
Abstract
Aims: The present study sought to examine the prevalence, clinical characteristics and one-year outcomes of patients undergoing percutaneous coronary intervention (PCI) to complex lesions (multivessel PCI, ≥3 stents, ≥3 lesions, bifurcation with ≥2 stents, total stent length >60 mm or chronic total occlusion [CTO]) in a prospective multicentre registry.
Methods and results: Using the e-Ultimaster multicentre registry, a post hoc subgroup analysis was performed on 35,839 patients undergoing PCI, stratified by procedure complexity, and further by number and type of complex features. Overall, complex PCI patients (n=9,793, 27.3%) were older, more comorbid and were associated with an increased hazard ratio (HR) of the composite endpoint at one year (target lesion failure [TLF]: 1.41 [1.25; 1.59]), driven by an increased hazard of cardiac death (1.28 [1.05; 1.55]), target vessel myocardial infarction (1.48 [1.18; 1.86]) and clinically driven target lesion revascularisation. The hazard of complications increased with the rising number of complex features (3-6 vs 1-2 vs none) for all outcomes. All individual complex features were associated with an increased hazard of composite complications (except CTO) and definite/probable stent thrombosis.
Conclusions: Overall, complex PCI is associated with an increased risk of mortality and complications at one year. The number and types of complex features have differing impacts on long-term outcomes.
Introduction
Advances in procedural and imaging techniques, stent platforms and operator experience have led to an increase in PCI in patients with complex coronary lesions1,2,3. Complex PCI is often used to describe interventions on lesions with challenging anatomical characteristics, including left main involvement, heavily calcified lesions, heavily thrombotic lesions, chronic total occlusions (CTO), bifurcation lesions or multivessel disease2,4. Several high-risk features for stent-related recurrent ischaemic events have been described in the 2017 European Society of Cardiology (ESC) update on dual antiplatelet therapy (DAPT), including prior stent thrombosis despite adequate antiplatelet therapy, stenting of the last remaining patent coronary artery, diffuse multivessel disease, chronic kidney disease (CKD), ≥3 stents implanted, ≥3 lesions treated, bifurcation lesions treated with two stents, total stent length >60 mm, and treatment of CTO4,5.
Previous studies examining outcomes of complex, high-risk lesions have either analysed all complex lesions collectively or only focused on specific lesion types (e.g., CTO or bifurcation disease)4,6,7,8,9,10. Furthermore, many studies were performed on highly selected cohorts, included stent platforms that are not commonly used such as bare metal stents (BMS) or involved early-generation drug-eluting stents (DES)4,11. Furthermore, differences in stent platforms used for particular lesion subsets may confound comparative outcomes reported amongst different complex lesion subtypes4,7. There are limited data on the prevalence and clinical outcomes of complex lesions in the real world, and whether the clinical outcomes from individual complex features vary either by type or by number.
The present study sought to examine the effect of lesion complexity on one-year clinical outcomes in a large and unselected cohort of PCI procedures from the e-Ultimaster multicentre registry, stratified by number and type of complex PCI features.
Methods
STUDY DESIGN AND PATIENT POPULATION
The e-Ultimaster is a prospective, multicentre, observational registry with a primary objective to evaluate further the safety and performance of the Ultimaster® DES system (Terumo Corporation, Tokyo, Japan) in an all-comer patient population. There were no further inclusion or exclusion criteria in order to enrol an unselected patient cohort. The selection process for our study cohort is illustrated in Supplementary Figure 1. For this post hoc subgroup analysis, complex PCI patients were identified based upon the presence of one or more of the following characteristics in the index procedure: multivessel PCI, ≥3 stents implanted, ≥3 lesions treated, bifurcation PCI with ≥2 stents, total stent length >60 mm or chronic total occlusion (CTO). The complex procedural characteristics defined in our study were those described in the 2017 ESC guidelines on DAPT, based on the pooled patient-level meta-analysis by Giustino et al4,5. Further information on trial registration, study device and follow-up is available in Supplementary Appendix 1. A full list of participating centres is presented in Supplementary Appendix 2.
OUTCOMES AND DEFINITIONS
The primary outcome is target lesion failure (TLF), defined as a composite of cardiac death, myocardial infarction (MI) that could not be clearly attributed to a vessel other than the target vessel (target vessel MI) and clinically driven target lesion revascularisation (CD-TLR) at one year. All primary outcome-related adverse events were adjudicated by an independent clinical events committee. Subcategories of death (cardiac death, vascular death and non-cardiovascular) were adjudicated according to the Academic Research Consortium (ARC) definitions12. For MI, the extended historical myocardial definition was applied that primarily uses creatine kinase myocardial band (MB) as cardiac biomarker criterion but, if not measured, troponin values for the determination of a periprocedural (<48 hours post PCI), reinfarction (<48 hours post PCI) or spontaneous MI (>48 hours post PCI)13.
Revascularisations and stent thrombosis were based upon the ARC definitions12. Bleeding was defined according to the Bleeding Academic Research Consortium (BARC) definitions14. Target vessel failure (TVF) was defined as a composite of cardiac death, target vessel MI and TVR, and the patient-oriented composite endpoint (POCE) as the composite of any death, any MI and any coronary revascularisation.
FOLLOW-UP
Follow-up was performed either by a direct phone contact with the patient or by visit of the patient to the outpatient clinic of the hospital. Collection of adverse events was done through a web-based database. At each follow-up, there was a specific question regarding whether any adverse event had occurred. If answered positively, all events had to be reported, i.e., death, MI, re-PCI, coronary artery bypass grafting (CABG), bleeding, vascular complication, stent thrombosis or other. Further relevant information was collected per event type.
