Abstract
Background: For women undergoing drug-eluting stent (DES) implantation, the individual and combined impact of chronic kidney disease (CKD) and diabetes mellitus (DM) on outcomes is uncertain.
Aims: We sought to assess the impact of CKD and DM on prognosis in women after DES implantation.
Methods: We pooled patient-level data on women from 26 randomised controlled trials comparing stent types. Women receiving DES were stratified into 4 groups based on CKD (defined as creatine clearance <60 mL/min) and DM status. The primary outcome at 3 years after percutaneous coronary intervention was the composite of all-cause death or myocardial infarction (MI); secondary outcomes included cardiac death, stent thrombosis and target lesion revascularisation.
Results: Among 4,269 women, 1,822 (42.7%) had no CKD/DM, 978 (22.9%) had CKD alone, 981 (23.0%) had DM alone, and 488 (11.4%) had both conditions. The risk of all-cause death or MI was not increased in women with CKD alone (adjusted hazard ratio [adj. HR] 1.19, 95% confidence interval [CI]: 0.88-1.61) nor DM alone (adj. HR 1.27, 95% CI: 0.94-1.70), but was significantly higher in women with both conditions (adj. HR 2.64, 95% CI: 1.95-3.56; interaction p-value <0.001). CKD and DM in combination were associated with an increased risk of all secondary outcomes, whereas alone, each condition was only associated with all-cause death and cardiac death.
Conclusions: Among women receiving DES, the combined presence of CKD and DM was associated with a higher risk of the composite of death or MI and of any secondary outcome, whereas alone, each condition was associated with an increase in all-cause and cardiac death.
Introduction
Chronic kidney disease (CKD) and diabetes mellitus (DM) are 2 common comorbid conditions in patients undergoing percutaneous coronary intervention (PCI) and are associated with increased morbidity and mortality, especially when both are present123. Among CKD patients, concomitant DM is frequent and one of the main causes of CKD4. Additionally, patients with DM frequently develop CKD due to DM-related microvascular changes within the kidney, an entity referred to as diabetic kidney disease5.
Women undergoing PCI sustain poorer clinical outcomes than men, due to their older age and a higher burden of comorbidities, including CKD and DM6. Prior studies assessing how the presence of DM and CKD affect cardiovascular outcomes in women undergoing PCI have considered the prognostic impact of these conditions separately and not in combination78910.
The WIN-DES (Women in Innovation and Drug-Eluting Stents) database was formed by pooling patient-level data of women treated with coronary stents from 26 randomised controlled trials (RCTs) to obtain new sex-specific evidence concerning drug-eluting stents (DES) and to address the underrepresentation of women in RCTs11.
In this study, we sought to examine the individual as well as the combined effects of CKD and DM on adverse events among women undergoing PCI included in this large pooled dataset.
Methods
Study population
The study design and rationale of the WIN-DES database have been previously described11. In summary, patient-level data of female participants from 26 randomised trials comparing different stent types (bare metal stent [BMS], first-generation or current-generation drug-eluting stents [DES]) in patients with acute coronary syndrome (ACS) or chronic coronary syndrome (CCS) undergoing PCI were pooled. Characteristics of the included trials are described in Supplementary Table 1. Dual antiplatelet therapy (DAPT) duration ranged from 2 to 24 months. All included studies complied with the provisions of the Declaration of Helsinki, and the institutional review board at each site approved the study protocol. All patients provided written informed consent before enrolment in each study.
For the purpose of the present analysis, women who received either first- or current-generation DES were stratified into 4 groups based on the presence of CKD and DM at baseline: 1) without CKD/DM, 2) with CKD alone, 3) with DM alone, and 4) with both CKD and DM. CKD was defined as baseline creatinine clearance (CrCl) <60 mL/min/1.73 m2 at the time of enrolment, according to the Kidney Disease Improving Global Outcomes (KDIGO) definition12. Patients were labelled as diabetic if this condition was present at baseline and irrespective of the type of antidiabetic treatment. Patients who received BMS or had no available data on baseline CrCl or DM were excluded.
Clinical outcomes
The primary outcome was the composite of all-cause death or myocardial infarction (MI) at 3-year follow-up after index PCI. Secondary outcomes included the individual components of the primary outcome, cardiac death, definite or probable stent thrombosis (ST), and target lesion revascularisation (TLR). The endpoint definitions used in each included study are listed in Supplementary Table 2.
