
Abstract
Aims: Newer-generation drug-eluting stents (DES) have been shown to be superior to first-generation DES. Current-generation DES have zotarolimus, everolimus or biolimus as antiproliferative drugs. Novolimus, a metabolite of sirolimus, has been specifically developed to provide efficacy similar to currently available agents at a lower dose and thus requires a lower polymer load. We report the final five-year outcomes of the EXCELLA II trial comparing a zotarolimus-eluting stent (ZES) with a novolimus-eluting stent (NES).
Methods and results: EXCELLA II is a prospective, multicentre, single-blind, non-inferiority clinical trial. Patients (n=210) with a maximum of two de novo lesions in two different epicardial vessels were randomised (2:1) to treatment with either NES (n=139) or ZES (n=71). At five-year follow-up, patients in the NES group had a significantly lower incidence of the patient-oriented (HR 0.53, 95% CI: 0.32-0.87, p=0.013) and device-oriented (HR 0.38, 95% CI: 0.17-0.83, p=0.011) composite endpoints. There was no difference in cardiac death and definite/probable stent thrombosis between the two groups; however, there was a trend towards reduction in myocardial infarction and repeat revascularisation in the NES group at five-year follow-up.
Conclusions: At five-year follow-up, the incidence of device- and patient-oriented events was significantly lower in the NES group. Further studies, adequately powered for clinical outcomes, are warranted. Trial Registration: ClinicalTrials.gov number NCT00792753.
Introduction
Percutaneous coronary intervention (PCI) using coronary stents has revolutionised the treatment of patients with coronary artery disease; however, the clinical outcomes with bare metal stents remained affected by a high risk of in-stent restenosis1-3. Drug-eluting stents (DES) substantially reduced neointimal proliferation2-4, but first-generation DES eluting sirolimus or paclitaxel from a durable polymer raised safety concerns about late and very late stent thrombosis, possibly due to delayed endothelialisation by the antiproliferative drugs and chronic inflammation or delayed hypersensitivity reaction caused by the polymers in these DES5-8. Newer-generation DES have sirolimus-derivative antiproliferative drugs (including zotarolimus, everolimus and biolimus) and biocompatible durable or biodegradable polymers9,10. These newer stents have shown promising results and improved clinical outcomes compared with first-generation DES11,12. It is therefore conceivable that a potent antiproliferative drug requiring a lower dose and lower polymer load may further improve outcomes. The DESyne® novolimus-eluting stent (NES) system (Elixir Medical Corporation, Sunnyvale, CA, USA) has been specifically developed with this objective in mind.
Novolimus™, a metabolite of sirolimus, represents a specifically manufactured macrocyclic lactone that favourably requires lower concentrations of drug (and consequently polymer) to inhibit neointimal proliferation effectively. The feasibility of using novolimus on a DES was assessed in the first-in-man EXCELLA study (n=15), which demonstrated satisfactory angiographic and clinical outcomes13. Further assessment of the NES has been performed in the single-blind, prospective EXCELLA II study, which randomised patients to treatment with either NES or the Endeavor® (Medtronic, Santa Rosa, CA, USA) zotarolimus-eluting stent (ZES)14. At nine months, the in-stent late lumen loss was significantly lower in the NES arm (NES 0.11±0.32 mm vs. ZES 0.63±0.42 mm, p<0.0001). However, there was no significant difference in the device-oriented endpoints (NES 2.9% vs. SES 5.6%, p=0.45)14. We report the final five-year follow-up of this trial, which represents the longest follow-up of the largest randomised assessment of a coronary stent eluting novolimus.
Methods
The study complies with the Declaration of Helsinki. Study protocol was approved by the relevant ethics committees, and informed consent was obtained from all participants (or their guardians).
