Abstract
Aims: To assess the safety and performance of the XIENCE V everolimus-eluting stent (EES) versus the TAXUS paclitaxel-eluting stent (PES) in the treatment of patients with de novo coronary artery lesions after a five-year follow-up period. Second-generation drug-eluting stents (DES) were developed with the aim of improving the safety profile of DES, after reports of stent thrombosis (ST) with first-generation devices. However, long-term follow-up data are scarce.
Methods and results: SPIRIT II was a multicentre, prospective, single-blind, clinical trial, randomising 300 patients with up to two de novo coronary artery lesions in a ratio of 3:1 to either a EES or a PES. The five-year clinical follow-up was completed in 244 patients (81%). At five-year follow-up, 19.5% of patients were on thienopyridine in the EES arm, while 30.5% were on the same therapy in the PES arm. Cardiac mortality was significantly lower in EES than in PES (1.5% vs. 7.3%, p=0.015). There was a trend towards lower cardiac death and MI (4.8% vs. 11.4%) and lower ID-TLR (4.7% vs. 9.4%) in EES than in PES. As a result, there was a consistent reduction in ID-MACE for EES vs. PES (ID-MACE 8.0% vs. 18.1%, p=0.018). In addition, the ARC-defined stent thrombosis rate was numerically lower in EES compared to PES (0.9% vs. 2.8%). No definite stent thrombosis events were observed after two years in the EES arm.
Conclusions: Five-year clinical follow-up of the SPIRIT II trial demonstrated the continuing long-term safety and efficacy of EES. (Clinical trials government number: NCT00180310)
Introduction
Second-generation drug-eluting stents (DES), such as the XIENCE V™ everolimus-eluting stent (EES) (Abbott Vascular, Santa Clara, CA, USA), were developed with the aim of improving the safety profile of DES, after reports of very late stent thrombosis (ST) with first-generation devices1. Current results from randomised studies evaluating the EES indicate favourable clinical outcomes, together with lower rates of ST when compared with first-generation DES; however, results are limited to only medium-term follow-up2-5. Ensuring that these benefits are sustained in the long term is particularly important for DES since long-term efficacy is no longer a consequence of drug elution, but is more dependent on the stent platform and polymer. The five-year results of the SPIRIT FIRST first-in-man trial, albeit in a small population, were consistent with the results from other studies of the EES with shorter follow-up6. This article presents the five-year long-term clinical outcome of patients enrolled in the SPIRIT II trial randomised to treatment with either EES or the TAXUS™ paclitaxel-eluting stent (PES) (Boston Scientific, Natick, MA, USA).
Materials and methods
STUDY DESIGN
The study design and outcomes at six months, one, two, three, and four years follow-up are reported elsewhere2,3,7-9. In brief, this multicentre, prospective, single-blind study randomised 300 patients with up to two de novo coronary artery lesions in a ratio of 3:1 to treatment with either EES (n=223) or PES (n=77). The primary endpoint was in-stent late loss at 180 days.
ENDPOINTS
Clinical endpoints assessed at five years included ischaemia-driven major adverse cardiac events (ID-MACE), a composite of cardiac death, myocardial infarction (MI), and ischaemia-driven target lesion revascularisation (ID-TLR) by percutaneous coronary intervention or coronary artery bypass graft surgery, and its individual components. All events up to five years were adjudicated by an independent clinical events committee.
DEFINITIONS
Complete definitions are provided elsewhere2,3,7-9. All deaths were considered cardiac unless an undisputed non-cardiac cause was present. Q-wave MI was defined as the development of new pathological Q-waves. A non-Q-wave MI was defined as a typical rise and fall of creatine kinase myoglobin band (CK-MB), with at least one of the following: ischaemic symptoms; electrocardiographic changes indicative of ischaemia (ST-segment elevation or depression); or an associated coronary artery intervention. For a non-procedural or spontaneous MI, the CK-MB was required to be ≥2 times the upper limit of normal (ULN). A CK-MB ≥3 times the ULN or ≥5 times the ULN was required for an MI to be defined post-percutaneous coronary intervention or post-coronary artery bypass graft, respectively. ID-TLR was defined as revascularisation of the target lesion in association with any of the following: a positive test of ischaemia, using either exercise testing or fractional/coronary flow reserve; ischaemic symptoms and an angiographic diameter stenosis ≥50% by on-line quantitative coronary angiography (QCA); or a diameter stenosis ≥70% by on-line QCA without ischaemic symptoms or a positive functional study. ST was classified according to the Academic Research Consortium classification10.