Statistical analysis
A prespecified extraction sheet was used for the collection of patient-level data from all 26 randomised trials. Baseline clinical and procedural characteristics were compared across all 4 groups using the chi-square test for categorical variables and the Student’s t-test for continuous variables. The Kaplan-Meier method was used to estimate the cumulative event rates for the primary and secondary endpoints, and the log-rank test was used to compare the event rates across the 4 groups. Multivariable Cox proportional hazards regression models were used to calculate the risk of primary and secondary endpoints using patients without DM and CKD as the reference group. The models included a frailty term (γ) to assess random effects in the trials1314. Risks were expressed as hazard ratios (HR) and 95% confidence intervals (CI). The multivariable model was adjusted for the following clinically relevant characteristics: age, body mass index (BMI), hypertension, hypercholesterolaemia, prior MI, prior revascularisation (including PCI or coronary artery bypass graft [CABG] surgery), indication for PCI (CCS vs ACS), stent type (first- vs current-generation DES), number of stents implanted, and multivessel disease. A p-value for interaction between DM/CKD status was calculated. The total follow-up time was defined as the time from index procedure until death or last follow-up date (whichever occurred first). In addition, a sensitivity analysis was performed to assess risks associated with CKD or DM individually according to their severity (based on CrCl and on concomitant insulin treatment, respectively). We reported 2-sided p-values and considered p-values <0.05 to be significant. The consistency of the effect across the 4 groups was assessed with an interaction test. All analyses were performed with STATA version 16.0 (Stata Corp).
Results
Study population
A total of 11,557 women were included in the pooled dataset of 26 RCTs. After exclusion of 1,108 patients who received BMS and 6,180 with missing data on CrCl or DM, 4,269 women were included in this analysis (Figure 1). The mean age was 67.7±10.5 years, 45.1% presented with ACS, 27.3% had multivessel disease, and 50.6% received a newer-generation DES. Within the included population, 1,822 (42.7%) had no CKD/DM, 978 (22.9%) had CKD alone, 981 (23.0%) had DM alone, and 488 (11.4%) had both CKD and DM. The prevalence of insulin-dependent DM and CKD severity were similar in the groups that included DM or CKD patients, respectively (Table 1).
Women with CKD were on average 10 years older, with lower BMI and higher creatinine values irrespective of concomitant DM. As compared to the group without CKD/DM, women with CKD and/or DM were more likely to have hypertension, hypercholesterolaemia, established coronary artery disease (i.e., prior MI, prior PCI or prior CABG), multivessel disease, type B2 or C lesions or lesions with moderate or severe calcification, and to have a greater total stent length implanted, particularly in women with both CKD and DM (Table 1). Presentation with CCS at the time of index PCI was more common in diabetic patients with or without CKD. Current-generation DES were implanted in about 50% of women across the 4 groups.
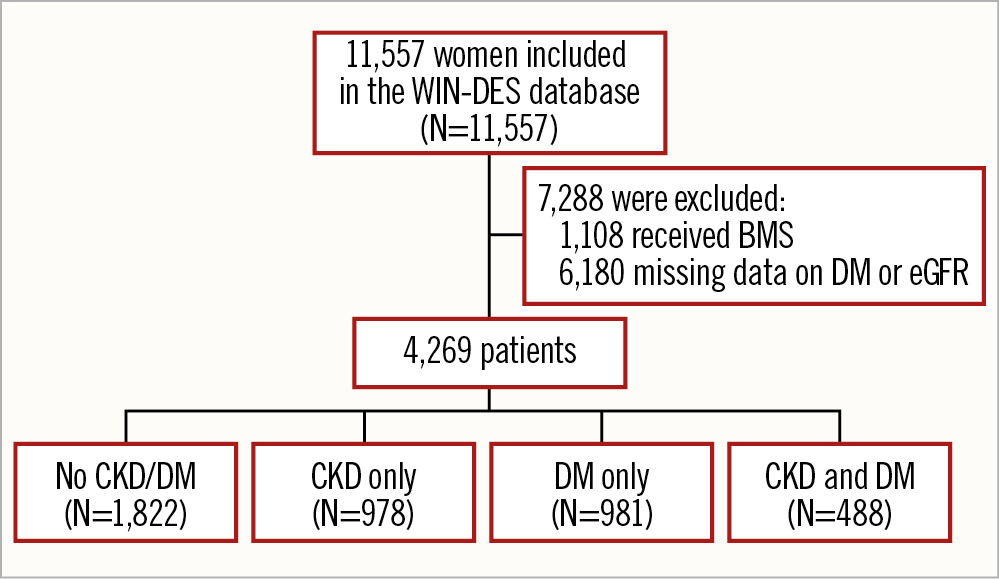
Figure 1. Patient flowchart. BMS: bare metal stent; CKD: chronic kidney disease; DM: diabetes mellitus; eGFR: estimated glomerular filtration rate; WIN-DES: Women in Innovation and Drug-Eluting Stents
Table 1. Baseline and procedural characteristics.
