Abstract
Aims: The impact of age on outcomes following everolimus-eluting stent (EES) or paclitaxel-eluting stent (PES) implantation was evaluated in a patient-level pooled analysis of the SPIRIT III (n=1,002) and SPIRIT IV (n=3,687) trials.
Methods and results: Clinical outcomes with EES compared to PES in elderly (≥65 years, n=2,071) and younger (<65 years, n=2,617) patients were evaluated at one year. At one year, elderly patients treated with EES rather than PES showed a significant reduction in target lesion failure (TLF) (3.9% EES vs. 6.8% PES, p=0.006), major adverse cardiac events (MACE) (4.0% EES vs. 7.1% PES, p=0.005), and ischaemia-driven target lesion revascularisation (ID-TLR) (2.0% EES vs. 4.0% PES, p=0.01). Younger patients treated with EES rather than PES also had significantly reduced one-year rates of TLF (4.9% EES vs. 7.9% PES, p=0.003), MACE (5.0% EES vs. 8.0% PES, p=0.004), target vessel myocardial infarction (MI) (2.0% EES vs. 3.4% PES, p=0.04), ID-TLR (3.3% EES vs. 5.5% PES, p=0.01) and stent thrombosis (0.5% EES vs. 1.6% PES, p=0.01).
Conclusions: In a pooled analysis from the SPIRIT III and IV trials, EES was safer and more effective than PES in both younger and older cohorts as evidenced by lower rates of TLR, TLF and MACE.
Introduction
Compared to their younger counterparts, elderly patients have significantly greater comorbidities, including more extensive coronary artery disease, and a more frequent history of prior myocardial infarction and coronary artery bypass grafting1,2. Older patients account for an increasing proportion of percutaneous coronary interventions (PCI) and have a higher risk of periprocedural complications2. Drug-eluting stents (DES) are now widely used in all age groups, but there are limited studies with sufficient statistical power that compare outcomes in elderly patients with those from younger patients, or that identify whether there are age-specific differences between different DES. The SPIRIT III (n=1,002) and IV (n=3,687) trials randomised a total of 4,689 patients to everolimus-eluting stents (EES) vs. paclitaxel-eluting stents (PES3,4). Of the 4,689 patients, 44.2% were ≥65 years and the median age was 63.2 years. The United States is projected to experience rapid growth in its older population (over the age of 65) between 2010 and 2050 with the baby boomers beginning to cross into this category in 20115. Most people aged 65 or older are eligible for Medicare hospital insurance in the United States. The standard age of eligibility to pension benefits is set at 65 years in two-thirds of Organisation for Economic Co-operation and Development (OECD) countries6. We therefore examined data from the pooled SPIRIT III and SPIRIT IV databases to determine the effect of age on outcomes in patients treated with PCI using EES and PES.
Methods
Patients and protocol
The design of the SPIRIT III and IV trials, which were both prospective, multicentre, randomised, single-blind, active-controlled studies, have previously been described3,4. In brief, SPIRIT III randomised 1,002 patients with either one or two de novo native coronary artery lesions (maximum, one lesion per epicardial coronary artery) in a 2:1 ratio to receive either EES (XIENCE V™; Abbott Vascular, Santa Clara, CA, USA) or PES (TAXUS™ EXPRESS2™; Boston Scientific, Natick, MA, USA), respectively. SPIRIT IV randomised 3,687 patients with one, two or three previously untreated native coronary artery lesions (maximum two lesions per epicardial vessel) in a 2:1 ratio to EES vs. PES. Clinical and angiographic inclusion criteria were similar between studies and included study lesion diameter stenosis of ≥50% and <100%, reference vessel diameter ≥2.5 mm and ≤3.75 mm, and lesion length ≤28 mm. For both trials, EES were available in diameters of 2.5 to 4.0 mm and lengths of 8, 18 and 28 mm, while the full range of commercially-available PES diameters of 2.5 to 3.5 mm and lengths of 8, 12, 16, 20, 24, 28 and 32 mm were used. Both studies were approved by the institutional review boards at participating centres, and consecutive eligible patients signed informed consent.
Medication administration and clinical follow-up
In both trials, aspirin ≥300 mg was administered prior to catheterisation and an oral clopidogrel load of ≥300 mg was recommended pre-procedure and required within one hour after stent implantation. In both trials, aspirin ≥80 mg was administered daily indefinitely, and clopidogrel 75 mg daily was prescribed for ≥6 months (SPIRIT III) or for ≥12 months (SPIRIT IV). In both trials, clinical follow-up was scheduled at 30, 180, 270, and 365 days and then yearly for five years.