STUDY DESIGN AND POPULATION
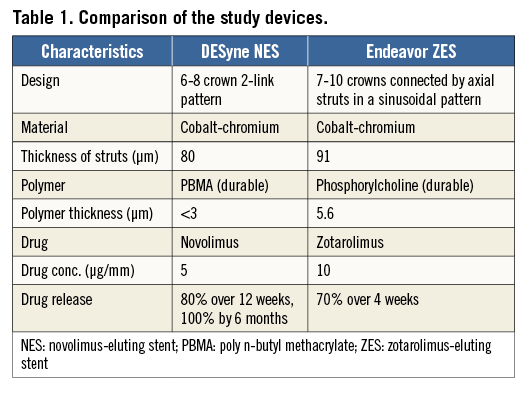
The EXCELLA II study was a prospective, single-blind, multicentre trial enrolling 210 patients who were randomised in a ratio of 2:1 to receive either an Elixir NES (n=139) or an Endeavor ZES (n=71). The characteristics of the two devices are shown in Table 1. All patients were over the age of 18, with a maximum of two target lesions in native epicardial vessels with a reference vessel diameter between 2.5 and 3.5 mm, lesion length <24 mm, percentage diameter stenosis (DS) between 50% and 99%, and a Thrombolysis In Myocardial Infarction (TIMI) flow grade ≥1 by visual estimation.
Patients with documented evidence of recent myocardial infarction (
STUDY PROCEDURE
Standard interventional techniques were used to treat the lesion: in particular, predilatation was mandatory, and stent implantation was performed at a pressure not exceeding the rated burst pressure. Post-dilatation was left to the operator’s discretion; however, if performed, balloons were required to be shorter than the length of the deployed stent. In the event of a bail-out procedure and the need for an additional stent, this was required to be of the same type as the first implanted stent if possible. If not, then a stent comprising the same base material and drug family was recommended. In patients with two de novo lesions, attempts at the second lesion were only permitted if an optimal result, defined as a residual DS <20%, TIMI 3 flow, absence of thrombus or edge dissection, was seen after PCI of the first lesion. Periprocedural pharmaceutical treatment was administered according to standard hospital practice. Procedural anticoagulation was achieved with unfractionated heparin or bivalirudin. The use of glycoprotein IIb/IIIa inhibitors was left to the operator’s discretion. All patients enrolled in the study were to receive ≥75 mg of aspirin and 75 mg of clopidogrel daily for a minimum of twelve months following the index procedure.
FOLLOW-UP AND CLINICAL ENDPOINTS
Patients’ clinical status was reviewed at one, six, nine, 12 months, and annually thereafter up to five years. Angiographic follow-up for all patients was planned at nine months, with IVUS follow-up planned in a subset of 65 consecutive patients (from selected centres). Prior to follow-up angiography, physicians were required to perform a clinical evaluation of the patients and prospectively record in the case record form whether any revascularisation, if required, was clinically indicated.
The primary endpoint of the study was in-stent late lumen loss as assessed by quantitative coronary angiography (QCA) at nine-month follow-up. Secondary clinical endpoints included the device-oriented composite endpoint (DoCE, including cardiac death, MI not clearly attributable to a non-intervened vessel, and clinically indicated target lesion revascularisation). We have also presented a comprehensive patient-oriented composite endpoint (PoCE, including all death, all MI and all revascularisation), the individual clinical endpoints, and stent thrombosis as defined by the Academic Research Consortium (ARC)15. An independent blinded clinical events committee (CEC) adjudicated all clinical endpoints.
STATISTICAL ANALYSIS
Categorical variables are presented as counts and percentages and compared using Fisher’s exact test. Continuous variables are presented as means±standard deviation and compared using the Student’s unpaired t-test. Survival curves were constructed using Kaplan-Meier estimates and compared using the log-rank test. A p-value <0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
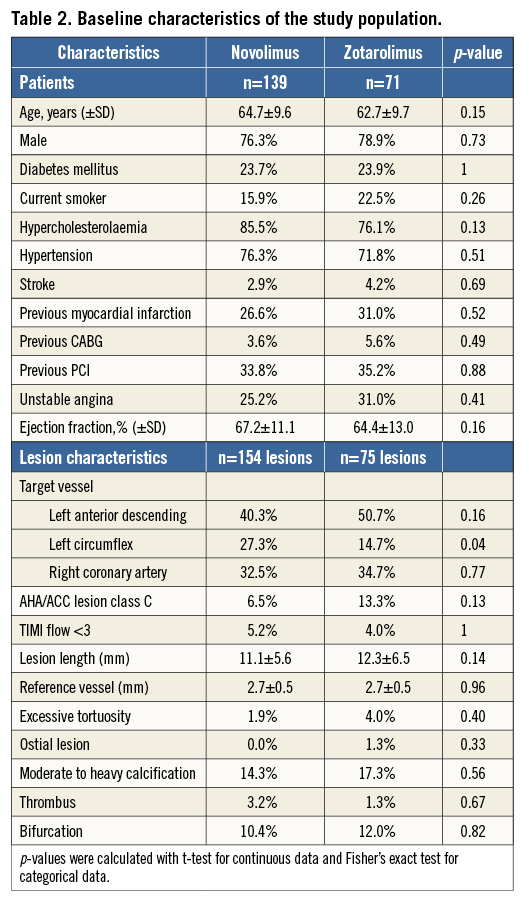
A total of 210 patients were enrolled and randomly assigned to treatment with NES (n=139) or ZES (n=71). The two groups were well matched for the baseline demographical, clinical and angiographic characteristics (Table 2). The mean age was 64±10 years, approximately two thirds of the patients were male, and 24% were diabetics. The angiographic and IVUS follow-up at nine months has been reported previously, showing both non-inferiority and superiority of NES for in-stent late lumen loss and superiority for IVUS-derived neointimal volume14.