STATISTICAL ANALYSIS
Complex PCI patients were compared to patients without any of the complex features (non-complex patients). The complex patient subgroup was further divided into patients with 1-2 complex features and 3-6 complex features. Patients’ demographics, comorbidities, medical history, target lesion characteristics and procedural characteristics are summarised with mean±standard deviation for continuous variables and with frequencies and percentages for categorical variables. The chi-square test was used to compare categorical variables and the t-test to compare continuous variables. For non-normally distributed data, non-parametric tests (i.e., Kruskal-Wallis test) were used, as appropriate. The Kaplan-Meier method was used to construct survival curves for time-to-event variables, which were compared by means of the log-rank test. Cox proportional hazard ratios (HRs) were calculated for all complex subgroups (overall complex, 1-2 and 3-6 components, and each individual component) using the non-complex group as the reference category, adjusting for the following factors: age, sex, diabetes, hypertension, hypercholesterolaemia, smoking history, renal impairment, clinical presentation (acute coronary syndrome [ACS] vs chronic coronary syndrome) and a previous history of MI, percutaneous transluminal coronary angioplasty (PTCA) or CABG. A two-sided p-value <0.05 was considered to indicate statistical significance. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Out of 37,261 patients recruited in the e-Ultimaster study, a total of 35,839 patients with one-year follow-up data were included in the final analysis, of whom 9,793 (27.3%) underwent complex PCI. Within the complex PCI group, the majority of patients had 1-2 complex PCI features (73.3%, n=7,174), whereas only 26.7% (n=2,619) had 3-6 complex features. The distribution of different complex PCI features is illustrated in Figure 1. The most prevalent features were multivessel PCI (16.3%) followed by ≥3 stents implanted (12.3%) and ≥60 mm stent length (8.8%).
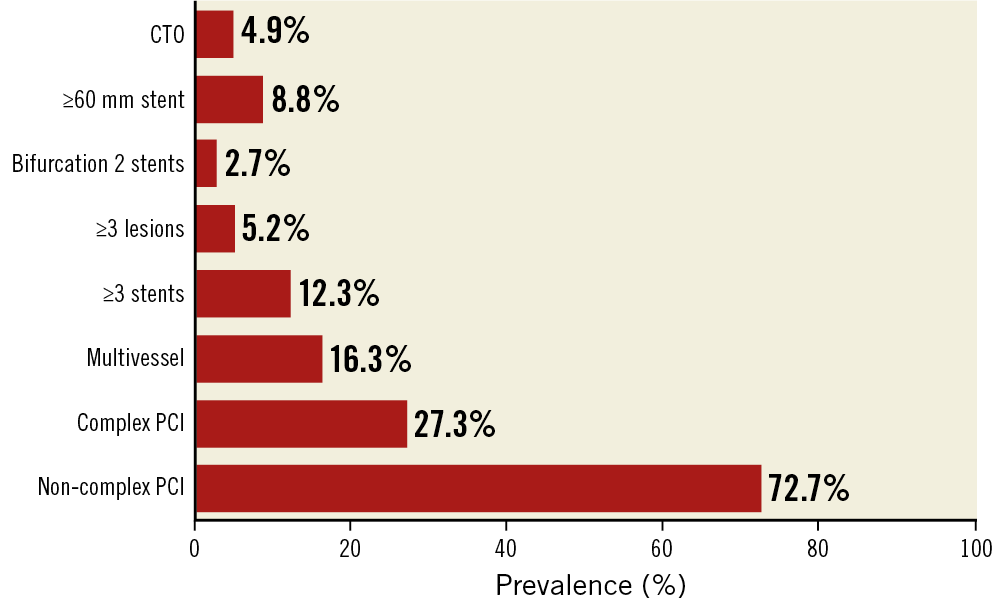
Figure 1. Prevalence of individual complex PCI components. CTO: chronic total occlusion; PCI: percutaneous coronary intervention
PATIENT CHARACTERISTICS
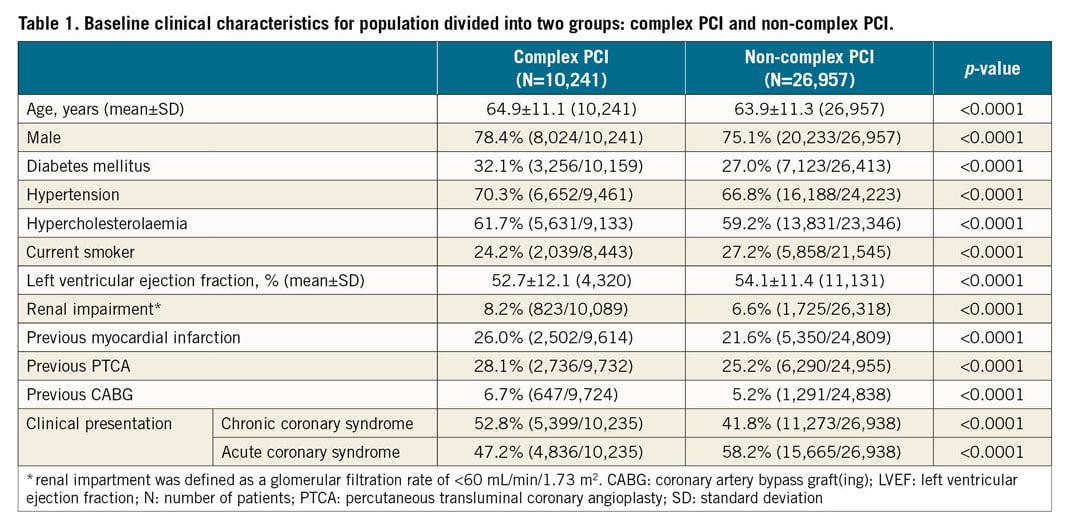
Several key differences in patient characteristics were observed between complex and non-complex PCI groups, all at a p-value <0.0001 unless otherwise stated (Table 1). In comparison to the non-complex PCI group, patients undergoing complex PCI were older (64.9±11.1 vs 63.9±11.3 years), more often male (78.4% vs 75.1%), and had a higher prevalence of diabetes mellitus (32.1% vs 27.0%), hypercholesterolaemia (61.7% vs 59.2%), hypertension (70.3% vs 66.8%) and renal impairment (8.2% vs 6.6%). Previous MI (26.0% vs 21.6%) and coronary revascularisation (PCI: 28.1% vs 25.2%; CABG: 6.7% vs 5.2%) were more frequent in the complex PCI group. The indication for the PCI was more commonly chronic coronary syndrome in the complex PCI group (52.8% vs 41.8%). The observed differences in patient characteristics were more pronounced as the number of complex PCI factors increased (Supplementary Table 1).