Clinical endpoints and definitions
Endpoint definitions have been described previously and were similar in both trials3,4. Target lesion failure (TLF) at one year was defined as the composite of cardiac death, target vessel myocardial infarction (MI) or ischaemia-driven target lesion revascularisation (ID-TLR) by either PCI or coronary artery bypass surgery (CABG). Additional endpoints for analysis included the composite of cardiac death or target vessel MI, ID-TLR, major adverse cardiac events (MACE; defined as the composite of cardiac death, MI or ID-TLR). Stent thrombosis was adjudicated using the Academic Research Consortium (ARC) definite or probable criteria7. Bleeding (haemorrhagic) complications for both SPIRIT III and IV included haematoma requiring transfusion or surgical repair, and any bleeding event associated with haemoglobin drop >5 g/dL or requiring transfusion or surgical repair (e.g., retroperitoneal bleed, gastrointestinal [GI] bleed, access site bleed).
Statistical analysis
Since examination of subgroup data is inherently under-powered, the SPIRIT III and IV databases were pooled, justified on the basis of similar inclusion and exclusion criteria, randomisation ratios, endpoint definitions and clinical follow-up schedules. Pooling of patient-level data from both trials yielded a total of 4,689 randomised patients. Data were analysed in two groups, younger (<65 years) vs. older (>65 years) cohorts.
Binary variables are presented as percentages, and were compared by Fisher’s exact test. Continuous variables are presented as mean±standard deviation, and were compared by t-test. All analysis was performed for the intent-to-treat population, consisting of all patients randomised, regardless of the treatment actually received. Only patients who had follow-up information and/or occurrence of an event are included in the denominator calculation for the binary endpoints. In other words, patients lost to follow-up in whom no event had occurred by a certain time-point were excluded from the analysis of binary endpoints. Kaplan-Meier curves representing the estimated cumulative incidence rates were also constructed for time-to-event variables and compared by log-rank test. Univariable and multivariable logistic regression analyses were performed for older and younger patient groups, respectively. Included in these analyses are demographic, clinical and procedural variables. Final models were selected and presented with selection criteria based on both statistical significance and clinical consideration. Logistic regression was also conducted to evaluate the interaction between the treatment and different age groups. A two-sided a=0.05 was used for all superiority testing. All p-values are displayed for descriptive purposes only. All statistical analyses were performed by SAS® version 9.1.3 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient population and characteristics
A total of 4,688 patients from SPIRIT III and IV were randomised. Of these, 2,071 (44.2%) were ≥65 years of age, and 2,617 (55.8%) were <65 years old. In the ≥65 year cohort, 1,378 and 693 patients were randomised to EES vs. PES, respectively. In the <65 year cohort, 1,749 and 858 patients were randomised to EES vs. PES, respectively. Data on patient and lesion characteristics in older and younger patients treated with EES vs. PES are provided in Table 1. Median age was 56 years in the younger cohort and 73 years in the older cohort. Younger patients had lower rates of hypertension, but were more likely to be male and smokers compared to the older population. In both older and younger cohorts, patient and lesion characteristics were similar in EES vs. PES patients. While procedural factors (Table 1) were similar in older and younger cohorts, the number of stents per lesion, total stent length per lesion and total stent to lesion length ratio were all higher for EES compared to PES, in both cohorts.