Follow-up data at five years were available for 97% of patients (Figure 1). There was no significant difference in usage of dual antiplatelet therapy between the two groups throughout the study period and at the final five-year follow-up (NES 5.5% vs. ZES 12.7%, p=0.1).
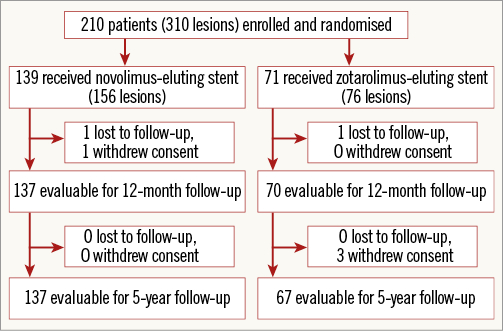
Figure 1. Study flow chart.
At five-year follow-up, patients in the NES group had a significantly lower incidence of DoCE (Figure 2). There was no difference in cardiac death and target vessel myocardial infarction. However, patients in the NES group had a trend towards reduction in clinically indicated target lesion revascularisation (Figure 2). The detailed comparison of clinical outcomes is shown in Table 3.
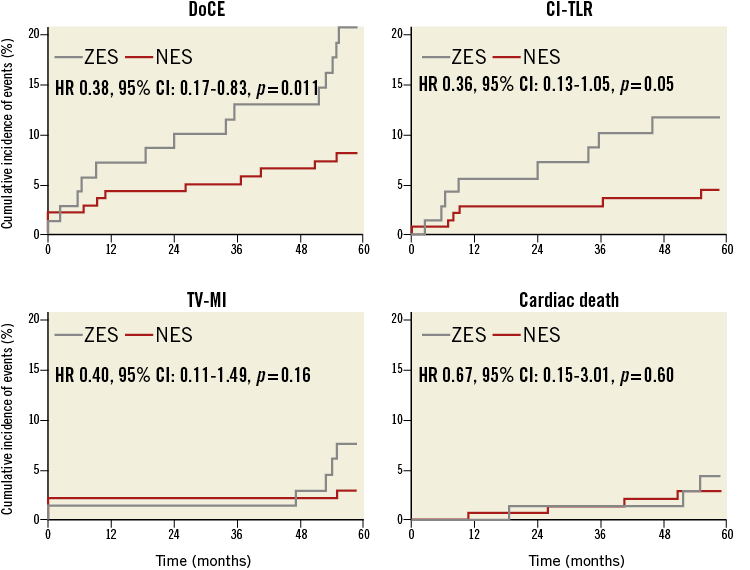
Figure 2. Kaplan-Meier curves for device-oriented composite endpoints. The novolimus-eluting stent (NES) was associated with a lower incidence of the device-oriented composite endpoint (DoCE) and clinically indicated target lesion revascularisation (CI-TLR) with no effect on cardiac mortality and a trend towards a reduction in target vessel myocardial infarction (TV-MI), compared with the zotarolimus-eluting stent (ZES), during five-year follow-up.
