- everolimus-eluting stent
- paclitaxel-eluting stent
- PCI
- elderly
Abstract
Aims: Age is an important determinant of outcomes in patients treated with percutaneous coronary intervention (PCI). This report from the randomised multicentre SPIRIT III trial compares the outcomes in elderly and younger patients treated with everolimus-eluting stent (EES) versus paclitaxel-eluting stent (PES).
Methods and results: A total of 1,002 patients with stable or unstable angina or inducible ischaemia undergoing PCI were randomised in a 2:1 ratio to receive EES or PES. Outcomes were examined across the randomised groups as a function of age and stent type. Patients ≥65 years of age (elderly) treated with EES vs. PES had lower in-segment late lumen loss (0.11±0.32 mm vs. 0.38±0.55 mm, respectively, p=0.0002) and lower rates of binary in-segment restenosis (3.4% vs. 15.5%, p = 0.004) at eight months, along with a 48% lower incidence of 3-year target vessel failure (TVF=cardiac death, myocardial infarction and ischaemia-driven target vessel revascularisation [TVR]; 10.8% vs. 20.8%, p=0.009), mainly due to a lower incidence of TVR (5.4% vs. 9.2%, p=0.20). Among EES patients, elderly compared to younger patients had comparable rates of binary in-segment restenosis (3.4% vs. 5.6%, p=0.44) at eight months but paradoxically lower rates of TVF (10.8% vs. 17.1%, p=0.03) at three years. Among PES patients, elderly compared to younger patients had a higher rate of binary in-segment restenosis (15.5% vs. 3.4%, p=0.01) at eight months and no difference in the rate of 3-year TVF (20.8% vs. 19.4%, p=0.77) .There was a significant interaction between stent assignment, age ≥65 years and 8-month angiographic in-segment late loss (p=0.001).
Conclusions: Implantation of both EES and PES appeared to be safe in elderly patients, however EES compared to PES was more effective due to enhanced 3-year MACE- and TVF-free outcomes. Further research should clarify age-specific mechanisms of neointimal response after treatment with drug-eluting stents.
Introduction
Developed nations are facing a transition in demographics in which the proportion of the population over the age of 65will double in the next few decades.1,2 Elderly patients have a high prevalence of cardiovascular disease, with approximately 25% of the population older than 65 years of age exhibiting symptomatic cardiovascular disease accounting for most hospital admissions.3 As part of the aging process, coronary arteries in the elderly are prone to excessive tortuosity, calcification, aneurysmal dilation, and endothelial dysfunction.4-6
A significant proportion of the elderly population requires percutaneous coronary intervention (PCI), and although drug-eluting stents (DES) are now widely used in all age groups, the clinical and vascular responses to DES as a function of age have been incompletely characterised. In the large-scale, prospective randomised SPIRIT III trial, an everolimus-eluting stent (EES) compared with a paclitaxel-eluting stent (PES) resulted in reduced angiographic late loss, non-inferior rates of target vessel failure (TVF), and fewer major adverse cardiac events (MACE) during one and two years of follow-up.7,8 This report examines the effect of age on 3-year clinical outcomes in patients undergoing PCI using EES versus PES in the SPIRIT III trial.
Methods
The SPIRIT III protocol, inclusion and exclusion criteria, and principal results have been reported.7,8 Briefly, 1,002 patients with stable or unstable angina or inducible ischaemia undergoing PCI for ≤2 de novo lesions ≤28 mm in length and reference vessel diameter 2.5-3.75 mm in a native coronary artery were randomised in a 2:1 ratio to receive the polymer-based everolimus-eluting stent (XIENCE V®; Abbott Vascular, Santa Clara, CA, USA) or the polymer-based paclitaxel-eluting stent (TAXUS® Express2; Boston Scientific, Natick, MA, USA). Other inclusion and exclusion criteria, as well as the stent implantation protocol have been previously described.7,8
Clinical follow-up was performed at 30 days, 180 days, 240 days, 270 days, one year, and then yearly to five years. Data are currently available to three years. The primary endpoint was in-segment late loss at 240 days. The co-primary endpoint was ischaemia-driven TVF at 270 days, defined as the composite of cardiac death (death in which a cardiac cause could not be excluded), myocardial infarction (MI) (Q-wave or non–Q-wave), and ischaemia-driven target vessel revascularisation (TVR) by either PCI or bypass graft surgery. Target vessel (or lesion) revascularisation was considered to be ischaemia-driven if associated with a positive functional study result of ≥50% diameter stenosis by core laboratory quantitative analysis with ischaemic symptoms, or ≥70% diameter stenosis with or without documented ischaemia. An additional pre-specified secondary endpoint included MACE at nine months and one year, defined as the composite of cardiac death, MI, or ischaemia-driven target lesion revascularisation (TLR). Definitions of other clinical endpoints have been previously described.7 Events were adjudicated by an independent clinical events committee. A second clinical events committee blinded to randomisation performed a post hoc adjudication of stent thrombosis using the Academic Research Consortium (ARC) definitions.9 Angiographic follow-up was pre-specified at eight months in 564 patients and completed in 436 patients. Outcomes were examined across the randomised groups as a function of age and stent type. The Medicare eligible age (65 years) was used as a cut-off for analysing clinical outcomes for the elderly population.