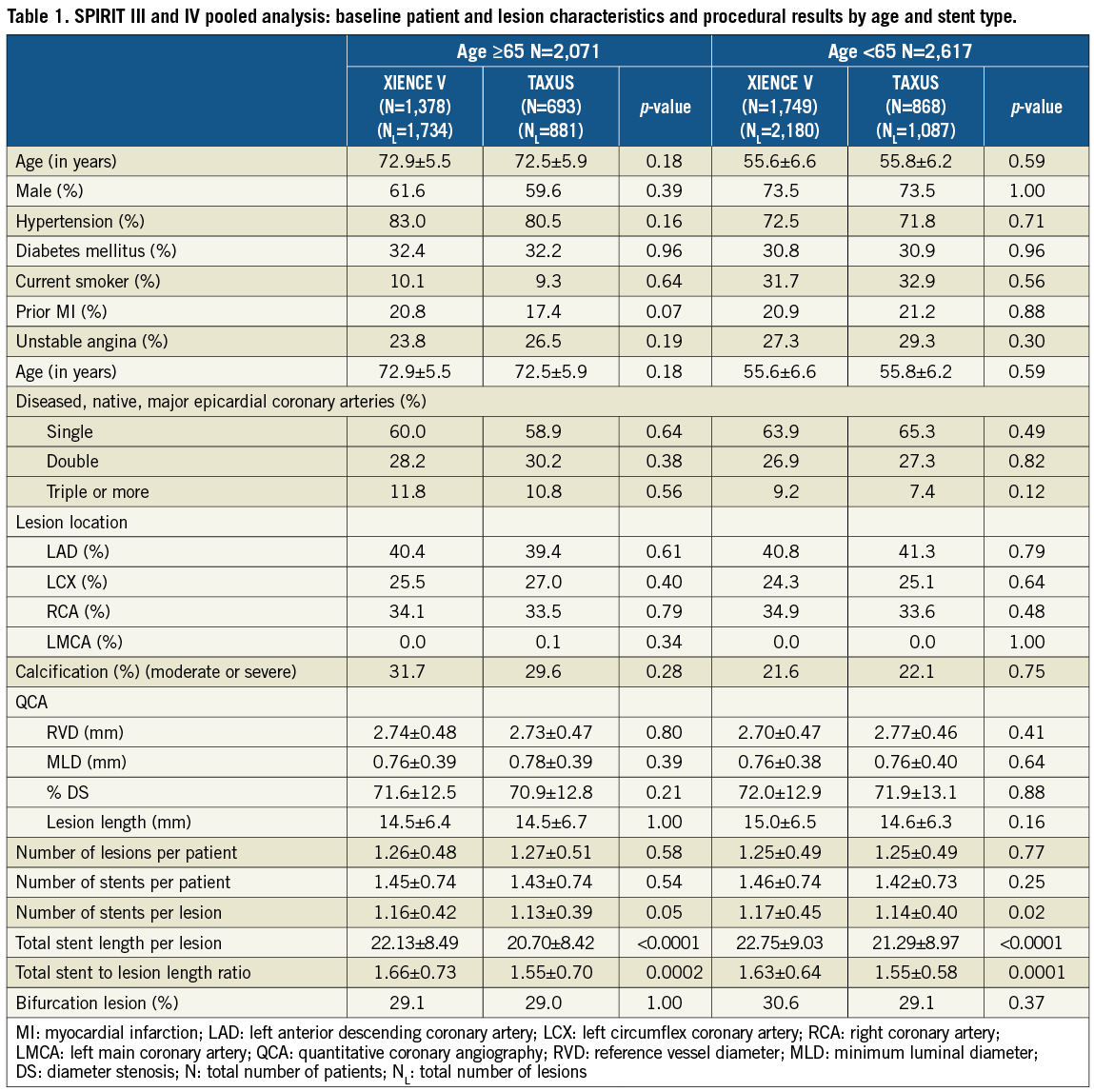
Clinical outcomes at one year
TLF and MACE rates at one year were significantly lower in the EES compared to PES subgroup in both older and younger patients (Table 2). Kaplan-Meier (KM) estimates of TLF rates showed a difference of 2.9% between EES and PES in both cohorts at one year (Figure 1A and Figure 1B). The rate of target vessel MI was significantly reduced with EES compared to PES in the younger cohort, driven by fewer Q-wave and non-Q-wave MI with EES. Ischaemia-driven target lesion revascularisation (ID-TLR) rates were significantly lower for EES in both younger and older cohorts (Table 2). There were no significant interactions by treatment type between older and younger subgroups for the one-year rates of TLF, MACE or ID-TLR (p-interaction=0.65, 0.74 and 0.71, respectively). Stroke rates were similar in both treatment arms in both older (EES vs. PES: 1.2% vs. 1.9%, p=0.232) and younger (EES vs. PES: 0.8% vs. 0.7%, p=1.000) cohorts.