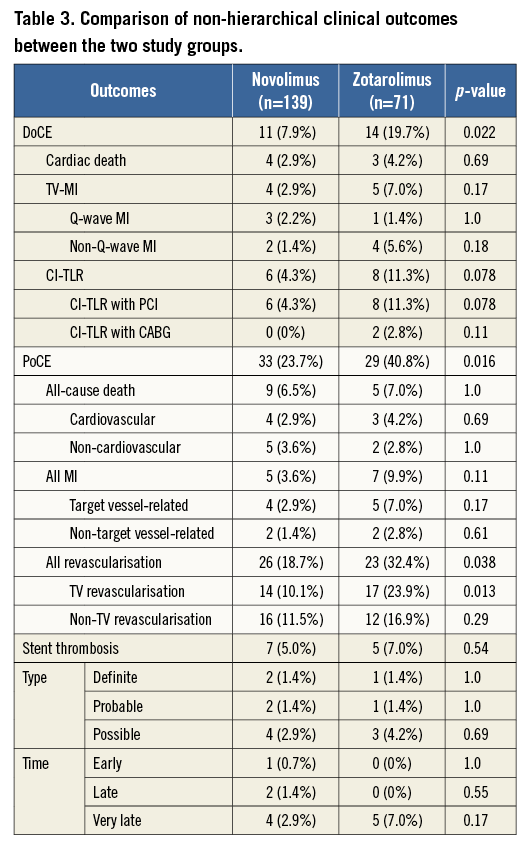
Patients in the NES group had a significantly lower incidence of PoCE (Figure 3). There was no difference in all-cause death, but a trend towards reduction in myocardial infarction (Figure 3) and significant reduction in repeat revascularisation (Figure 3). There was no difference in the incidence of stent thrombosis at five-year follow-up (Table 3).
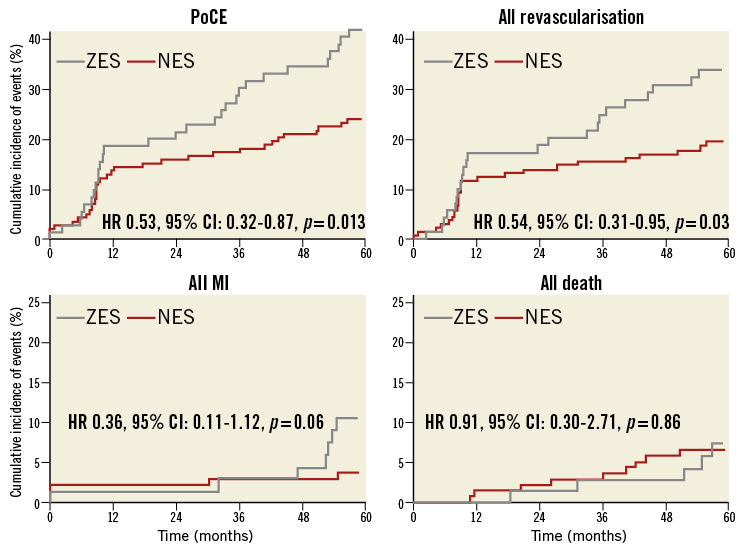
Figure 3. Kaplan-Meier curves for patient-oriented composite endpoints. The novolimus-eluting stent (NES) was associated with a lower incidence of the patient-oriented composite endpoint (PoCE) and all revascularisation, compared with the zotarolimus-eluting stent (ZES), during five-year follow-up.
Discussion
The EXCELLA II trial compared the performance of two newer-generation stents over a long follow-up period. The main finding of the present study is that, at five-year follow-up, NES outperformed ZES in clinical efficacy and safety including patient-oriented and device-oriented composite endpoints.
Novolimus, a novel mTOR (mammalian target of rapamycin) inhibitor macrocyclic lactone, is a potent antiproliferative drug (IC50 of 0.5 nM). The dose of drug and the polymer thickness on NES are 5 µg/mm and <3 µm respectively, compared to 10 µg/mm and 4.1 µm for ZES, 10 µg/mm and 16 µm for the TAXUS™ paclitaxel-eluting stent (Boston Scientific, Marlborough, MA, USA), 14 µg/mm and 12.6 µm for the CYPHER® sirolimus-eluting stent (Cordis, Johnson & Johnson, Warren, NJ, USA), and 10 µg/mm and 7.6 µm for the XIENCE V® everolimus-eluting stent (Abbott Vascular, Santa Clara, CA, USA). NES, with absence of a primer coating, together with lower doses of drug and polymer, has potential advantages to improve both safety and efficacy14.
