Abstract
Aims: The DIABETES (DIABETes and sirolimus-Eluting Stent) trial is a prospective, multicentre, randomised, controlled trial aimed at demonstrating the efficacy of sirolimus-eluting stent (SES) as compared to bare metal stent (BMS) implantation in diabetic patients. The aim of the present analysis was to assess the five-year clinical follow-up of the patients included in this trial.
Methods and results: One hundred and sixty patients (222 lesions) were included: 80 patients were randomised to SES and 80 patients to BMS. Patients were eligible for the study if they were identified as non-insulin-dependent diabetics (NIDDM) or insulin-dependent diabetics (IDDM), with significant native coronary stenoses in ≥1 vessel. There was a sub-randomisation according to diabetes status. Clinical follow-up was extended up to five years. Five-year clinical follow-up was obtained in 96.2%. Overall, MACE at five years was significantly lower in the SES group as compared with the BMS arm, mainly due to a significant reduction in TLR. There were no significant differences in cardiac death or myocardial infarction (MI). This was also observed in both prespecified subgroups IDDM and NIDDM. In the SES group, the incidence density of definite/probable stent thrombosis was 0.53 per 100 person-years, whereas in the BMS group it was 0.8 per 100 person-years. Independent predictors of MACE were: SES implantation (p<0.001), multivessel stent implantation (p=0.04), and creatinine levels (p=0.001).
Conclusions: Five-year follow-up of the DIABETES trial suggests the effect of SES in reducing TLR is similar in both IDDM and NIDDM. No major safety concerns in terms of ST, MI or mortality were observed.
Introduction
Sirolimus-eluting stent (SES) implantation reduces the need for target vessel revascularisation in patients with coronary artery disease1. However, due to the antiproliferative effects of sirolimus and the pro-inflammatory effects of the polymer, long-term follow-up studies are warranted to rule out the presence of very late complications. In this regard, a meta-analysis2 showed a significant increase in mortality in diabetic patients treated with SES compared with those treated with bare metal stents (BMS). Recently, the five-year follow-up of the SIRIUS (Sirolimus-Eluting Stent in De-Novo Native Coronary Lesions) trial3 confirmed the sustained efficacy of SES in reducing the need for repeat revascularisation as compared to BMS with a similar safety profile. However, in the diabetic subgroup those treated with SES presented a twofold cumulative incidence of cardiac death as compared to those treated with BMS.
Long-term concerns after the use of SES include late stent thrombosis (ST) and late restenosis (namely “late catch-up” phenomenon). Overall, there is still a paucity of data regarding the long-term outcome of SES in diabetic patients. The DIABETES (DIABETes and sirolimus-Eluting Stent) trial4 was the first specifically designed to assess the performance of SES versus BMS in diabetics. We herein report the five-year follow-up of the patients included in this trial including the long-term outcome of patients with late stent malapposition detected by intravascular ultrasound (IVUS) at nine-month follow-up5.
Methods
PATIENT SELECTION
The DIABETES trial was a prospective, randomised, double-blind, controlled trial aimed at demonstrating the efficacy of DES vs. BMS in diabetic patients. The study design has been described previously4. In brief, 160 diabetics with one or more de novo stenoses were randomly assigned to receive either SES (Cypher™; Cordis, Johnson & Johnson, Warren, NJ, USA) or BMS (Bx Velocity/Sonic; Cordis, Johnson & Johnson) in a 1:1 ratio. Main exclusion criteria were diabetic patients treated with diet, acute ST-elevation myocardial infarction (MI), lesions located in the left main, saphenous or bypass graft, in-stent restenosis, true bifurcations, or patients treated with intracoronary brachytherapy. The study protocol was approved by the local ethics committee from each centre and all patients signed the informed consent. The study was initially designed with a follow-up of two years. However, due to concerns of potential ominous very late outcomes the investigators of the DIABETES trial decided to extend the clinical follow-up up to five years. This additional clinical follow-up was approved by the ethics committee of each centre. The main trial was investigator-initiated without any support from the industry. The extended follow-up was supported by an unrestricted grant from Cordis, J&J, Spain.
CLINICAL FOLLOW-UP
Clinical follow-up of the DIABETES trial was performed at one, nine, 12, 13 and 24 months4,6. An additional clinical visit was scheduled at five years. All data were sent to the coordinating centre (Hospital Clínico San Carlos, Madrid, Spain). All clinical data were reviewed and adjudicated by an independent clinical events committee blinded to the type of stent implanted.