STATISTICAL METHODS
All analyses were conducted according to the intention-to-treat principle. Binary variables are presented as percentages (counts). Continuous variables are presented as mean and standard deviation. Cumulative events curves were generated using the Kaplan–Meier method, and the two groups were compared using the log-rank test. Hazard ratios with 95% confidence intervals were calculated using the Cox proportional hazards model.
Results
Clinical follow-up was available in 244 (81%) patients (EES 185 [83.0%] and PES 59 [76.6%]) with a similar proportion of patients (EES 15 [6.7%] and PES 5 [6.5%]) lost because of failure to complete a new consent form, which was required after a change to the initial protocol to allow extended follow-up from two years to five years. Other reasons for incomplete follow-up included death (EES 13 and PES 10), loss to follow-up (EES 5 and PES 1) and withdrawal (EES 5 and PES 3).
BASELINE DEMOGRAPHIC DATA
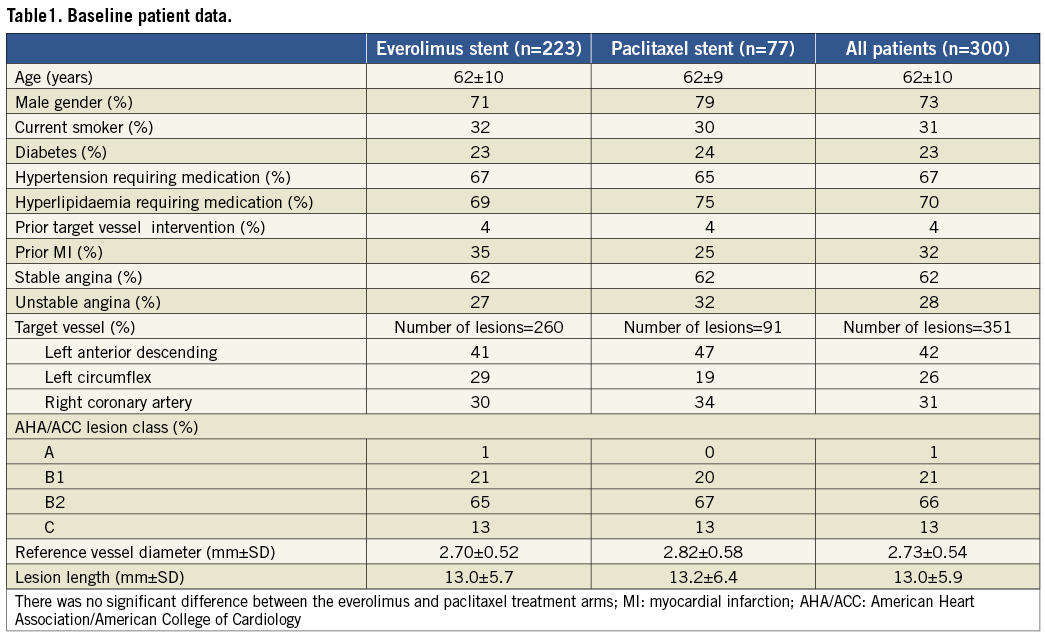
Baseline demographic, clinical and angiographic characteristics, which were comparable between both treatment groups, are described in full elsewhere7 and summarised in Table 1.
CLINICAL OUTCOMES
Clinical outcomes in terms of cardiac death, MI, ID-TLR and ID-MACE at five-year follow-up are shown in the Kaplan–Meier cumulative curves (Figure 1A, Figure 1B, Figure 1C and Figure 1D).