| Variable | No DM/CKD n=1,822 | CKD onlyn=978 | DM onlyn=981 | DM and CKD n=488 | p-value |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Age, years | 63.8±9.9 | 75.1±7.8 | 64.2±9.3 | 74.6±8.3 | <0.01 |
| BMI, kg/m2 | 28.2±5.2 | 24.4±4.1 | 31.0±6.2 | 26.3±5.0 | <0.01 |
| Hypertension | 1,227 (67.3) | 778 (79.6) | 831 (84.7) | 438 (89.8) | <0.01 |
| Hypercholesterolaemia | 1,208 (66.3) | 645 (66) | 740 (75.4) | 346 (70.9) | <0.01 |
| Insulin-dependent DM | 0 (0) | 0 (0) | 307 (31.3) | 161 (33.0) | <0.01 |
| Smoking | 793 (43.5) | 259 (26.5) | 313 (31.9) | 81 (16.6) | <0.01 |
| Family history of CAD | 697 (39.3) | 263 (28.1) | 321 (34.3) | 104 (22.7) | <0.01 |
| Prior MI | 323 (17.7) | 209 (21.4) | 218 (22.2) | 123 (25.2) | <0.01 |
| Prior PCI | 277 (15.2) | 204 (20.9) | 240 (24.5) | 160 (32.8) | <0.01 |
| Prior CABG | 54 (3.0) | 63 (6.4) | 68 (6.9) | 38 (7.8) | <0.01 |
| Creatinine, mg/dL | 0.8±0.2 | 1.2±1.0 | 0.8±0.2 | 1.5±1.5 | <0.01 |
| Chronic kidney disease | 0 (0) | 978 (100) | 0 (0) | 488 (100) | <0.01 |
| Mild (CrCl 45-59 mL/min) | 0 (0) | 565 (57.8) | 0 (0) | 246 (50.4) | <0.01 |
| Moderate-severe (CrCl <45 mL/min) | 0 (0) | 381 (39.0) | 0 (0) | 222 (45.5) | <0.01 |
| LVEF, % | 52.5±23.2 | 54.9±18.0 | 54.2±19.6 | 53.0±17.8 | 0.06 |
| Indication for PCI | 0.04 | ||||
| ACS | 835 (46.5) | 447 (47) | 404 (42.7) | 195 (41.2) | |
| CCS | 960 (53.5) | 505 (53) | 543 (57.3) | 278 (58.8) | |
| Procedural characteristics | |||||
| Multivessel disease | 379 (20.8) | 292 (29.9) | 285 (29.1) | 209 (42.8) | <0.01 |
| Lesions per patient | 1.3±0.6 | 1.3±0.6 | 1.3±0.6 | 1.4±0.7 | <0.01 |
| Number of stents per patient | 1.5±0.9 | 1.5±0.9 | 1.5±0.9 | 1.6±1.0 | <0.01 |
| Mean stent diameter, mm | 3.0±0.4 | 2.9±0.4 | 2.9±0.4 | 2.9±0.4 | <0.01 |
| Mean stent length, mm | 29.0±19.8 | 29.9±19.5 | 29.8±19.0 | 33.2±22.7 | <0.01 |
| Moderate/severe calcification | 410 (24.2) | 283 (33.5) | 246 (26.8) | 139 (32.3) | <0.01 |
| At least one type B2 or C lesion | 1,042 (58.1) | 635 (66.5) | 602 (62.6) | 344 (72.7) | <0.01 |
| At least one bifurcation lesion | 120 (22.3) | 72 (20.1) | 81 (23.0) | 46 (21.0) | 0.78 |
| Type of stent implanted | |||||
| First-generation DES | 870 (47.7) | 506 (51.7) | 491 (50.1) | 244 (50.0) | 0.23 |
| Current-generation DES | 952 (52.3) | 472 (48.3) | 490 (49.9) | 244 (50.0) | |
| Data are presented as n (%) or mean±SD. ACS: acute coronary syndrome; BMI: body mass index; CABG: coronary artery bypass graft; CAD: coronary artery disease; CCS: chronic coronary syndrome; CKD: chronic kidney disease; CrCl: creatinine clearance; DES: drug-eluting stent; DM: diabetes mellitus; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation | |||||
Clinical outcomes at 3-year follow-up
In the transition from patients without CKD/DM to those with CKD or DM alone and to patients with both these conditions, a significant stepwise increase in 3-year rates was observed for all-cause death or MI (7.2%, 11.9%, 8.8% and 23.4%, respectively; p<0.001) (Figure 2), all-cause death (2.4%, 7.6%, 4.5%, 15.7%; p<0.001) (Figure 3) and cardiac death (1.2%, 4.0%, 2.3%, 10.1%; p<0.001). Rates of MI were similar between women without CKD/DM (5.1%), with CKD only (5.7%) and DM only (5.4%) but significantly higher in patients with CKD and DM (12.4%) (Figure 3). Similarly, definite or probable ST was generally rare in patients without CKD or DM (1.0%) or with only one of these conditions (CKD only: 0.9%; DM only: 1.6%) but significantly increased in patients with both CKD and DM (4.0%). TLR occurred in 5.0% of women without CKD/DM or with CKD only; however, the incidence of TLR was significantly increased in patients with DM only (9.1%) and those with both CKD and DM (9.4%) (Figure 4).