Statistical analysis
Categorical variables were compared with the Fisher exact test. Continuous variables are presented as means ±SD, and were compared using the Wilcoxon two-sample test. All analyses were performed in the intent-to treat population. Follow-up outcomes were estimated by the Kaplan-Meier method and compared by log-rank test.
Results
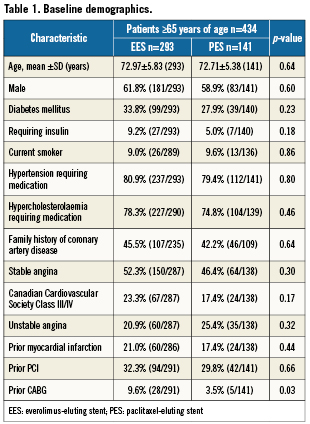
Among a total of 1,002 patients enrolled at 65 US sites between June 22, 2005 and March 15, 2006 in the SPIRIT III trial, 434 patients were 65 years of age or older representing the study population (elderly). A total of 293 and 141 patients ≥65 years of age were assigned to receive EES and PES, respectively. The mean age of the study population was 72.9±5.7 years, 60.8% were male, and 31.9% had diabetes mellitus. The indication for PCI was stable angina in approximately half of the patients (50.4%). The majority of patients (61.8%) had single vessel disease, and the target lesion was in the left anterior descending artery in 42.0% of patients. Mean reference vessel diameter (RVD) was 2.78±0.47 mm and mean lesion length was 14.5±5.7 mm.
As shown in Table 1, baseline clinical and angiographic characteristics were well matched between patients ≥65 years of age assigned to EES or PES, apart from a higher prevalence of previous coronary artery bypass grafting in EES patients.
Procedural results and angiographic outcomes
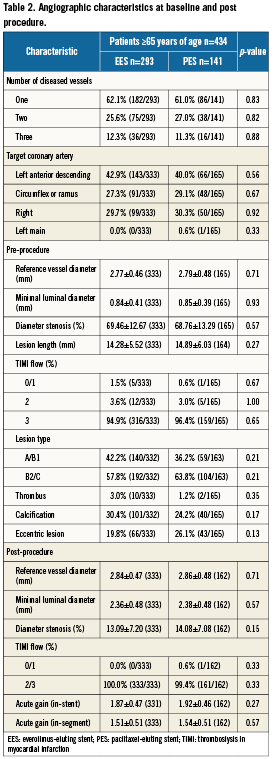
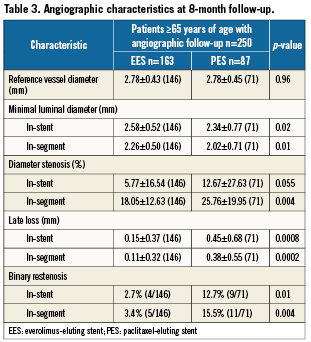
Lesion length, post-procedural minimal lumen diameter, percent diameter stenosis, and acute gain were similar among EES and PES treatment arms (Table 2). At 8-month angiographic follow-up obtained in 146 EES patients and in 71 PES patients, treatment with EES was associated with significantly lower in-segment late lumen loss, and significantly lower rates of binary angiographic in-stent restenosis and in-segment restenosis (Table 3).