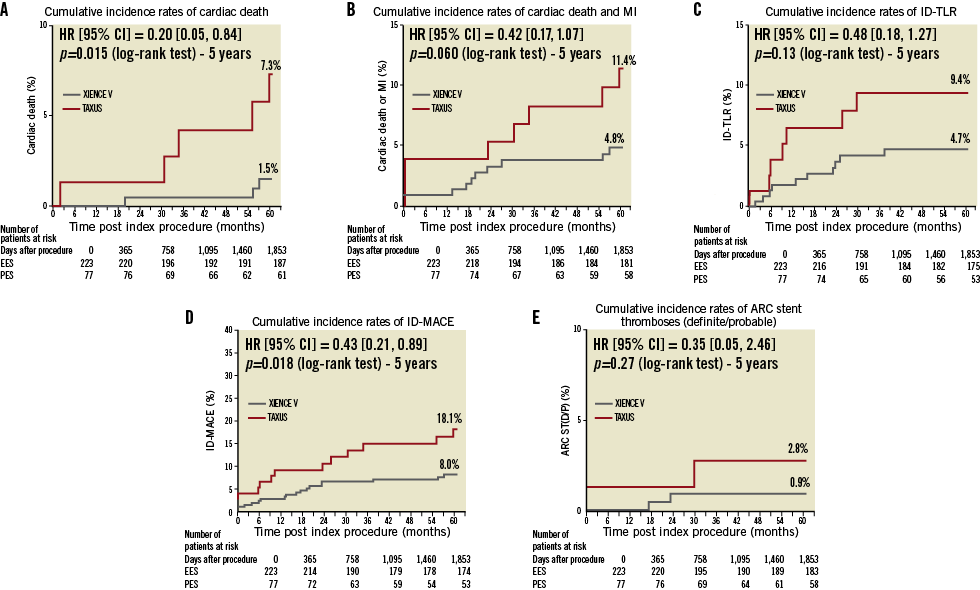
Figure 1. Cumulative Kaplan-Meier estimates of the rates of key study endpoints. Cumulative risk of events at 1,853 days for (A) cardiac death; (B) cardiac death or MI; (C) ischaemia-driven target lesion revascularisation (ID-TLR); (D) ischaemia-driven major adverse cardiac events (ID-MACE), a composite of cardiac death, non-fatal myocardial infarction (MI), and ischaemia-driven target lesion revascularisation (ID-TLR); (E) definite/probable stent thrombosis. HR: hazard ratio.
Five-year cardiac mortality was significantly lower in EES than in PES (1.5% vs. 7.3%, p=0.015). There were four additional cardiac deaths documented after four years. In the EES arm, one patient died from acute myocardial infarction on day 1,716. Since resuscitation was unsuccessful, the relationship between the death and the implanted stent was unknown. The other patient died on day 1,664 from an unknown cause. In the PES arm, a patient with liver cirrhosis died from an unknown cause on day 1,655 and another patient on regular dialysis died due to heart failure on day 1,796.
There was a trend towards lower cardiac death and MI (4.8% vs. 11.4%) and lower ID-TLR (4.7% vs. 9.4%) in EES than in PES. As a result, there was a consistent reduction in ID-MACE for EES vs. PES (ID-MACE 8.0% vs. 18.1%, p=0.018).
The rate of definite or probable ST out to five-year follow-up was 0.9% and 2.8% in patients treated with EES and PES, respectively (p=0.27, Figure 1E). Of note, no ST events were observed in the EES arm after 24 months of follow-up, whereas only one ST event was observed in the PES arm around month 30. The ST events are detailed elsewhere3.
At five-year follow-up, 19.5% of patients were on thienopyridine therapy in the EES arm, while 30.5% were on the same therapy in the PES arm.