ANGIOGRAPHIC AND IVUS DATA
All angiograms and IVUS studies were performed at the index procedure and at nine months follow-up as previously described4. All studies were analysed by an independent core lab (University of Florida, Jacksonville, FL, USA) which was blinded to the assigned treatment.
STUDY ENDPOINTS AND DEFINITIONS
The primary endpoint of the DIABETES trial was late lumen loss assessed by quantitative coronary analyses at nine-month follow-up. Secondary endpoints included other angiographic and IVUS data, and clinical follow-up at one, nine, 12, and 24 months4. For the purpose of this five-year analysis, we have assessed the incidence of major adverse cardiac events (MACE), the occurrence of very late ST, and the long-term outcomes of patients with stent malapposition diagnosed by IVUS at nine-month follow-up5.
MACE was defined as the composite of cardiac death, MI, and TLR (in-segment zone)4. Revascularisation due to atherosclerosis progression was defined as the need for revascularisation secondary to the appearance of new significant coronary stenoses, remote from the stent and both edges present only in the follow-up angiogram, accompanied by symptoms or evidence of ischaemia6.
For the purposes of this long-term follow-up study, ST was defined according to the Academic Research Consortium definition7. Incomplete stent apposition (ISA) was defined as ≥1 strut clearly separated from the vessel wall, with evidence of blood speckling behind the stent struts without overlapping side branches and was classified into persistent and late acquired5.
STATISTICAL ANALYSES
Continuous variables are expressed as mean±standard deviation and categorical variables as percentages. After the confirmation of the normal distribution with the Kolmogorov-Smirnov test, all comparisons between groups were performed by Student’s t-test for quantitative variables and Fisher’s exact test for categorical variables. In this particular study we have calculated the incidence density considering as the numerator the number of events and the sum of the person-time as denominator, expressed in years. The rate of survival free from MACE, TLR and MI during the five-year follow-up period was analysed with the use of Kaplan-Meier analyses, and the difference between rates was assessed by the log-rank test. The analysis of ST was performed as per intention-to-treat. However, for the purposes of this study, any ST that might have occurred in non-target stents implanted in non-target vessels before the patient was enrolled in the trial was excluded from this final analysis. Stratified analyses were performed to assess the clinical efficacy in the following prespecified variables: diabetes status, gender, left anterior descending artery, use of glycoprotein IIb/IIIa inhibitors, chronic total occlusion, lesion length, and stent size. To identify factors potentially associated with MACE, backward Cox regression models were used including those variables with a p<0.1 or those clinically relevant. The assumption of the proportional hazard was verified. The variables finally included in the model were: SES implantation, multivessel disease, multivessel stent implantation, left anterior descending, circumflex artery, serum creatinine levels, in-stent minimal luminal diameter after the procedure, and diabetes status. All statistical analyses were performed with the use of SPSS (version 15.0) or STATA (version 9.0) software, and all reported p-values were two-sided. We assumed significance at the 5% level (p<0.05).
Results
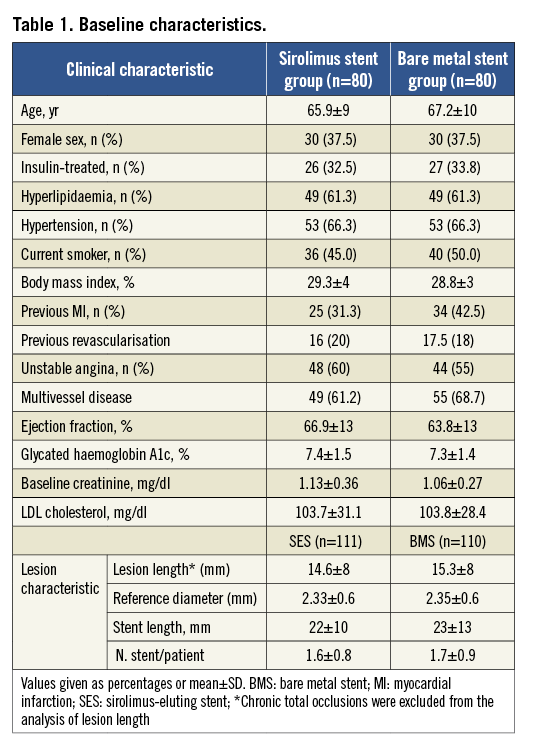
BASELINE CHARACTERISTICS
One hundred and sixty diabetic patients were included in the study. The flow diagram of the study population is depicted in Figure 1. Baseline clinical, angiographic and procedural characteristics were similar between groups (Table 1).