PROCEDURAL CHARACTERISTICS
Procedural characteristics differed between the two groups; all p-values were <0.0001. There was a lower rate of utilisation of radial access in complex compared to non-complex PCI procedures (76.7% vs 84.3%) (Supplementary Table 2). The majority of PCI procedures were performed on the left anterior descending artery (LAD), more so in the complex PCI group (63.5% vs 47.0%). Overall, there were higher rates of intervention for in-stent restenosis lesions (7.5% vs 4.5%) as well as bifurcation (20.3% vs 8.6%) and ACC/AHA type B2/C lesions (47.2% vs 39.3%) in the complex PCI group. Complex PCI lesions required longer stents on average, both per patient (48.3±26.7 mm vs 24.5±10.3 mm) and per lesion (30.0±18.3 mm vs 23.3±9.6 mm). The differences in lesion characteristics between the complex and non-complex PCI groups were more pronounced with increasing number of complex factors (3-6 factors >1-2 factors >none) (Supplementary Table 3). Adherence to DAPT and lipid-lowering therapy (including statins) was higher in the complex PCI than in the non-complex PCI group (DAPT: 70.3% vs 65.8%; lipid-lowering therapy: 78.8% vs 74.1%, p<0.0001 for both) (Supplementary Table 2), and increased in proportion to the number of complex features (3-6 features >1-2 features >none: DAPT: 71.3% vs 70.0% vs 65.8%; lipid-lowering therapy: 80.1% vs 78.4% vs 74.1%, p<0.0001 for both) (Supplementary Table 3).
30-DAY AND ONE-YEAR OUTCOMES
The rates of composite endpoints (TLF, TVF and POCE) were all significantly higher in the complex PCI than in the non-complex PCI groups, both at 30 days (TLF and TVF: 1.4% vs 0.8% each; POCE: 1.9% vs 1.1%, p<0.0001 for all) and at one year (TLF: 4.2% vs 2.8%; TVF: 4.8% vs 3.3%; POCE: 7.9% vs 6.0%, p<0.0001 for all) (Table 2). Similarly, rates of all-cause and cardiac death at 30 days and one year were both higher in the complex PCI group, as were the rates of ischaemic outcomes. Although there was no difference in all-cause and BARC 3-5 bleeding between the complex and non-complex PCI groups at 30 days, both events were higher in the complex group at one year (any bleeding: 2.4% vs 2.0%, p=0.03, and major bleeding: 0.8% vs 0.5%, p<0.01). There was no difference in rates of Q-wave MI and clinically driven target vessel CABG revascularisation between complex and non-complex PCI groups at 30 days and one year.
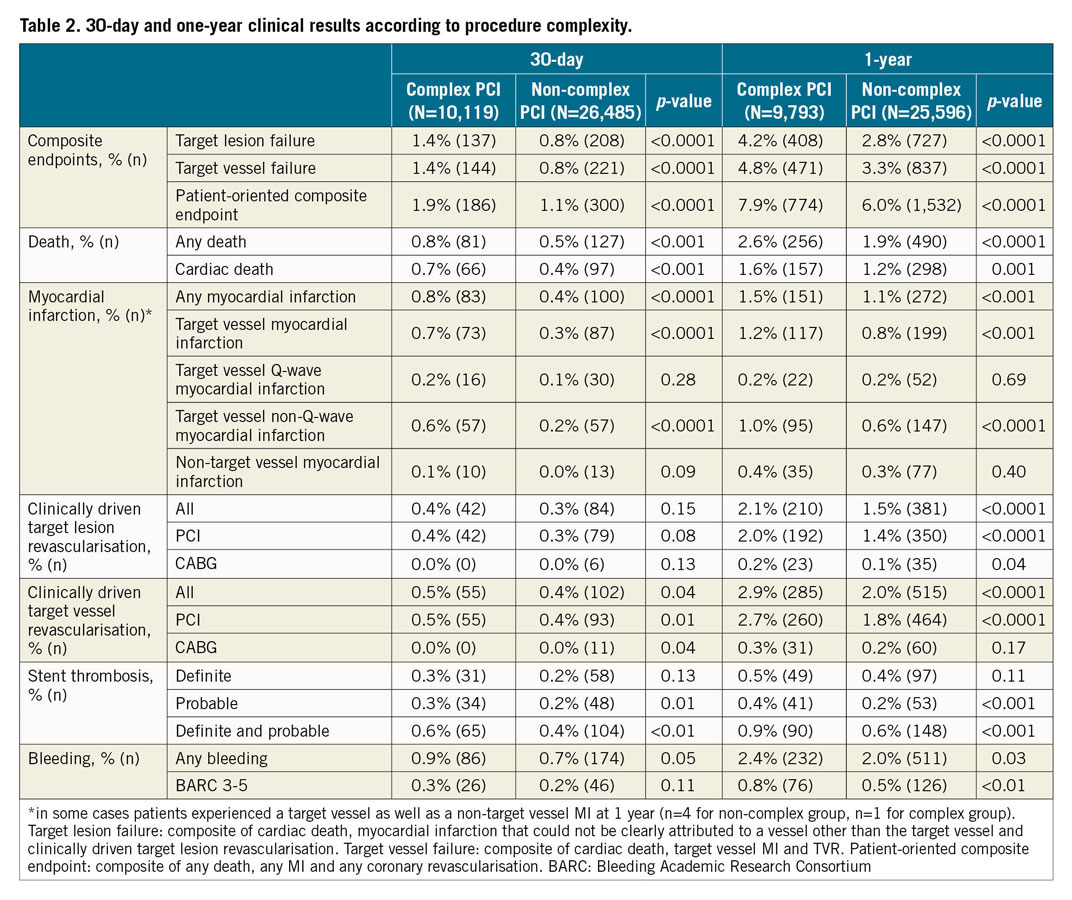
After adjustment for baseline differences, the complex PCI group remained at a significantly increased one-year hazard of composite endpoints (TLF: HR 1.41 [1.25; 1.59], TVF: HR 1.47 [1.27; 1.69]), as well as individual outcomes (cardiac death: HR 1.28 [1.05; 1.55], target vessel MI: 1.48 [1.18; 1.86], clinically driven TLR: 1.42 [1.20; 1.68]) (Figure 2, Table 2).