Discussion
This study represents one of the longest available follow-ups of EES and thus provides essential data on the long-term efficacy and safety of the EES. This short report on five-year clinical outcomes of the SPIRIT II trial demonstrates the continuing long-term safety and efficacy of EES. At five years, EES continues to be clinically superior to PES, with an 80% reduction in cardiac death (1.5% vs. 7.3%), a 52% reduction in ID-TLR (4.7% vs. 9.4%), and a 57% reduction in overall ID-MACE (8.0% vs.18.1%).
Although a “late loss catch-up” with EES was suggested by the two-year imaging outcome data from SPIRIT II, there was no significant difference in angiographic and clinical outcomes between the EES and PES stents, with a numerically lower MACE rate observed in the EES arm. These late loss results raised concerns about the clinical implications of “late loss catch-up”9. The concerns were proven unfounded when the three-year data confirmed that this increase in neointimal hyperplasia did not translate into higher clinical events2. In fact, the EES showed a reduction in cardiac events, clinical restenosis, overall MACE and stent thrombosis rates at three-year follow-up compared to PES.
Furthermore, in this SPIRIT II trial, despite a lower proportion of patients taking DAPT at the time of follow-up (19.5% of patients on clopidogrel/thienopyridine in the EES arm and 30.5% in the PES arm), the ARC-defined stent thrombosis rate remained numerically lower with EES compared to PES at five years (0.9% vs. 2.8%), with no stent thrombosis events observed after two-year follow-up in the EES arm.
This favourable five-year long-term clinical outcome of the EES is consistent with the results from other studies of the EES with shorter follow-up. Likewise, the two-year, the three-year and the four-year follow-up data from SPIRIT III have shown improvements in event-free survival, and lower rates of stent thrombosis with the use of an EES11-13.
The small sample size of the current study must be taken into account; however, the absence of very late ST events during prolonged follow-up is nevertheless important. It will be of importance to observe whether similar findings are seen during long-term follow-up of the more complex patient groups enrolled in the SPIRIT III and IV studies.
Limitations
The loss of patients during clinical follow-up, which was largely due to the failure of some patients to complete a new consent form that was required following a protocol amendment prolonging follow-up duration of the study from two years to five years, impacts on the power of the study to detect differences in clinical events. The clinical event rates might therefore be underestimated. Moreover, interpretation of the current clinical results must take into account that the study was powered for in-stent late loss at six-month follow-up, and therefore not specifically designed to detect differences in clinical outcomes or ST.
Conclusions
The five-year clinical follow-up of the SPIRIT II trial demonstrated the continuing long-term safety and efficacy of EES. At five years, EES continues to be clinically superior to PES, with an 80% reduction in cardiac death, a 52% reduction in ID-TLR, and a 57% reduction in overall ID-MACE, which is consistent with other randomised studies of EES, albeit at shorter follow-up. Overall stent thrombosis rates were low, with the absence of recent stent thrombosis events after two-year follow-up in the EES arm.
Acknowledgements
We would like to thank Cécile Dorange and Susan Veldhof (Abbott Vascular) for their intellectual input in reviewing the manuscript.
Funding
This study was sponsored by Abbott Vascular (Santa Clara, CA, USA). The sponsor was involved in the design and conduct of the study, the collection, management, initial analysis, and interpretation of the data, and they had the right to a non-binding review of the manuscript. Full monitoring ensured accuracy of data collection. The senior author had full access to all the data and had final responsibility for the decision to submit for publication. This study is registered with ClinicalTrials.gov, number NCT00180310.
Conflict of interest statement
K. Miquel-Hebert is a full-time employee of Abbott Vascular. The other authors have no conflicts of interest to declare.
Appendix
Clinical events committee: C. Hanet, MD, PhD, Brussels, Belgium; D. McClean, MD, Christchurch, New Zealand; A. Hoye, MD, PhD, Hull, UK; V. Umans, MD, PhD, Alkmaar, The Netherlands
Data safety monitoring board: J. Tijssen, PhD, Amsterdam, The Netherlands; T. Lefèvre, MD, Massy, France; P. Urban, MD, Geneva, Switzerland