FIVE-YEAR MACE RATE
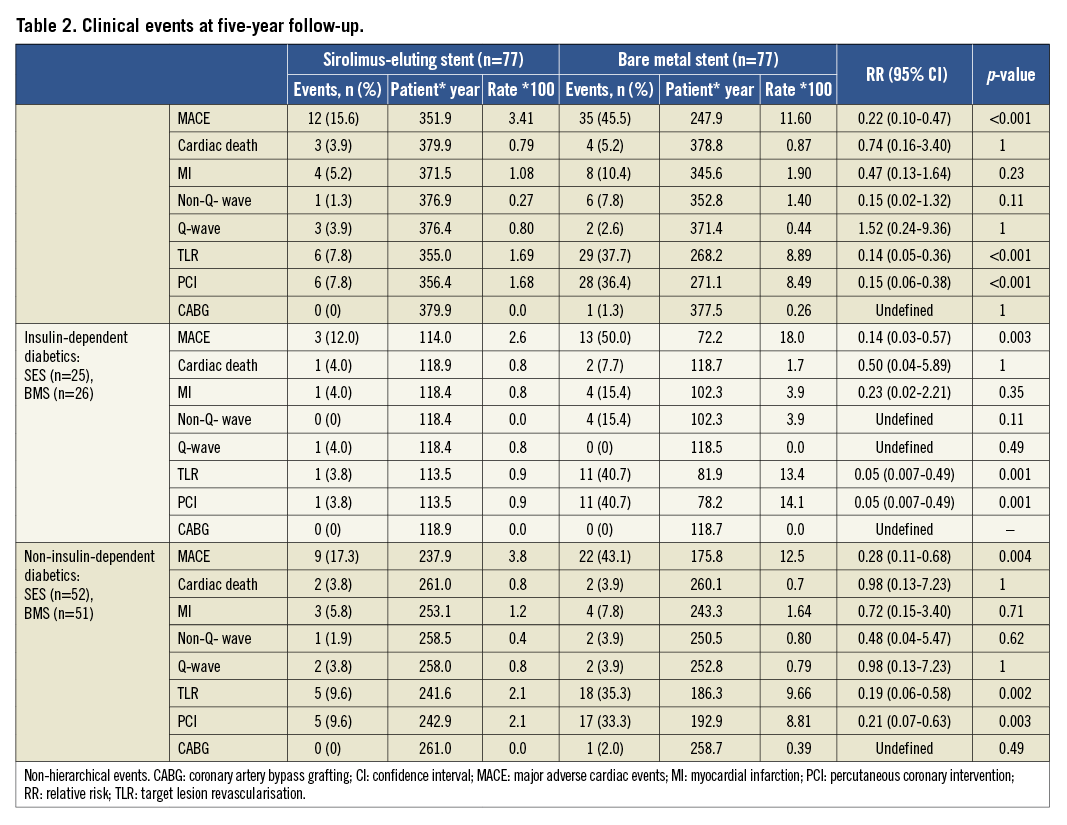
Five-year clinical follow-up (57±18 months) was obtained in 154 (96.2%) of the patients (Figure 1). Overall, MACE at five years was significantly lower in the SES group as compared with the BMS arm mainly due to a significant reduction in TLR. There were no significant differences in cardiac death and MI (Table 2).
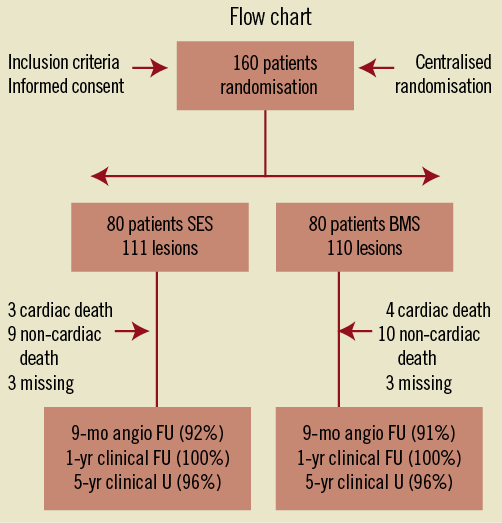
Figure 1. Flow chart of the study. The randomisation was centralised and a sub-randomisation by insulin or no insulin diabetics was performed.