This is the first study to report the long-term safety and efficacy of novolimus-eluting stents. Our data suggest an improvement in both device-oriented and patient-oriented outcomes with NES, which has not been shown in any other trials comparing newer-generation DES. We have shown that NES tended to improve both safety (myocardial infarction) and efficacy (revascularisation) endpoints. The incidence of myocardial infarction seen in the ZES arm was comparable to that seen in other contemporary trials of DES, including the RESOLUTE All Comers and LEADERS trials16,17. Nevertheless, NES reduced myocardial infarction in our study, whereas no difference was observed in the RESOLUTE All Comers (XIENCE V 6.8% vs. Resolute 7.1%) and LEADERS (CYPHER 8.4% vs. BioMatrix [Biosensors International, Singapore] 8.0%) trials at five-year follow-up16,18. Additionally, the composite endpoint of patient-oriented endpoints (PoCE) was significantly lower in the NES group (24.0%) compared with the ZES group (40.8%) at five-year follow-up.
We have also shown a reduction in the incidence of repeat revascularisation, which could be explained by both superior efficacy of NES and inferiority of Endeavor, compared with other newer-generation DES. The Endeavor stent has been shown to be superior to the first-generation DES in the PROTECT trial19; however, it has shown a high in-stent late lumen loss of 0.60 mm and 0.67 mm at eight-month follow-up in the ENDEAVOR III and ENDEAVOR IV studies, respectively20,21. However, despite this higher late loss, there was no significant difference in clinically indicated target lesion revascularisation between Endeavor and SES (ENDEAVOR III)21 or paclitaxel-eluting stents (PES; ENDEAVOR IV)20,22. Furthermore, reductions in the absolute difference in repeat revascularisation between short- and long-term follow-up in favour of Endeavor indicate that the Endeavor stent may not be susceptible to the delayed restenosis phenomenon observed with other mTOR-inhibiting DES such as SES20,23 and EES24.
There was no difference in cardiac death at five-year follow-up, consistent with other recent trials of newer-generation DES (RESOLUTE All Comers: XIENCE V 5.7% vs. Resolute 6.5%; LEADERS: CYPHER 10.5% vs. BioMatrix 9.9%) at five-year follow-up16,18. Meta-analyses of clinical trials have also not shown a definite mortality benefit of DES25,26. The incidence of definite stent thrombosis was also not different between the two stents studied, but was in an acceptable range (1.4% in both groups) and comparable with other newer-generation DES (Resolute 2.0% and XIENCE V 0.9% in RESOLUTE All Comers, XIENCE V 1.1% in SPIRIT III, and BioMatrix 2.6% in LEADERS) at five-year follow-up16,18,27. Our study, however, was not powered to detect any differences in these clinical endpoints. Considering the potential benefits of this device, further larger studies are warranted. DESyne has achieved CE mark in Europe. The EXCELLA III trial comparing NES with Resolute ZES has received approval from the FDA to enrol approximately 2,000 patients in a 2:1 fashion at up to 50 institutions in the USA and internationally.
Limitations
This study’s powered primary endpoint was in-stent late lumen loss at nine months; the device-oriented composite endpoint at five years was a non-powered secondary endpoint. However, this was a pre-specified secondary endpoint, with all events adjudicated by an independent clinical events committee.
Conclusion
At five-year follow-up, the incidence of device- and patient-oriented events was significantly lower in the DESyne novolimus-eluting stent compared with the Endeavor zotarolimus-eluting stent. Further studies, adequately powered for clinical outcomes, are warranted to confirm the potential of this novel drug-eluting stent.
| Impact on daily practice Selection of a drug-eluting stent to implant in a patient undergoing percutaneous coronary intervention remains a subjective choice of individual operators, as trials comparing various newer-generation stents have not shown definite superiority of one over the others. The EXCELLA II trial has shown that the incidence of device- and patient-oriented events was significantly lower with the DESyne novolimus-eluting stent, compared with the Endeavor zotarolimus-eluting stent, at five-year follow-up. Further trials, powered for clinical outcomes, are warranted before a change in clinical practice can be recommended. |
Guest Editor
This paper was guest edited by William Wijns, MD, PhD, Cardiovascular Research Center Aalst, Onze-Lieve-Vrouw Hospital, Aalst, Belgium.
Funding
The EXCELLA II trial was funded by Elixir Medical Corp., Sunnyvale, CA, USA.
Conflict of interest statement
L. Morrison and S. Toyloy are full-time employees of Elixir Medical. The other authors have no conflicts of interest to declare. The Guest Editor receives institutional research grants to the Cardiovascular Center Aalst from Medtronic.

