frequent follow-up and aggressive adjunctive pharmacological therapy to amend or prevent the recurrence of adverse events for at least a year. Even though patients enrolled in this trial received follow-up with scheduled visits at 1, 6, and 12 months, non-fatal PMAE still impacted the 1-year mortality. Future studies with protocols mandating specific algorithms for patients with non-fatal PMAE are warranted. Of note, when we stratify patients according to the number of PMAE, non-fatal PMAE ≥2 was an independent predictor for all-cause 10-year mortality.
The benefit of OMT on mortality after non-fatal PMAE
A substudy of the SYNTAXES trial previously reported that at 5 years, patients on OMT, defined as the combination of 4 types of medications, had significantly lower mortality at 10 years compared with those on ≤2 types of medications (13.1% vs 19.9%; adjusted HR 0.470, 95% CI: 0.292-0.757; p=0.002), and similar mortality to those on 3 types of medications24. Our results suggest that in patients with non-fatal PMAE, OMT with guideline-recommended pharmacological therapy may provide survival benefits during the first year post-procedure as well as in the long term (Supplementary Figure 9, Supplementary Figure 10).
Does PMAE affect mortality predicted by SSII-2020?
The SSII-2020, which was derived from cross-correlation and has been externally validated in randomised trials and registries, predicts death and MACCE at 5 and 10 years following PCI and CABG in patients with 3VD and/or LMCAD456726.
Calibration plots for 10-year all-cause mortality suggest that PMAE synergistically increase the predicted (and observed) rate of all-cause death compared to preprocedural risk assessment (Figure 4).
Although the patients with PMAE had higher SSII-2020 at baseline (Supplementary Table 10), absolute risk differences show that PMAE disrupted the accuracy in predicting their 10-year mortality (Figure 5). Our findings suggest that the parameters predicting mortality after PMAE need to be re-evaluated given that the SSII-2020 no longer accurately predicts all-cause death when a PMAE occurs in the first 30 days.
Limitations
This study reports a post hoc analysis of the SYNTAX Study and, hence, is merely hypothesis-generating. Periprocedural events, except for MACCE, were adjudicated post hoc. In the SYNTAX Study, PCI was performed with a first-generation drug-eluting stent (DES) (TAXUS; Boston Scientific), and it has been widely demonstrated that novel current-generation DES have the potential to reduce MACCE components of non-fatal PMAE. The SYNTAX II Trial (ClinicalTrials.gov: NCT02015832) demonstrated that contemporary PCI, using current-generation DES, physiology-guided treatment, and intravascular ultrasound optimisation of stent deployment, has improved clinical outcomes including all-cause death, periprocedural MI, subacute stent thrombosis, and repeat revascularisation27. Indeed, in comparison with the landmark SYNTAX Study, the SYNTAX II registry, in a prospective propensity analysis based on the SYNTAX score II, demonstrated an absolute reduction in all-cause death of 5.7% at 5 years28. While the impact of the suggested combination of OMT agents was found beneficial in reducing mortality post-PMAE, case-specific clinical judgment should prevail, as the current guidelines propose the best management strategies for an individual patient with a given condition2930. Considering the long follow-up in this study and the age of the patients at enrolment, despite Cox regression adjustment, age (i.e., mortality due to age) may have led to an underestimation of the impact of PMAE at long-term follow-up.
Conclusions
In patients with 3VD and/or LMCAD, non-fatal PMAE were more common following CABG than PCI, but their prognostic impact was similar, being significant in the first year and then diminishing out to 10 years. Patients with non-fatal PMAE may therefore require more careful follow-up and additional preventive treatment in the first-year post-procedure.
Impact on daily practice
The prognostic impact of non-fatal PMAE occurring within 30 days of PCI or CABG in patients with 3VD and/or LMCAD was significant in the first year and then diminished out to 10 years. Patients with non-fatal PMAE may require more careful follow-up and additional preventive treatment in the first year post-procedure. Further studies incorporating contemporary PCI using current-generation DES with physiological and imaging guidance, and contemporary CABG with multiple arterial
grafting, fractional flow reserve guidance, and a minimally invasive approach, are needed to address the prognostic impact and management strategy of PMAE for patients with complex CAD.
Funding
SYNTAXES was supported by the German Foundation of Heart Research. The SYNTAX Study, up to 5-year follow-up, was funded by Boston Scientific. Both sponsors had no role in the study design, data collection, data analyses, or interpretation of the study data and were not involved in the decision to publish the final manuscript. The principal investigators and authors had complete scientific freedom.
Conflict of interest statement
N. Kotoku has received a grant for studying overseas from Fukuda Foundation for Medical Technology. P.W. Serruys has received consultancy fees from Philips/Volcano, SMT, Novartis, Xeltis, and Meril Life, outside of the submitted work. K. Ninomiya has received a grant from Abbott Medical, Japan, outside the submitted work. S. Masuda has received a grant from Terumo, outside the submitted work. M-C. Morice is the CEO and a shareholder of CERC, a contract research organisation based in Paris, which had no role in this trial; and a minor shareholder of Electroducer, which had no role in this trial. A.P. Kappetein has been an employee of Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