At five years all patients were on aspirin alone. Only eight patients suffered an event between one and five-year follow-up (Table 3). Two patients from the SES arm presented sudden cardiac death. In the BMS group, one patient died due to end-stage heart failure and one patient died during cardiac surgery indicated for atherosclerosis progression in another segment. MI was observed in two patients in the SES group (both Q-wave MI due to ST) and in two patients in the BMS arm (one Q-wave MI due to stent thromboses and one spontaneous Q-wave MI due to atherosclerosis progression in another artery).
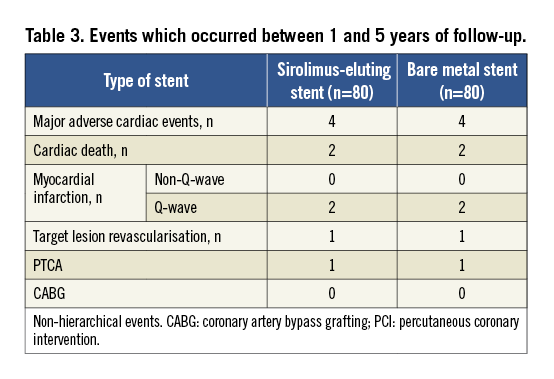
Overall, there was not a single TLR due to clinical restenosis (late catch-up). However, one patient in the SES group and one patient in the BMS arm underwent TLR in the setting of the treatment of a very late ST. All TLR were clinically driven but one was due to an extensive stent malapposition. Event-free survival curves are shown in Figure 2. The mean number of patients needing treatment with SES to prevent one TLR and one MACE was 13(11-19) and 15(12-26), respectively.
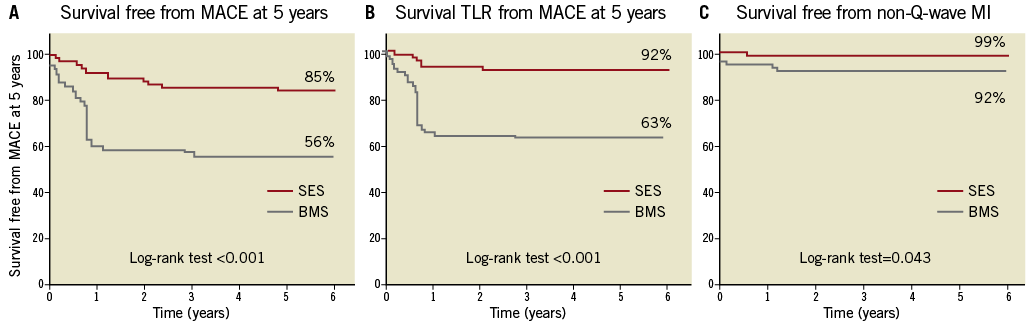
Figure 2. A) Actuarial rate of survival free from major adverse cardiac events at five years among patients treated with sirolimus-eluting stent (n=80) or bare metal stent (n=80). B) Actuarial rate of survival free from non-Q-wave myocardial infarction at five years. C) Actuarial rate of survival free from target lesion revascularisation at five years. The analyses were performed on a per patient basis. BMS: bare metal stent; SES: sirolimus-eluting stent
Factors independently associated with the occurrence of MACE at five years included SES implantation (OR: 0.13; 95% CI: 0.06-0.28; p<0.001), multivessel stent implantation (OR: 1.87; 95% CI: 1.02-3.44; p=0.04), and creatinine levels (OR: 2.43; 95% CI: 1.42-4.17; p=0.001).The cumulative rate of MACE at five years was significantly lower in all of the prespecified variables for subgroup analyses (Figure 3). Both insulin-dependent diabetics (IDDM) and non-insulin dependent diabetics (NIDDM) presented favourable results in terms of MACE and TLR after treatment with SES. Of note, the subgroup of IDDM treated with SES presented a significantly lower rate of non-Q-wave MI as compared with that of the BMS group (Table 2).