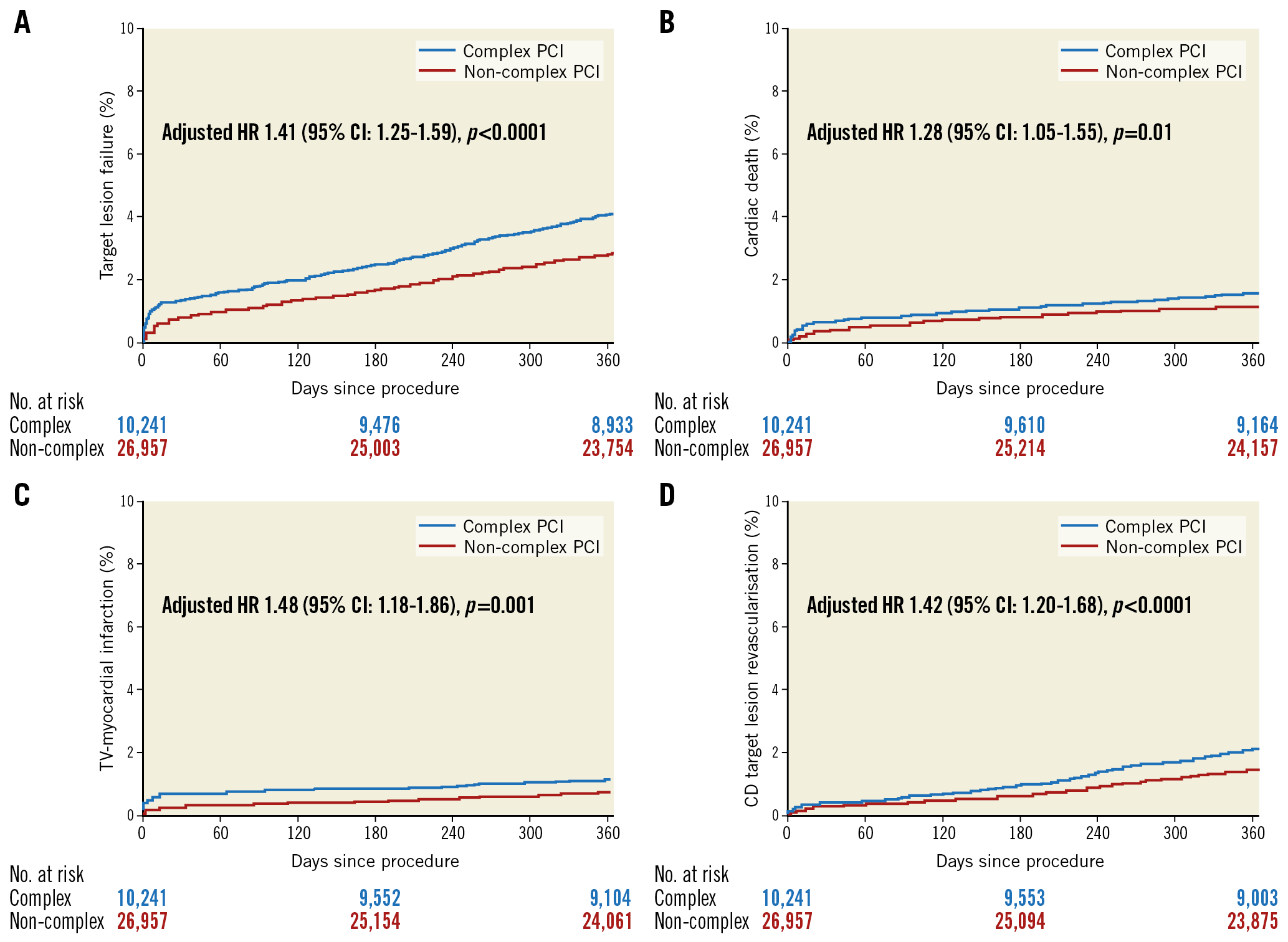
Figure 2. Kaplan-Meier curves for the complex and non-complex PCI groups. A) TLF. B) Cardiac death. C) Target vessel MI. D) CD-TLR. CD-TLR: clinically driven target lesion revascularisation; MI: myocardial infarction; TLF: target lesion failure
A stepwise increase in the one-year event rate of TLF was observed as the number of complex PCI factors increased, driven by increasing rates of cardiac death (none vs 1-2 vs 3-6: 1.2% vs 1.5% vs 1.8%), target vessel MI (none vs 1-2 vs 3-6: 0.8% vs 1.1% vs 1.6%) and clinically driven TLR (none vs 1-2 vs 3-6: 1.5% vs 2.0% vs 2.4%) (Supplementary Table 4). Similarly, the rates for the other composite endpoints TVF and POCE increased in line with the rising number of complex PCI factors. Definite/probable stent thrombosis rates increased from no complex feature to 1-2 complex features and further to 3-6 complex features (0.6% vs 0.9% vs 1.1%, respectively). These findings persisted after adjustment for baseline differences, with an incremental rise in HR with an increasing number of complex features (Figure 3, Table 3).