After multivariable adjustment for potential confounders, DM and CKD in combination were independently associated with an increased risk of all-cause death or MI (HR 2.64, 95% CI: 1.95-3.56; p<0.001), MI (HR 2.00, 95% CI: 1.36-2.94; p<0.001) and definite or probable ST (HR 3.28, 95% CI: 1.51-7.16; p=0.003) at 3 years (Figure 4). The risk of all-cause death and cardiac death was increased by about 2-fold in the presence of CKD or DM alone and by about 5- and 6-fold, respectively, in women with both conditions. The risk of TLR was enhanced in women with DM, irrespective of the concomitant presence of CKD. The increase in all-cause or cardiac death associated with CKD alone was largely determined by women with moderate/severe CKD (Supplementary Table 3). The risks associated with DM alone were consistent, irrespective of concomitant insulin treatment (Supplementary Table 4).
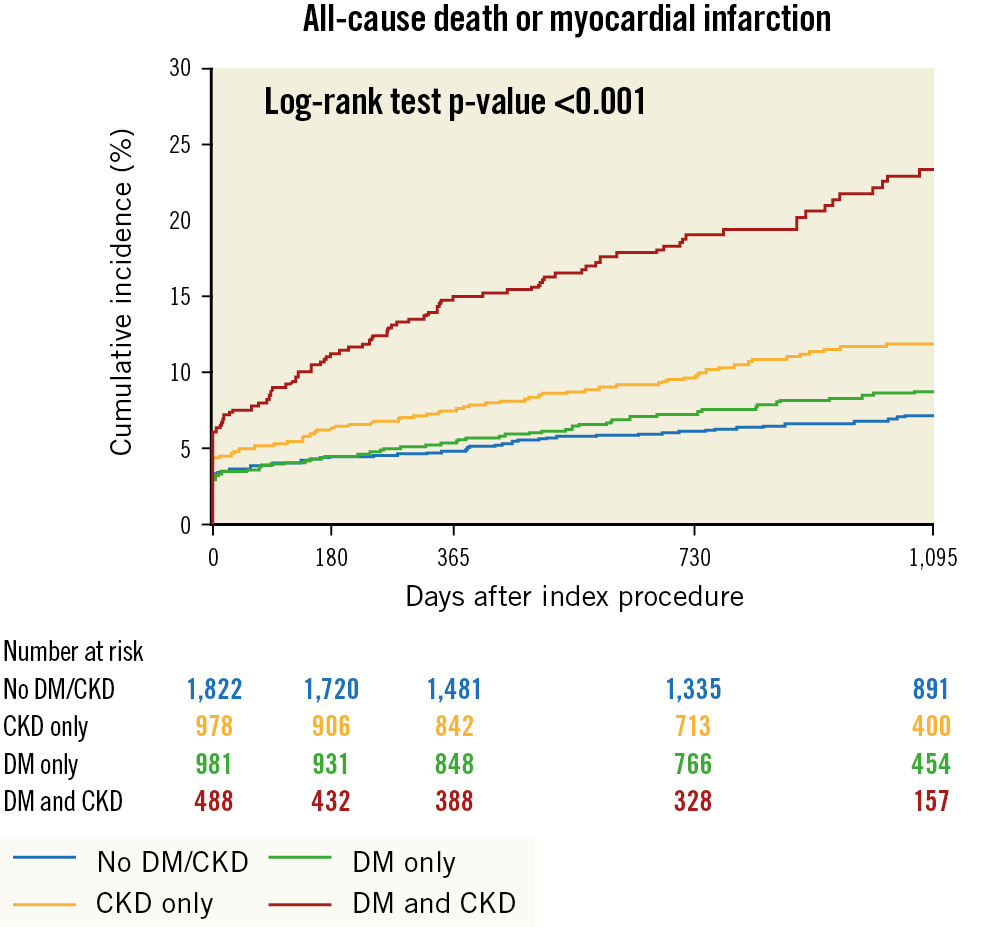
Figure 2. Kaplan-Meier curves for all-cause death or myocardial infarction at 3 years. CKD: chronic kidney disease; DM: diabetes mellitus
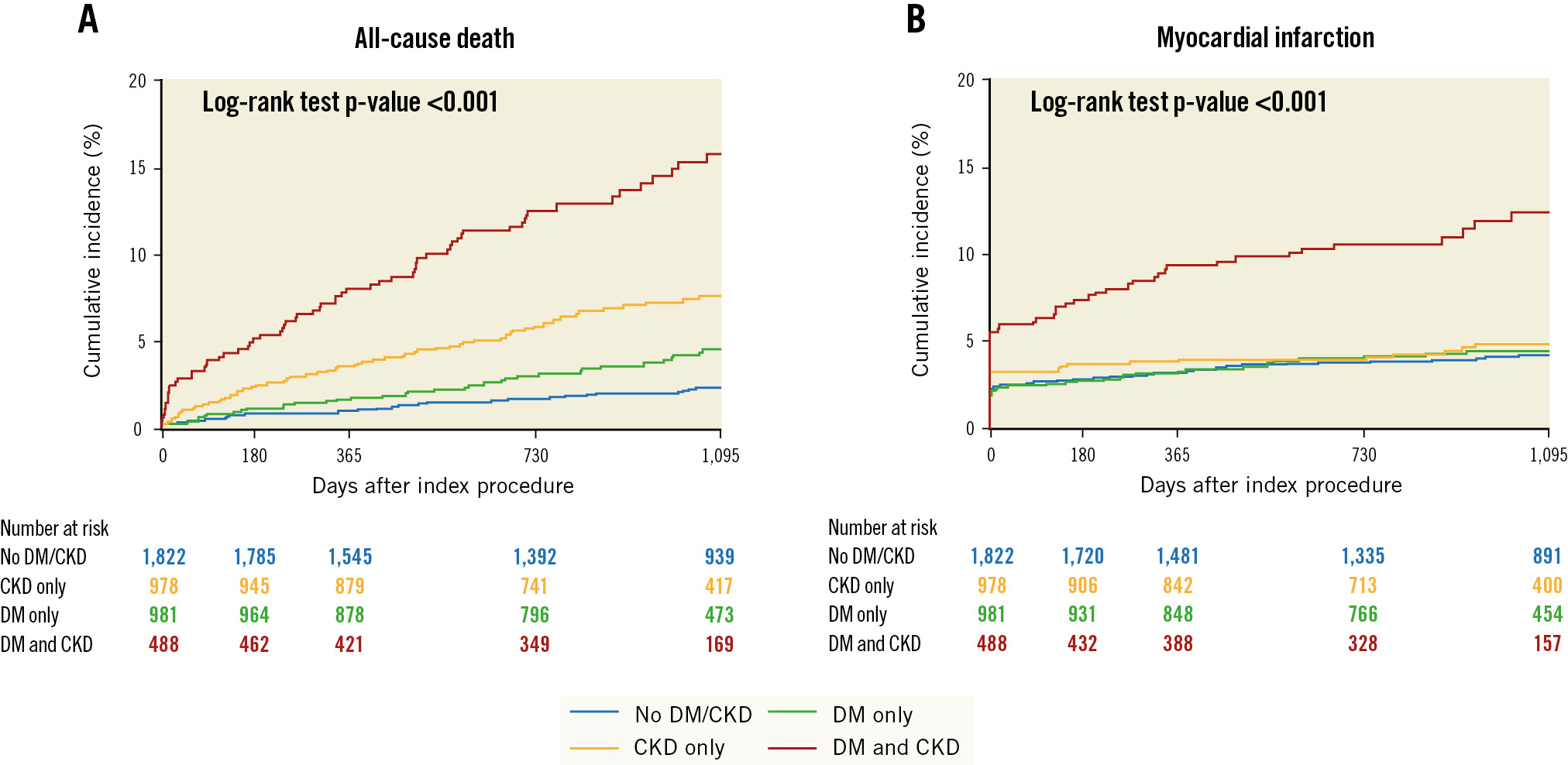
Figure 3. Kaplan-Meier curves for 3-year outcomes. Kaplan-Meier curves for all-cause death (A) and myocardial infarction (B). CKD: chronic kidney disease; DM: diabetes mellitus
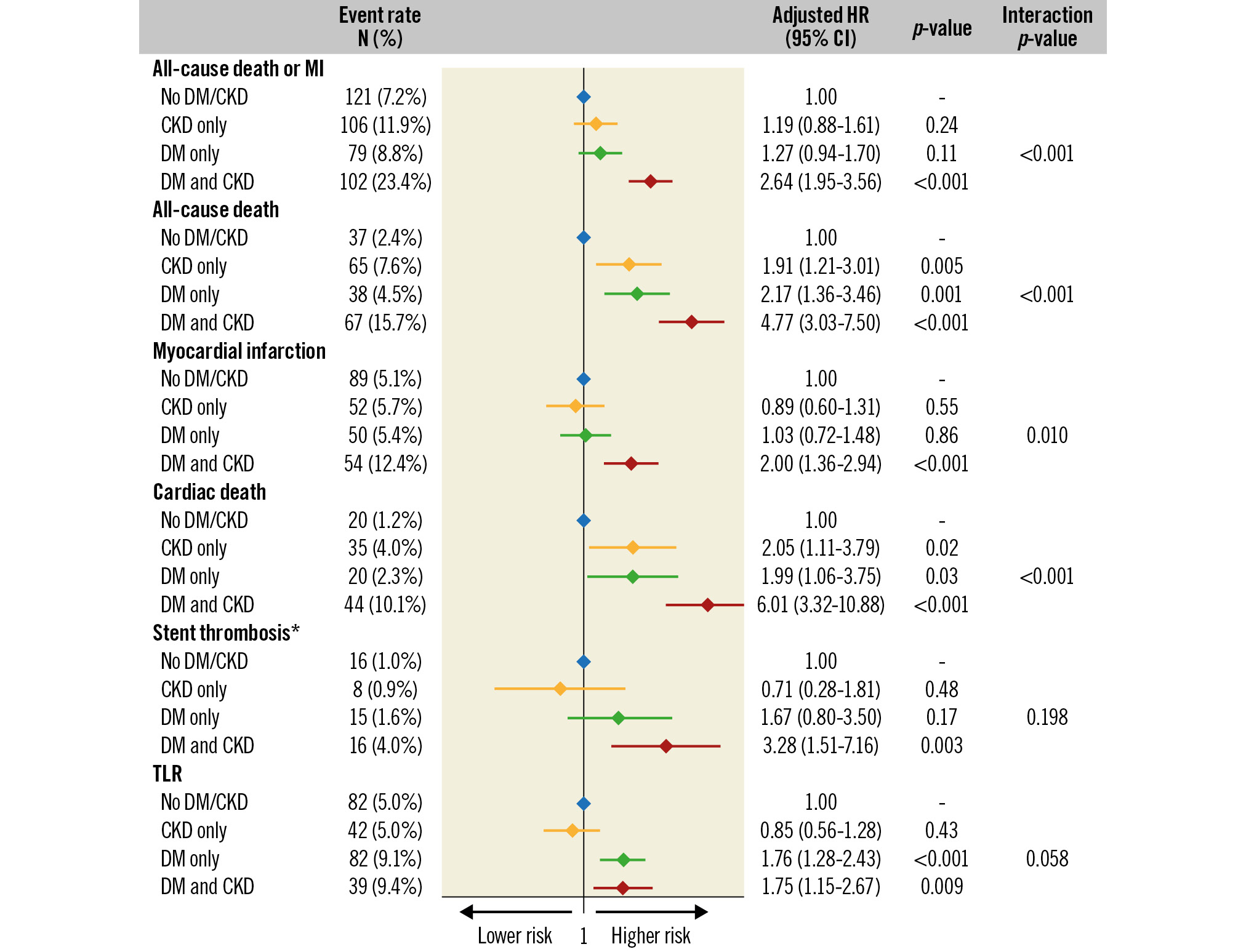
Figure 4. Adjusted risk of adverse events at 3 years. *definite or probable. CI: confidence interval; CKD: chronic kidney disease; DM: diabetes mellitus; HR: hazard ratio; MI: myocardial infarction; TLR: target lesion revascularisation
Discussion