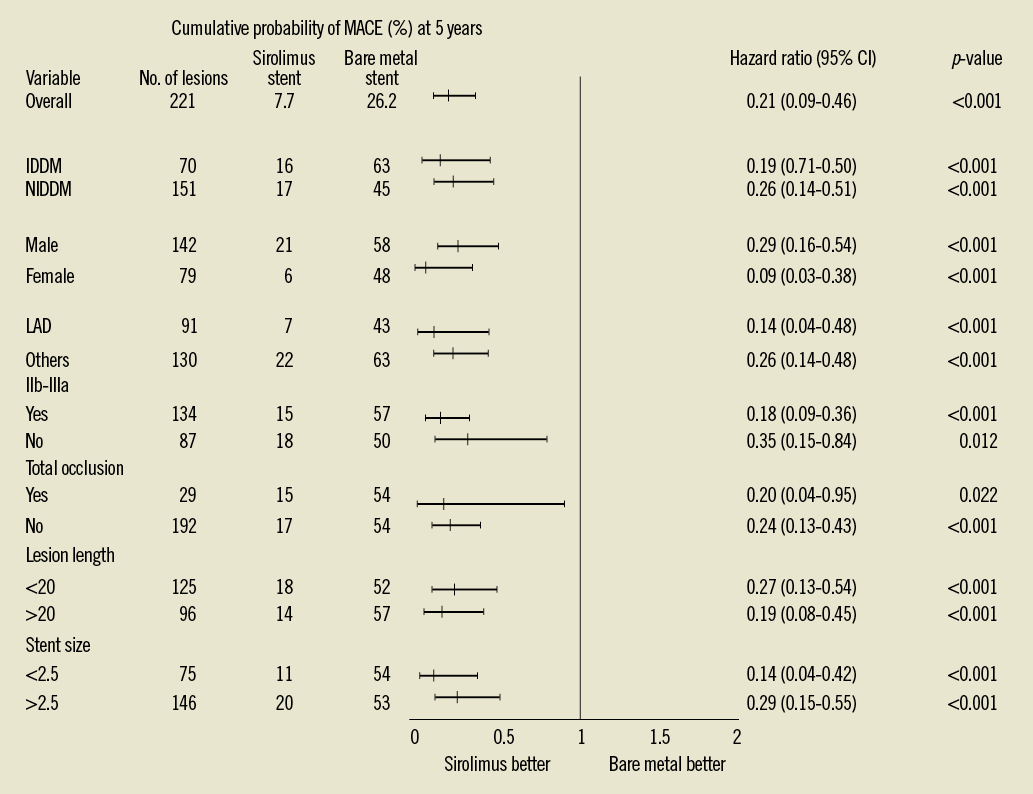
Figure 3. Cumulative probability of MACE and hazard ratios at 5 years for prespecified subgroups of patients. p<0.05 for all comparisons between sirolimus stent group and bare metal stent group. CI: confidence interval; MACE: major adverse cardiac events; IDDM: insulin-dependent diabetes mellitus; NIDDM: non-insulin-dependent diabetes mellitus; LAD: left anterior descending coronary artery; IIb-IIIa: IIb-IIIa glycoprotein inhibitors
FIVE-YEAR STENT THROMBOSIS RATE
The cumulative incidence of definite or probable ST was not different between groups (Table 4). One patient presented a definite ST due to the development of a coronary aneurysm at the site of the stent with a large ISA 15 months after the index procedure. In one patient, who had a terminal gastric cancer, possible ST occurred after cessation of aspirin because of continuous gastric bleeding, five years after stent implantation. Finally, two patients suffered from sudden cardiac death two years after the index procedure.
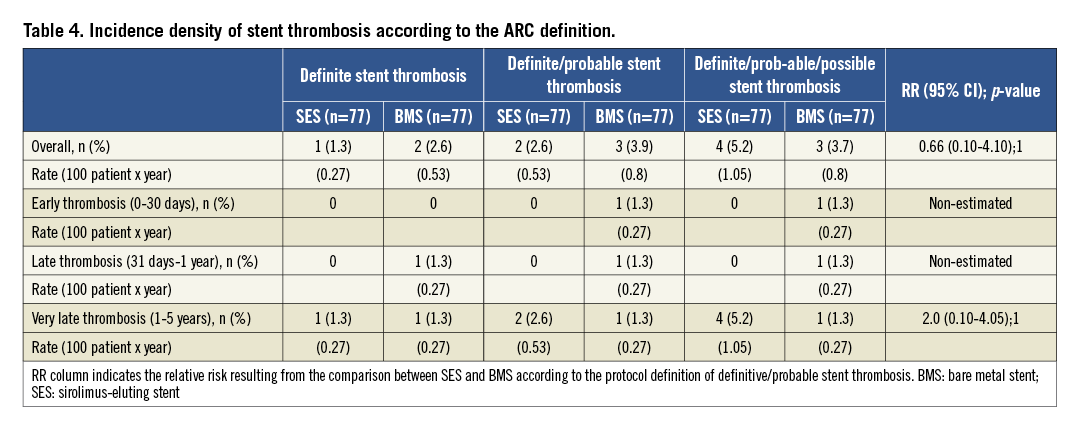
In the BMS group, one patient suffered from a definite thrombosis one week after clopidogrel withdrawal due to abdominal surgery two months after the index procedure, and one patient presented a sudden death 28 days after the stent implantation. In addition, one case of definite very late ST was recorded in this group. This patient did not have ISA or underexpansion on the IVUS study performed at nine months and was on aspirin at the time of thrombosis.