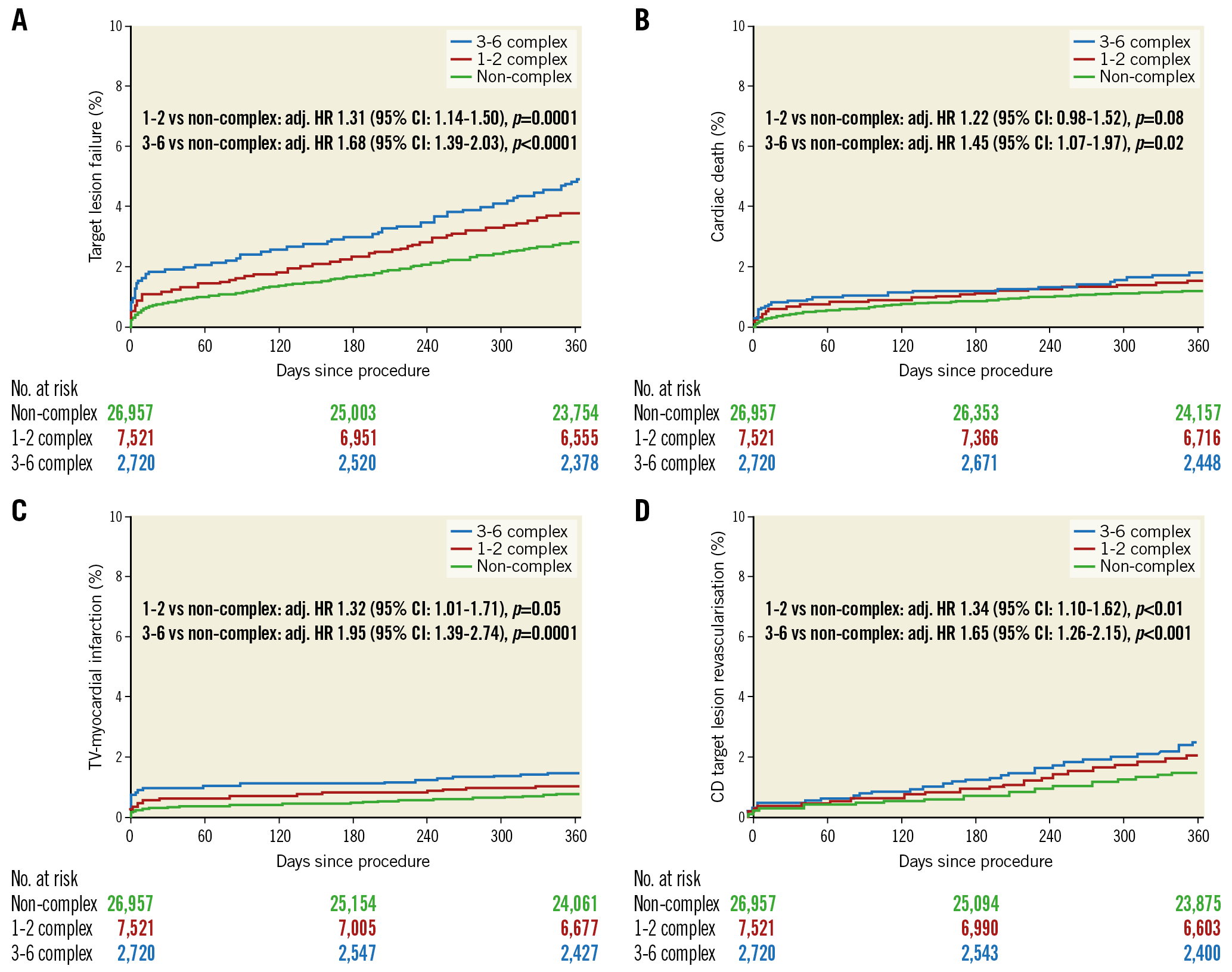
Figure 3. Kaplan-Meier curves according to the number of complex PCI features. A) TLF. B) Cardiac death. C) Target vessel MI. D) CD-TLR. CD-TLR: clinically driven target lesion revascularisation; MI: myocardial infarction; TLF: target lesion failure
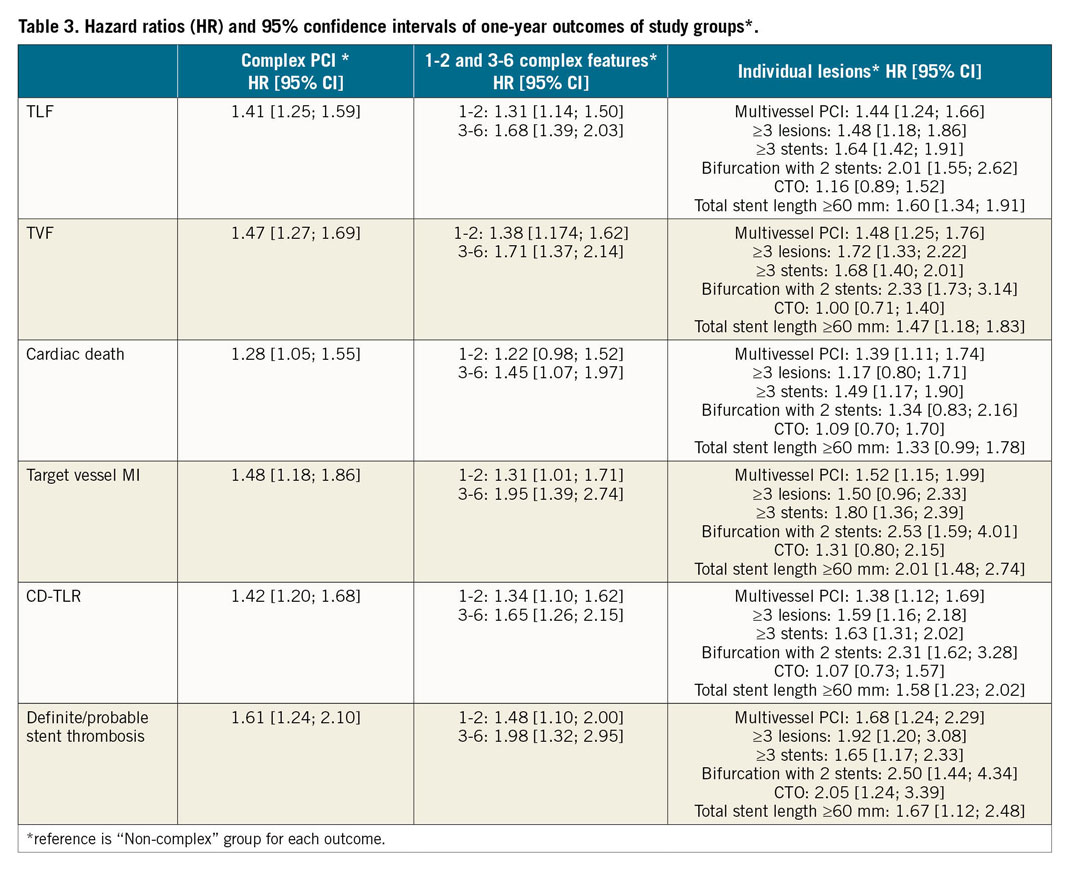
A subgroup analysis of one-year clinical outcomes of individual complex PCI features is summarised in Supplementary Table 5 and further illustrated in Figure 4. Compared to the non-complex PCI group, the rates of composite endpoints (TLF and TVF) were increased with all individual complex features other than CTO, driven primarily by a higher incidence of clinically driven TLR and target vessel MI (except in patients with ≥3 lesions stented). These findings persisted after adjustment for baseline differences, with the greatest hazard observed among bifurcation lesions with two stents (TLF: HR 2.01 [1.55; 2.62], and TVF: HR 2.33 [1.73; 3.14], clinically driven TLR: HR 2.31 [1.62; 3.28], target vessel MI: HR 2.53 [1.59; 4.01) (Figure 5, Table 3).