In this pooled analysis of patient-level data of 4,269 women from 26 randomised coronary stent trials, we evaluated the independent and combined impact of DM and CKD on adverse outcomes at 3 years after PCI.
The key findings for women undergoing PCI are as follows:
1) more than 50% had CKD or DM, and approximately 11% had both these conditions;
2) CKD and DM in combination were associated with an increased risk of death or MI and of any secondary outcomes;
3) CKD and DM in isolation predicted a higher risk of all-cause death and cardiac death;
4) DM was related to an increased risk of TLR irrespective of the CKD status.
Although the individual effect of DM and CKD on cardiac complications following PCI has been well demonstrated in women-specific cohorts, to date no report has examined the joint prognostic impact of these 2 conditions78910.
The prevalence of DM and CKD in combination in our women-only cohort (11.4%) was in keeping with that reported in prior male-predominant observational studies (10-22%)2315 and slightly higher than in previous RCTs (5-8%)161718, in which no more than 30% of participants were women. As previously observed, CKD patients were on average 10 years older than those without CKD31517. Women with CKD, DM or both conditions had a higher burden of comorbidities and were more likely to present with multivessel disease or complex lesions. The combination of CKD and DM conferred a significantly higher risk of the composite of all-cause death or MI and its individual components, of cardiac death, definite or probable ST and TLR at 3 years. Conversely, CKD or DM individually were associated with a 2-fold greater risk of all-cause or cardiac death, but were not related to an increased risk of the primary outcome, MI or ST. In women with DM, the hazard of TLR was significantly increased, irrespective of concomitant CKD status. The risks associated with CKD were largely determined by women with moderate/severe CKD.
Our results suggest that poor outcomes in women with either CKD or DM are largely driven by those with both coexisting conditions78910. The additive effect of CKD and DM observed in the present study is consistent with previous observational reports and RCTs enrolling predominantly men2315161718. In addition, pharmacodynamic studies revealed that the combination of DM and CKD confers a synergistic effect on residual platelet reactivity when compared to either condition alone1920.
Conversely, the prognostic effect of CKD alone and DM alone noted in the present women-specific analysis partly differs from previous studies that included mostly men. Indeed, in the present study, CKD alone as compared to DM alone was associated with higher crude rates of all-cause death (7.6% vs 4.5%) and cardiac death (4.0% vs 2.3%); however, after adjustment for baseline imbalances (including age), both CKD and DM were associated with a similar risk increase (about 2-fold) for these two outcomes. These findings are in keeping with one previous RCT that provided adjusted risks for adverse outcomes16 but are in contrast with observational studies in which CKD remained, after adjustment, a more relevant predictor of mortality than DM2315. Additionally, in the present analysis, DM alone was related to a higher risk of ST and TLR than CKD alone, whereas in previous observational studies, CKD alone and DM alone had a similar impact on these complications2315.