REVASCULARISATION DUE TO ATHEROSCLEROSIS PROGRESSION
At five years revascularisation due to atherosclerosis progression in the SES group was observed in seven patients (incidence density: 1.97 per 100 person-years) whereas the number was 15 patients (incidence density: 4.3 per 100 person-years) in the BMS group. Revascularisation for any cause, which included restenosis and progression of the atherosclerotic disease in other segments, was performed in 13 (3.94 per person-years) patients in the SES group and 36 (14.5 per person-years) patients in the BMS group. In addition, the progression of the atherosclerosis was the cause of MI in 33% of the patients.
LONG-TERM FOLLOW-UP OF PATIENTS WITH DOCUMENTED INCOMPLETE STENT APPOSITION
At nine-month follow-up, 24 patients (25 lesions) presented ISA assessed by IVUS (15 with late acquired ISA, all of them located in the SES group: p=0.001 and nine with persistent ISA), 22 patients in the SES group and two patients in the BMS group. DAPT was not prolonged beyond one year in patients with ISA. The baseline clinical, procedural and angiographic characteristics of patients with ISA were not significantly different compared to those without ISA. At five years, all patients with ISA were event-free except two who presented a sudden cardiac death two years after the index procedure. In addition, one patient had a non-clinically driven TLR based on the IVUS findings at nine months in the SES group. No events were observed in the two patients with ISA in the BMS arm.
Discussion
The main findings of the present study were: the overall reduction of MACE at five years by the use of SES with the absence of late catch-up phenomenon in diabetic patients treated with SES; the reassuring safety data in terms of ST; and the overall favourable long-term outcomes of patients in whom ISA was detected at the nine-month IVUS.
Previous reports have raised the concern that SES implantation in diabetic patients may be associated with an increase in mortality. In this regard, Spaulding et al2 performed a pooled analysis of four randomised trials evaluating the long-term safety of patients treated with SES compared with BMS. This study involved 787 patients treated with SES and 870 treated with BMS. The rate of death, MI or ST was comparable between the groups at four years. However, in the subgroup of diabetic patients (n=428; SES=195 and BMS=233) a significant increase in all-cause mortality was observed in patients treated with SES compared to those treated with BMS (87.8% vs. 95.6%; p=0.008). These data were somewhat rebutted in a more extended meta-analysis8 involving 3,852 diabetics, with similar rates of mortality and MI between DES and BMS when the duration of dual antiplatelet treatment was at least six months. Therefore, the mortality rate of diabetic patients treated with SES could be related to the short duration of dual antiplatelet treatment (two or three months) prescribed in the pivotal trials of drug-eluting stents (DES). Moreover, several non-randomised registries9,10 including more than 7,000 diabetic patients treated with DES confirmed our results, showing a clear benefit in terms of TLR and a similar risk or even a decrease in the risk of death and MI associated with DES.