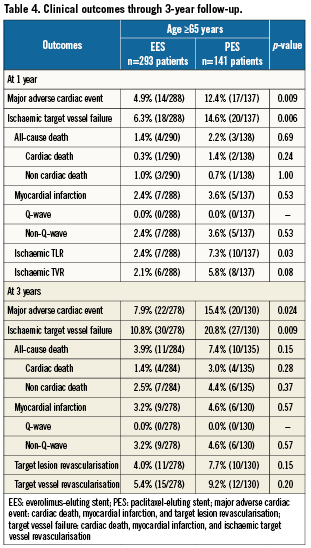
Clinical outcomes in patients ≥65 years of age
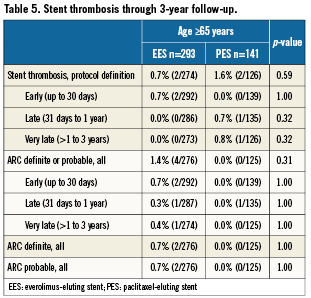
Among the elderly group, clinical follow-up for EES and PES patients was 97.9% and 97.2% at one year, and 94.9% and 92.2% at three years. As shown in Table 4, at one year, elderly patients treated with EES had similar rates of all-cause mortality, cardiac mortality and myocardial infarction but significantly lower rates of MACE and TVF apparently due to lower rates of TVR and TLR. These differences in MACE and TVF persisted at 2- and 3-year follow-up (Figures 1 A and B). Protocol-defined ST occurred within three years in two patients treated with EES and two patients treated with PES (Table 5). Definite or probable ST by ARC definition occurred in four patients in the EES arm (early ST in two patients, and late and very late ST each in one patient) and none of the patients in the PES arm.
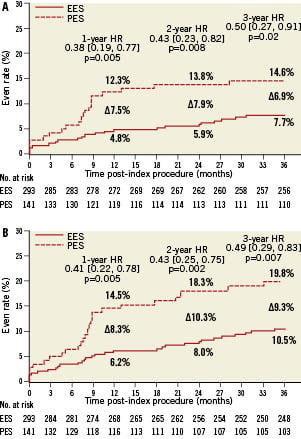
Figure 1. Cumulative estimated incidence of major adverse cardiac events (MACE) (A) and target vessel failure (TVF) (B) at three years for patients ≥65 years of age treated with EES vs. PES.
Outcomes in patients ≥65 years of age vs. <65 years of age
Elderly compared with younger patients were more frequently females (39.2% vs. 25.4%, p<0.0001) and less frequently smokers (9.2% vs. 33.6%, p<0.0001), and had higher prevalence of hypertension (80.4% vs. 71.7%, p=0.002) and hypercholesterolaemia (77.2% vs. 70.5%, p=0.02) along with a trend toward higher prevalence of diabetes mellitus (31.9% vs. 26.9%, p=0.09). Other clinical as well as angiographic and procedural characteristics were close between the two age groups. As shown in Figure 2, elderly compared with younger patients had no significant differences in rates of 1-year and 3-year cardiac mortality, MI, TLR, TVR, MACE, and TVF. Similarly, incidence of stent thrombosis did not differ significantly as a function of age at any of the study endpoints. The overall 3-year rates of stent thrombosis in elderly vs. younger patients were 1.0% vs. 1.4% (p=0.76), respectively, by protocol definition, and 1.0% vs. 1.7% (p=0.41) by ARC definition of definite/probable stent thrombosis.
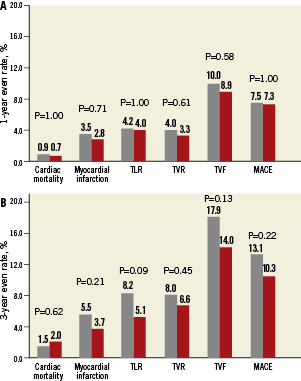
Figure 2. Clinical outcomes of patients younger than 65 years (grey bars) versus patients 65 years of age or older (red bars) at one year and at three years. TVR is non-target lesion.
Among patients assigned to angiographic follow-up in the EES arm, elderly as opposed to younger patients had a comparable magnitude of angiographic late loss (0.11±0.32 mm vs. 0.16±0.44 mm, p=0.22) and rates of binary in-stent (2.7% vs. 2.0%, p=0.73) and in-segment restenosis (3.4% vs. 5.6%, p=0.44). Among patients assigned to angiographic follow-up in the PES arm, elderly patients had significantly higher rates of angiographic late loss (0.38±0.17mm vs. 0.17±0.35 mm, p=0.006) along with significantly higher rates of binary in-stent (12.7% vs. 0.0%, p=0.0006) and in-segment restenosis (15.5% vs. 3.4%, p=0.01) compared to younger patients.