These discrepancies are mostly explained by the exclusion of CKD patients with advanced disease (i.e., glomerular filtration rate [GFR] <30 mL/min) from most of the RCTs, in which the CKD groups had a better prognosis. In the present study, moderate and severe CKD (i.e., GFR <45 mL/min) was associated with a higher mortality than mild CKD (i.e., GFR 45-59 mL/min) or DM alone. These differences in prior non-women-specific studies might also be due to a different prognostic impact of CKD and DM in women, but the mechanism is unclear.
In aggregate, our observations suggest that even though DM and CKD are interrelated and share common pathophysiological mechanisms that lead to accelerated atherosclerosis and enhanced blood thrombogenicity2122, DM alone might induce a faster progression of coronary artery disease (CAD) and be a stronger risk factor for stent-related complications than CKD alone. However, since the risks of MI and ST were not significantly increased in women with CKD or DM alone, the higher all-cause and cardiac mortality in these patients is probably explained by sudden cardiac death, heart failure or non-cardiac causes. Among CKD patients, anaemia, volume overload, and hypertension may also have a relevant impact on mortality2324.
Optimal medical therapy together with percutaneous or – especially in diabetic patients with extended CAD – surgical coronary revascularisation have been demonstrated to decrease morbidity and mortality related to CAD, irrespective of concomitant DM or CKD2526. A recent analysis of the TWILIGHT trial showed that ticagrelor monotherapy results in fewer bleeding complications without increasing ischaemic risk in patients with CKD, DM or both conditions16. Other interventions that attenuate the risks of adverse events in patients with CKD or DM include lipid-lowering agents, sodium-glucose cotransporter-2 inhibitors2728, as well as the use of new stent technologies like the ABLUMINUS DES+ (Concept Medical; a biodegradable polymer sirolimus-eluting stent)29.
In summary, our study provides valuable insights on how the prognosis of women undergoing PCI is affected by the interaction between DM and CKD and highlights the utility of stratifying women according to these 2 comorbidities for decision-making on therapy and further management.
Limitations
The study findings should be interpreted in light of the following limitations. First, since data were pooled from 26 RCTs mostly excluding patients with severe CKD, the prognostic impact of this condition might have been underestimated. Secondly, even if we had included "trial" as a random effect in our adjusted analyses, differences between the pooled RCTs concerning study design, inclusion/exclusion criteria, follow-up duration and enrolment at different time periods might have affected the results. Thirdly, despite adjusting for several clinically relevant baseline characteristics, residual confounding may still exist. For example, data on the type or duration of antithrombotic therapy, as well as adherence during follow-up, were lacking. Similarly, some confounders that reflect disease severity (e.g., haemoglobin A1C in DM) were not available. Of note, about 50% of the women included received a first-generation DES, devices that are no longer used in contemporary practice given their inferior safety and efficacy as compared to the current-generation stents. Therefore, the absolute rates of adverse events might be slightly higher than expected because of the use of first-generation DES. However, it is unlikely that the CKD- and DM-related risks have been significantly biased by the high proportion of these devices, the use of which was similar across the 4 CKD/DM categories. Furthermore, data on bleeding events were not available. Finally, these results apply only to women undergoing PCI with DES implantation.
Conclusions
Among women receiving DES, the combined presence of CKD and DM was associated with a higher risk of the composite of death or MI and of any secondary outcome. Each of these conditions alone increased the risk of all-cause and cardiac death, but had no impact on MI or ST. In addition, DM alone enhanced the risk of TLR.
The presence or absence of DM and CKD provides valuable information on the risk of adverse events in women undergoing PCI.
Impact on daily practice
Among women receiving drug-eluting stents (DES), the combination of CKD and DM was associated with a higher risk of all-cause death, cardiac death, MI, ST or TLR. CKD or DM alone were associated with a higher risk of all-cause and cardiac death, but were not predictors of MI or ST. DM alone increased the risk of TLR. The presence or absence of DM and CKD provides valuable information on the risk of adverse events in women undergoing DES implantation.