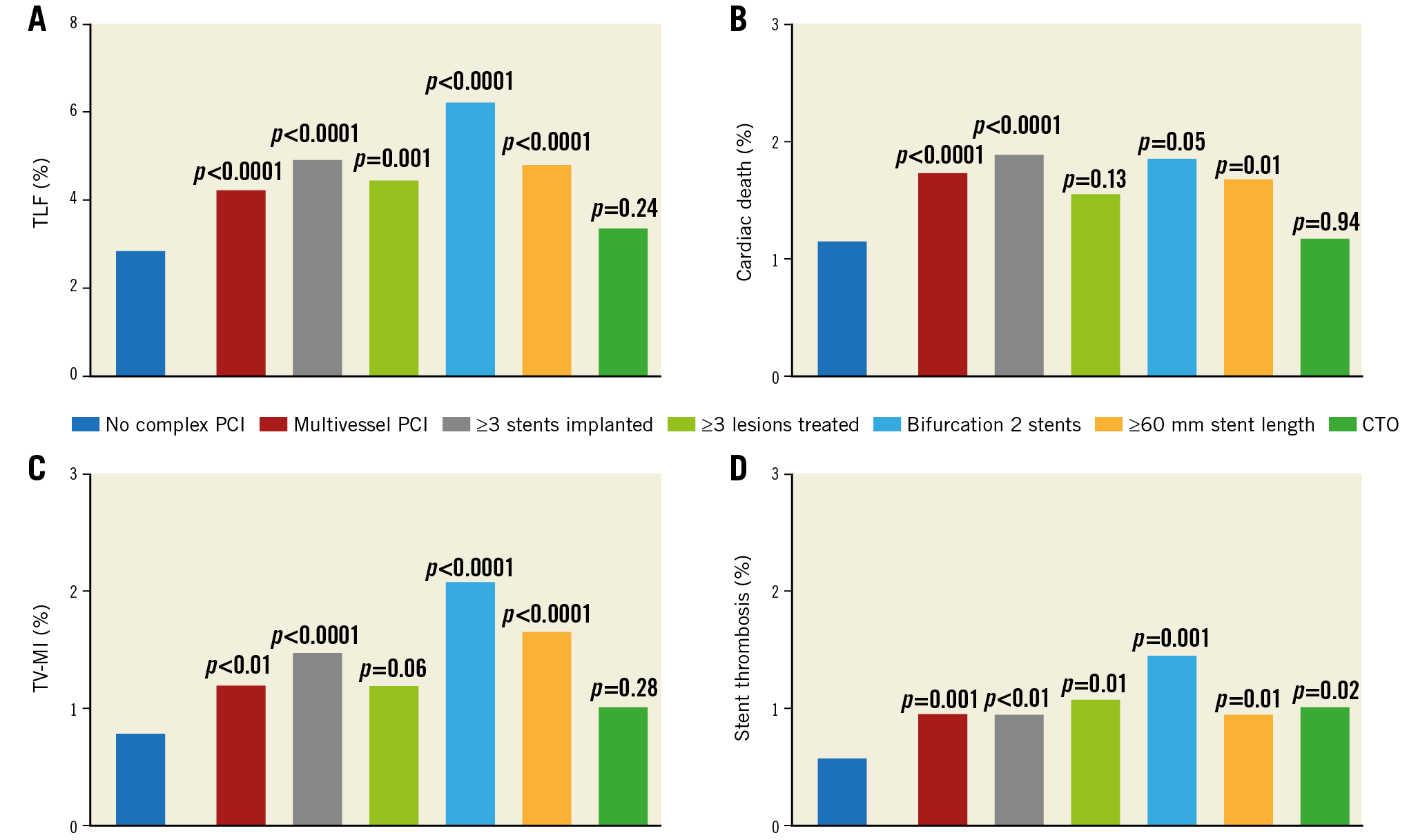
Figure 4. Crude rates at one year for individual complex risk factors. A) TLF. B) Cardiac death. C) Target vessel MI. D) Definite/probable stent thrombosis. All p-values refer to comparison with the non-complex PCI group. TLF: target lesion failure; TV-MI: target vessel myocardial infarction
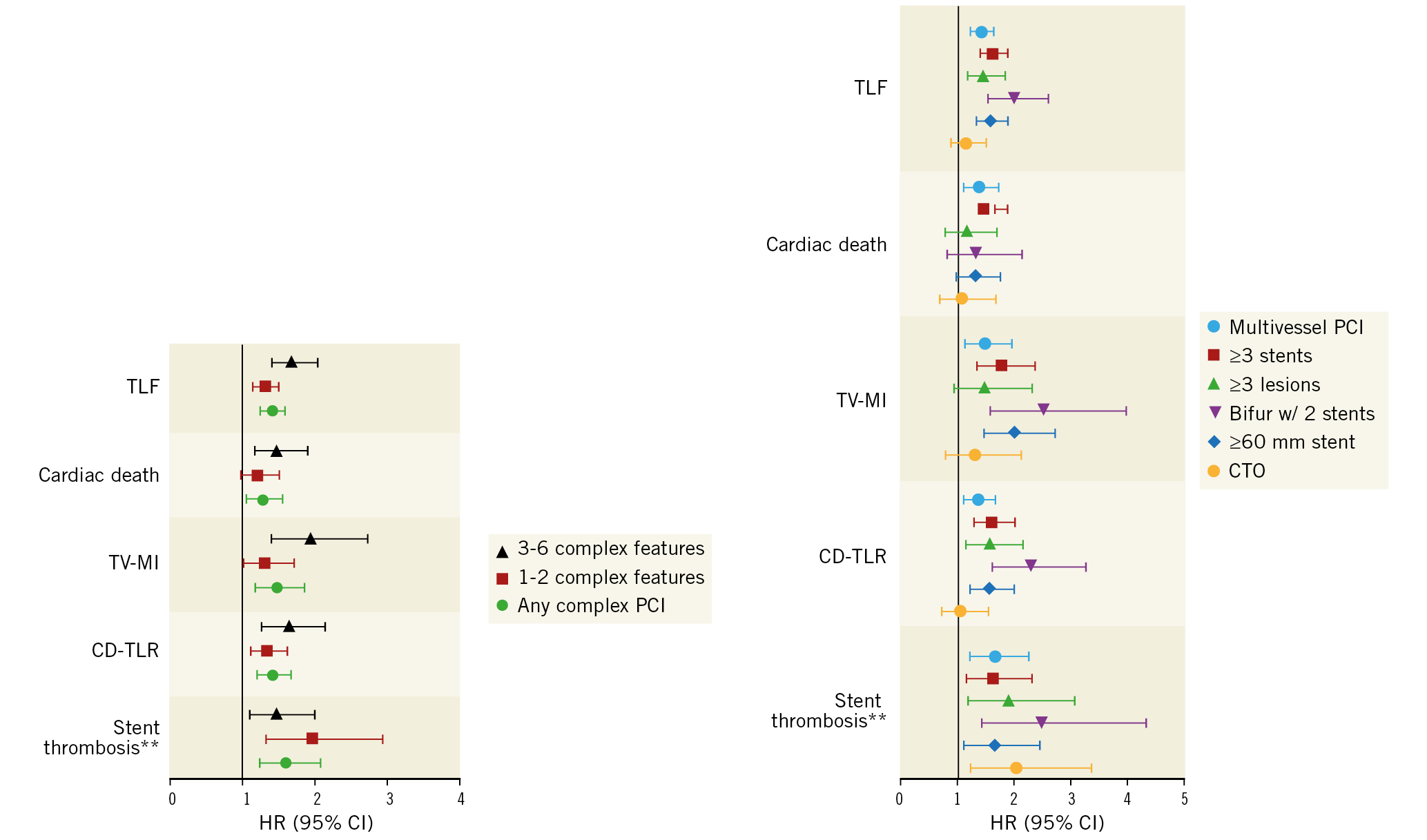
Figure 5. Adjusted hazard ratios (HR) and 95% confidence intervals (CI) of adverse events*. *reference is non-complex PCI for each outcome. **definite or probable stent thrombosis.
Discussion
This is the largest study to examine the effect of lesion complexity, in terms of both number and type, on one-year outcomes in a large and unselected cohort of more than 35,000 patients undergoing PCI using a single new-generation stent platform. Several important conclusions can be drawn from our findings. First, we show that patients with complex lesions undergoing PCI are often older, male, with a higher prevalence of traditional cardiovascular risk factors. Second, we found that, overall, complex PCI is associated with worse outcomes at 30 days and one year including composite endpoints (TLF, TVF and POCE), all-cause and cardiac deaths, any bleeding and BARC 3-5 bleeding, and the majority of ischaemic outcomes. Further, we demonstrate an incremental relationship between the number of complex features and adversity of clinical outcomes including the composite adverse outcomes of TLF, TVF and POCE as well as secondary outcomes, even in a cohort managed with latest-generation DES, emphasising the impact of lesion and procedure complexity on clinical outcomes. Finally, we highlight differences (or lack thereof) in specific outcomes between individual complex PCI features.