Discussion
This sub-study from the SPIRIT III randomised trial is the first systematic analysis of outcomes in elderly patients treated with different DES with latest follow-up covering more than 90% of the patients at 3 years. The key findings from this analysis are: 1) implantation of both EES and PES in elderly was safe, without increased rates of cardiac death or MI; 2) implantation of EES vs. PES in elderly resulted in a significant decline in late loss and lower incidence of binary angiographic restenosis and translated into a significant reduction in rates of TLR and TVR as well as enhanced 3-year MACE and TVF-free survival; 3) 8-month angiographic and 3-year clinical outcomes did not differ significantly as a function of age after the implantation of EES, while implantation of PES in elderly as opposed to younger patients was associated with significantly increased late loss and rates of binary angiographic restenosis within stent and within segment. Alexander et al. have shown that the use of certain therapies within elderly populations was lower in spite of the fact that ACC/AHA guidelines do not differ based on the age of the patient.10 The 3-year results from the SPIRIT III clinical trial underscore the safety and efficacy of the EES for treatment of coronary artery disease in the elderly and reinforce the use of PCI as a therapeutic alternative for older patients.
Age is an important determinant of outcomes in patients treated with PCI. In the early coronary angioplasty series, older age was associated with lower procedural success.11-14 Since the advent of stents, procedural success was no more related to the age exceeding 90% in any age group.15-17 In this analysis, patients ≥65 years of age represented 43.3% of the study population. Elderly patients generally suffer from a predisposition to higher incidences of multivessel disease, lower left ventricle function, and further complications including renal insufficiency. Because of these factors, older populations are typically less represented in clinical trials, and therefore the risks and benefits of stent implantation in the elderly are not well-characterised. Here we show that despite a higher prevalence of comorbidities, similar early adverse outcomes using either EES or PES were observed in elderly compared to younger patients, with low rates of mortality, MI, ischaemic TVR, and TVF at 30days, confirming early safety of PCI in elderly patients. Incidence of 3-year cardiac mortality, MI and stent thrombosis were low regardless of age without significant differences between elderly and younger patients, providing further evidence of the long-term safety of DES.
Despite the fact that a significant percentage of patients treated with a DES are elderly, no targeted assessment of late outcomes including rates of restenosis and repeat revascularisation after PCI has been reported in this patient subset. Scant and inconsistent data are available from several retrospective series lacking systematic follow-up. In the retrospective series by De Gregorio et al,15 higher rates of angiographic restenosis after implantation of bare metal stents were observed in elderly patients (≥75 years) compared with younger patients (47% vs. 28%; p=0.0007), while in the series by Muñoz et al,18 rates of angiographic restenosis did not differ significantly between these age groups (21% vs. 28%). Among 9,868 Medicare beneficiaries ≥65 years of age treated with PCI in 1998, clinically significant restenosis was discovered in >14% of the patients within the first year after PCI.19 In most reports, rates of TLR were not found to be related to age.13,15,16,18
In agreement with the overall results of the SPIRIT III trial, implantation of EES as opposed to PES in elderly patients ≥65 years of age was associated with significant reductions in neointimal tissue proliferation and restenosis rates translating into a significant reduction of TLR and TVR. There was a 48% reduction in TVF and 49% reduction in MACE at three years in elderly patients. Surprisingly, the reduction in late loss seen with EES as compared to PES was confined to elderly patients, while no significant differences in angiographic and clinical outcomes were observed between the two stents in younger patients. With PES treatment, however, elderly patients had significantly increased rates of late loss and binary angiographic restenosis, with comparable rates of revascularisation at three years. There was a significant interaction for in-segment late loss, older age, and stent type. The nature of this finding is not clear at this time and requires further research to clarify whether there is an age-specific mechanism of neointimal response after treatment with DES.
In conclusion, the current analysis of outcomes from the SPIRIT III trial through three years suggests that the use of EES and PES was safe in elderly patients while greater efficacy was achieved using EES as assessed by significant reductions in 3-year rates of MACE and TVF.
Limitations
This post hoc analysis was not pre-specified in the original trial design, and thus has inherent limitations. The number of elderly in this study makes it underpowered to detect small differences between the groups in low-rate events such as death and stent thrombosis. Patients with complex clinical and angiographic characteristics were excluded from the SPIRIT III trial, and therefore data of this analysis cannot be extrapolated to the entire spectrum of patients with coronary artery disease requiring coronary revascularisation. Finally, the observed in SPIRIT III relationship between age, different DES types and angiographic restenosis has not been previously described, and should therefore be confirmed in additional studies.
Acknowledgements
Abbott Vascular was the sole sponsor of this study.
Conflict of interest statement
Dr. JB Hermiller is a research consultant for Abbott Vascular. Dr. AJ Lansky has research support from Abbott Vascular. Dr. RJ Applegate has research support from and is part of the Advisory Board of Abbott Vascular. Dr. GW Stone is on the Advisory board for Abbott Vascular and Boston Scientific. Drs. Manejeh Yaqub, Poornima Sood, Krishnankutty Sudhir, and Ms. Sherry Cao are employees of Abbott Vascular. The other authors have no conflict of interest to declare.