Conflict of interest statement
A. Spirito received a research grant from the Swiss National Science Foundation (SNSF). R.V. Jeger reports research and educational grants to the institution from Abbott, Amgen, AstraZeneca, Bayer, Biosense Webster, B. Braun Melsungen AG, Biotronik, Boston Scientific, Bristol-Myers Squibb, Cordis, Daiichi Sankyo, Edwards Lifesciences, GE Medical Systems, MCM Medsys, Medtronic, Novartis, Pfizer, Terumo, and Vascular Medical GmbH. G.W. Stone has received speaker honoraria from Medtronic, Pulnovo, Infraredx, Abiomed, and Abbott; has served as a consultant to Daiichi Sankyo, Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Ancora, Elucid Bio, Occlutech, CorFlow, Apollo Therapeutics, Impulse Dynamics, Cardiomech, Gore, Amgen, Adona Medical, and Millennia Biopharma; and has equity/options from Ancora, Cagent, Applied Therapeutics, the Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, and Xenter; his daughter is an employee at IQVIA. G.W. Stone’s employer, Mount Sinai Hospital, receives research support from Abbott, Abiomed, Bioventrix, Cardiovascular Systems Inc, Philips, Biosense-Webster, Shockwave, Vascular Dynamics, Pulnovo, and V-Wave. P.G. Steg reports receiving research grants from Amarin, Bayer, Sanofi, and Servier; compensation for work on clinical trials (steering committee, clinical events committee or data safety and monitoring board) from Amarin, AstraZeneca, Bayer, Bristol-Myers Squibb, Idorsia, Novartis, PhaseBio, Pfizer, Sanofi, and Servier; for consulting or speaking from Amarin, Amgen, BMS/Myokardia, Merck, Novo Nordisk, and Regeneron; and is a Senior Associate Editor at Circulation. S. Windecker reports research, travel or educational grants to the institution from Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardinal Health, CardioValve, CorFlow Therapeutics, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Guerbet, InfraRedx, Janssen-Cilag, Johnson & Johnson, Medicure, Medtronic, Merck Sharp & Dohm, Miracor Medical, Novartis, Novo Nordisk, Organon, OrPha Suisse, Pfizer, Polares, Regeneron, Sanofi-Aventis, Servier, Sinomed, Terumo, Vifor, and V-Wave; and serves as advisory board member and/or member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Boston Scientific, Biotronik, Bristol-Myers Squibb, Edwards Lifesciences, Janssen, MedAlliance, Medtronic, Novartis, Polares, Recardio, Sinomed, Terumo, V-Wave, and Xeltis, with payments to the institution but no personal payments; he is also member of the steering/executive committee group of several investigator-initiated trials that receive funding from industry without impact on his personal remuneration. W. Wijns received institutional research grants and honoraria from MicroPort (Steering Committee TARGET-AC trial); is co-founder of Argonauts, an innovation facilitator; and is a medical adviser to Rede Optimus Research and Corrib Core Laboratory, NUI Galway. P.W. Serruys reports consultancy payments from Meril Life Sciences, Novartis, Philips, SMT, and Xeltic. M. Valgimigli reports grants and personal fees from Terumo; and personal fees from Abbott Vascular, Alvimedica/CID, AstraZeneca, Bayer, Biotronik, Boston Scientific, Bristol-Myers Squibb, Chiesi, CorFlow, Daiichi Sankyo, Idorsia Pharmaceuticals, Medtronic, Novartis, PhaseBio, Universität Basel (Department of Klinische Forschung), Vesalio, and Vifor. M-C. Morice is CEO and a shareholder of CERC (a CRO based in Massy, France) and also owns shares in Electroducer. B. Pileggi received research grant from the Brazilian Federal Foundation for Support and Evaluation of Graduate Education (CAPES). G. Weisz is a member of the medical advisory board for Filterlex, Intratech, Microbot, and Trisol; received equity from Filterlex, Intratech, and Microbot; and consulting fees from Cuspa, Filterlex, Intratech, Magenta, and Microbot. D.E. Kandzari reports institutional research/grant support from Ablative Solutions and Medtronic; and personal consulting honoraria from Medtronic. A. Kastrati is listed as an inventor in the patent application ─ PCT/EP2021/053116 “Calcium channel TRPC6 inhibitors using balloons, stents or other medical devices” ─ from his institution. C. von Birgelen reports institutional research grants (Thoraxcentrum Twente) from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. G.D. Dangas reports institutional research grants from Abbott Laboratories, AstraZeneca, Bayer, Boston Scientific, Medtronic, and Daiichi Sankyo; consultant fees from Biosensors and Boston Scientific; and speaker honoraria from Chiesi. P.C. Smits received institutional grants from Abbott Vascular, SMT and MicroPort; and received consultancy or speakers fees from Abbott Vascular, Abiomed, MicroPort, Terumo, Daiichi Sankyo, and AstraZeneca. T. Kimura reports research grants from Abbott Vascular and Boston Scientific. G.W. Mikhail is Course Director of the Imperial Valve and Cardiovascular Course (IVCC) to which Edwards Lifesciences is a major financial sponsor. A. Chieffo has received speaker fees from Abiomed and GADA. R. Mehran reports institutional research grants from Abbott, Abiomed, Applied Therapeutics, Arena, AstraZeneca, Bayer, Biosensors, Boston Scientific, Bristol-Myers Squibb, CardiaWave, CellAegis, CERC, Chiesi, Concept Medical, CSL Behring, DSI, Insel Gruppe AG, Medtronic, Novartis Pharmaceuticals, OrbusNeich, Philips, Transverse Medical, and Zoll; personal fees from ACC, Boston Scientific, California Institute for Regenerative Medicine (CIRM), Cine-Med Research, Janssen, WebMD, and SCAI; consulting fees paid to the institution from Abbott, Abiomed, AM-Pharma, Alleviant Medical, Bayer, Beth Israel Deaconess, CardiaWave, CeloNova, Chiesi, Concept Medical, DSI, Duke University, Idorsia Pharmaceuticals, Medtronic, Novartis, and Philips; has equity <1% in Applied Therapeutics, Elixir Medical, STEL, and CONTROLRAD (spouse); and serves on the scientific advisory board for AMA and Biosensors (spouse). The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