OVERALL PROCEDURAL COMPLEXITY
Although some previous studies have looked at the relationship between lesion complexity and long-term clinical outcomes after PCI, they were subject to the limitations previously described4,7,8,9,10. These findings are consistent with those of a recent analysis from the Bern PCI registry that showed an increased (unadjusted) hazard of cardiac death (HR 3.07 [2.43; 3.89]), target vessel MI (HR 1.92 [1.54; 2.39]) and stent thrombosis (HR 1.71 [1.10; 2.65]) in 5,323 patients with high-risk PCI features undergoing PCI compared to those without high-risk features11. However, their analysis was based on a smaller cohort derived from a single regional centre, and included patients treated with first-generation DES and bare metal stents (BMS). In a post hoc analysis of randomised controlled trials (RCTs), Giustino et al reported an increased hazard of cardiac mortality, definite or probable stent thrombosis and TVR, but no difference in stroke or bleeding, between patients undergoing complex and non-complex PCI4. However, their analysis was based on a modest number of complex PCI patients (n=1,680), derived from a highly selected cohort from RCTs, and included procedures performed with both early- and new-generation DES. Although some of these findings were observed in the present study, including higher rates of probable or definite stent thrombosis, MI, as well as cardiac death, we show that target lesion and vessel failure, target vessel MI and bleeding (any bleeding and BARC 3-5 bleeding) were also higher in patients with complex PCI. Several factors place complex PCI patients at a heightened risk of further ischaemic complications and mortality. Patients undergoing complex PCI are often older, with a higher burden of comorbidities and cardiovascular risk factors, as observed in our cohort. Patients undergoing complex PCI may also have a greater burden of residual coronary artery disease (CAD) which puts them at a risk of recurrent ischaemic events. Although there is limited literature to explain the higher incidence of major bleeding in complex than in non-complex PCI, this is possibly justified by several factors. First, risk factors for ischaemia (hypertension, diabetes, renal failure and advanced age) also increase the risk of bleeding15. Furthermore, complex PCI patients are more likely to receive prolonged DAPT therapy or more potent P2Y12 agents, which may have contributed to their higher bleeding rates.
NUMBER AND TYPES OF COMPLEX FEATURES
To the best of our knowledge, the present study is the largest to compare an expansive array of one-year outcomes after complex PCI according to number and type of complex features and informs operators of several important findings. Our analysis shows a positive correlation between the number of complex features (none vs 1-2 vs 3-6 features) and all adverse outcomes, driven primarily by higher rates of target vessel MI and clinically driven TLR. Our findings are in keeping with those reported by Ueki et al in their single-centre analysis of 5,323 patients with high-risk PCI features, where the number of complex features (1-2 and ≥3) correlated with the adjusted hazard of cardiac death, target vessel MI and definite/probable stent thrombosis11.
We also show prognostic differences between individual complex PCI features, with an increased hazard of TLF and TVF among all individual features, driven by increased hazards of clinically driven TLR and target vessel MI, especially in patients with bifurcation with two stents. Another important observation in our analysis is the increased hazard of stent thrombosis with all individual complex features (compared to non-complex PCI). Giustino et al demonstrated an increased hazard of definite or probable stent thrombosis in specific complex PCI subsets, namely ≥3 stents implanted and bifurcation lesions with two stents, whereas we found the hazard to be increased with all subsets4. It is possible that their analysis was underpowered to detect differences across all individual groups given their relatively small sample size. The underlying mechanisms behind stent thrombosis are multifactorial and include patient factors (chronic kidney disease, diabetes mellitus, smoking, type of coronary syndrome), type of stent platform and the type and duration of DAPT16.
Strengths and limitations
Our data are derived from a registry evaluating the efficacy of the Ultimaster stent which enrolled a large number of patients from several regions. Other than the novel findings we report, a strength in our study is that all events composing the primary endpoints were independently adjudicated. However, as with most registries, it is subject to several inherent limitations. First, there is a potential for selection bias and under-reporting of events. In particular, an underestimation of periprocedural MI cannot be excluded. Periprocedural biomarker collection, relevant for the detection of usually smaller MI, was per hospital practice and not mandated per protocol. Measures to ensure data quality included remote and on-site monitoring with a risk-based approach well as close communication with the sites to reinforce the importance of complete and accurate data entry. Second, vessel and lesion characteristics were assessed by operators, most commonly through visual estimation, and not measured centrally by a core lab. Third, the outcomes reported are based on the use of a single new-generation stent platform for all patients; these may potentially differ with the use of other new-generation DES. Finally, although we report a follow-up of one year, coronary stents are lifelong implants and it is possible that further differences between our study groups would be noted on longer follow-up.
Conclusions
In a real-world cohort of PCI patients, we found that patients with complex target lesions are at an increased risk of cardiac death and complications at one year as compared to non-complex PCI patients. Even with the use of latest-generation DES, we show that a greater number of complex PCI features correlates with higher mortality and worse outcomes. Finally, we demonstrate prognostic differences between individual complex lesions, with the worst outcomes observed among patients with bifurcation treatment using two stents, compared to non-complex PCI patients, and a lack of difference in outcomes other than stent thrombosis in CTO patients. The present findings provide operators with insight regarding the relationship between number and types of lesion complexity and one-year outcomes after complex PCI.
|
Impact on daily practice The present study highlights prognostic differences in one-year outcomes after complex PCI. The findings provide operators with novel insights regarding clinical outcomes of individual complex features and emphasise that the number and types of complex features both have an impact on procedural outcomes. Furthermore, our findings, drawn from a large and contemporary procedural cohort, support those from previous studies examining the overall effect of lesion complexity on PCI outcomes. |
Funding
The e-Ultimaster registry was funded by Terumo Europe, Middle East & Africa (Leuven, Belgium).
Conflict of interest statement
M. Mohamed receives funding in support of a PhD scholarship from Medtronic Ltd. Medtronic Ltd was not involved in the conceptualisation, design, conduct, analysis, or interpretation of the current study. C. von Birgelen reports institutional research grants from Abbott Vascular, Biotronik, Boston Scientific and Medtronic, not related to the present study. A. Aminian is a consultant for Terumo. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