Previous studies have suggested a limited efficacy of DES3,10 in the subgroup of IDDM. In the SIRIUS trial3, for instance, the restenosis rate of IDDM patients treated with SES was located mainly at the edges, and this was probably due to incomplete coverage by the stent of the segment treated with a balloon (i.e., geographical miss). In another registry10, the use of DES was not associated with a significant reduction in MACE at two years in the subgroup of IDDM. In contrast to the above-mentioned studies, in our study the clinical benefit was observed in both IDDM and NIDDM. In this regard, the relative reduction in TLR at five years was higher in the IDDM as compared with the NIDDM (90.9% vs.72.2%). Probably, the poor performance of the BMS used as comparator in the IDDM group may reinforce the benefit of SES. Previous studies have shown an inverse correlation between the vessel size and the restenosis rate after BMS implantation. In contrast, DES implantation may blunt this effect. In this regard, Stenestrand et al11 reported that the benefit of the treatment with DES in lesions located in small vessels is higher than in that of the large vessels. In accordance with these findings, vessel size in our study was rather small (mean value 2.35 mm)12. Furthermore, the vessel size of IDDM patients included in our study was even smaller than that of NIDDM patients (mean vessel diameter in IDDM: 2.23 mm vs. 2.39 mm in NIDDM, p=0.08). These factors could also have contributed to the beneficial effect observed in this subgroup of patients.
ST is a serious complication associated with a higher incidence of ST-segment elevation MI and mortality13. Diabetes mellitus has been consistently associated with an increased risk of ST14-16. In our study, the overall incidence of ST was very low, and similar between groups. However, in agreement with previous studies a different time pattern was observed. All episodes of ST recorded in the SES group occurred after one year. Per protocol all patients stopped clopidogrel at one-year follow-up. As has been mentioned above, there is a clear benefit of the treatment of small vessels with the first generation of DES. In the three-year follow-up of the BASKET trial17, a significant increase in serious events (death/MI) was observed in the group of patients with large vessels, and this correlated with an increase in the rate of ST. On the other hand, a significant reduction in MACE and early ST (0-6 months) was reported in the small stent group. Likewise, in the long-term analyses of the SCAAR registry11 in both the overall population and diabetic patients the greatest benefit of the treatment with DES was observed in patients treated with stents with a diameter <3 mm and a length ≥20 mm. These findings are consistent with our results in terms of TLR and may explain the low incidence of ST observed in our patients4,12. Recent studies have shown the development of atherosclerosis within stents (neoatherosclerosis) as a rare underlying cause of ST18. Interestingly, in the present study, one episode of very late ST (three years) was recorded in the BMS group, in a patient without any predisposing factors classically associated with this condition. A possible explanation of our finding may be the development of an unstable lesion in the context of neointimal atherosclerotic change, although this cannot be confirmed due to the absence of intracoronary imaging information.
Atherosclerosis progression is a serious problem in both diabetics and non-diabetic patients19. However, previous studies have demonstrated that progression of the atherosclerotic disease is more frequent in diabetics6,20. The increase in atherosclerotic progression, especially in diabetics, constitutes one of the reasons why CABG in multivessel disease had better outcome as compared with PCI in classic studies21,22. Of note, in the present study the incidence density of atherosclerosis progression is higher than the incidence density of TLR at five years in the SES group (1.97 vs. 1.69 per 100 person-years). As a result, the progression of atherosclerosis tarnishes the clinical benefits obtained following SES implantation and may generate a concern especially in patients with multivessel disease. In contrast, in the BMS group the incidence of progression is lower than TLR. Nevertheless, due to the high incidence of restenosis observed in this group, almost half of the patients treated with a BMS may need a second revascularisation (restenosis and/or progression) at five years. Thus, apart from the traditional concern of restenosis in diabetic patients, we believe that atherosclerosis progression represents another key issue in daily practice which warrants further studies. Until cellular, molecular and pathophysiological mechanisms are clearly understood, a close monitoring of other cardiovascular risk factors and metabolic parameters are needed in this population in order to reduce atherosclerotic burden.
Study limitations
Some limitations have to be acknowledged. First, the primary endpoint of the trial was angiographic. For this reason, the clinical parameters and comparisons are per definition underpowered. Thus, any conclusions regarding those issues have to be taken as hypothesis-generating. Both the SES and the BMS used in this trial represent an early generation of stents. Data cannot be extrapolated to current second-generation DES or BMS with different alloys and drugs. However, this trial represents the first report of the long-term results of a trial specifically designed in diabetic patients with the use of DES.
In conclusion, long-term follow-up of the DIABETES trial suggests the durable effect of SES in reducing TLR both in IDDM and in non-IDDM without major safety concerns in terms of ST, MI or mortality.
Funding
This extended follow-up was supported by an unrestricted grant from Cordis, J&J, Spain.
Conflict of interest statement
M. Sabaté is a consultant for Cordis, Medtronic and Abbott. The other authors have no conflicts of interest to declare.