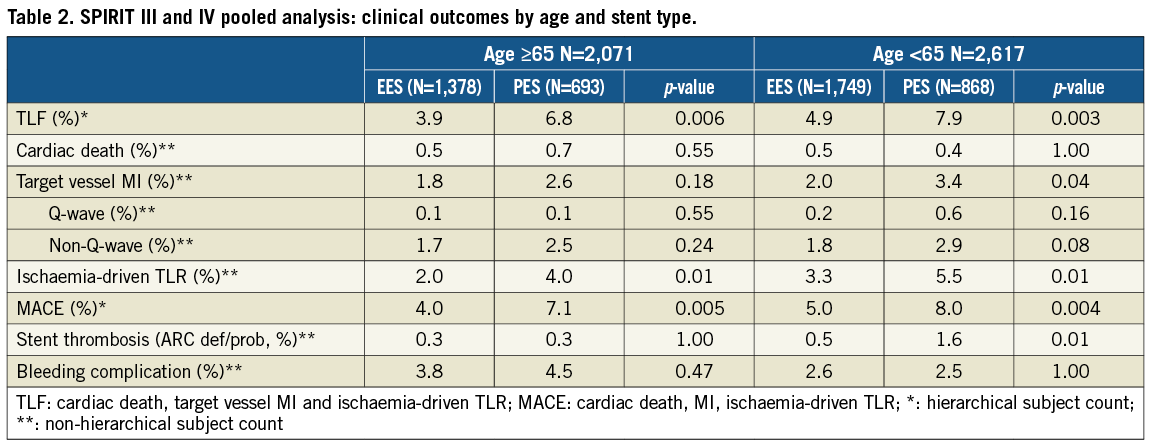
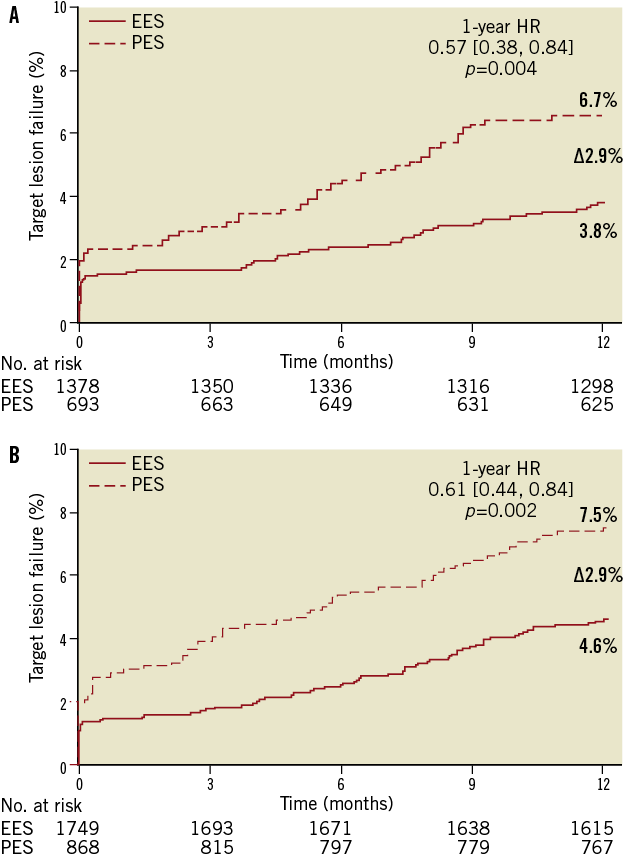
Figure 1. Kaplan-Meier time-to-event curves showing target lesion failure rates through one year for everolimus-eluting vs. paclitaxel-eluting stents in: (A) patients ≥65 years of age; and (B) patients <65 years of age.
In the older cohort, dual antiplatelet therapy (DAPT) use at one year was 87% in both EES- and PES-treated arms. Stent thrombosis rates in the ≥65 year cohort were similar: 0.3% in both treatment arms at one year (Figure 2). However, in the <65 year cohort, where DAPT use was 89% in both treatment arms at one year, stent thrombosis rates at one year were significantly lower with EES (0.5% vs. 1.6%, p=0.01). Both early (<30 days) and late (30 days-one year) rates of stent thrombosis were numerically lower with EES in younger patients. At one year, the older cohort appeared to have higher bleeding complications compared to the younger cohort for both stent types. The bleeding rates were not significantly different between EES and PES in the older cohort (EES vs. PES: 3.8% vs. 4.5%, p=0.47) or younger cohort (2.6% vs. 2.5%, p=1.00) (Table 2).
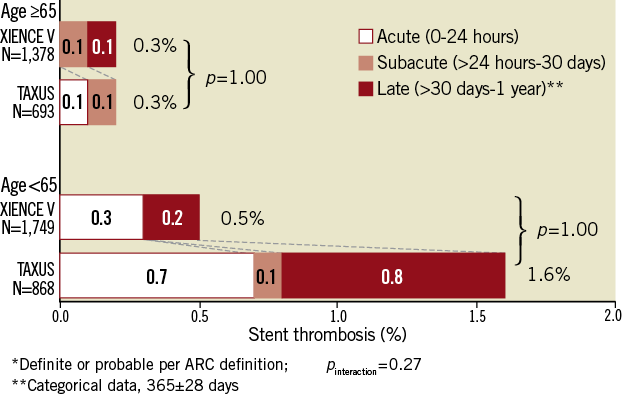
Figure 2. ARC-defined stent thrombosis rates through one year for everolimus-eluting vs. paclitaxel-eluting stents in patients ≥65 years of age (above); and patients <65 years of age (below).
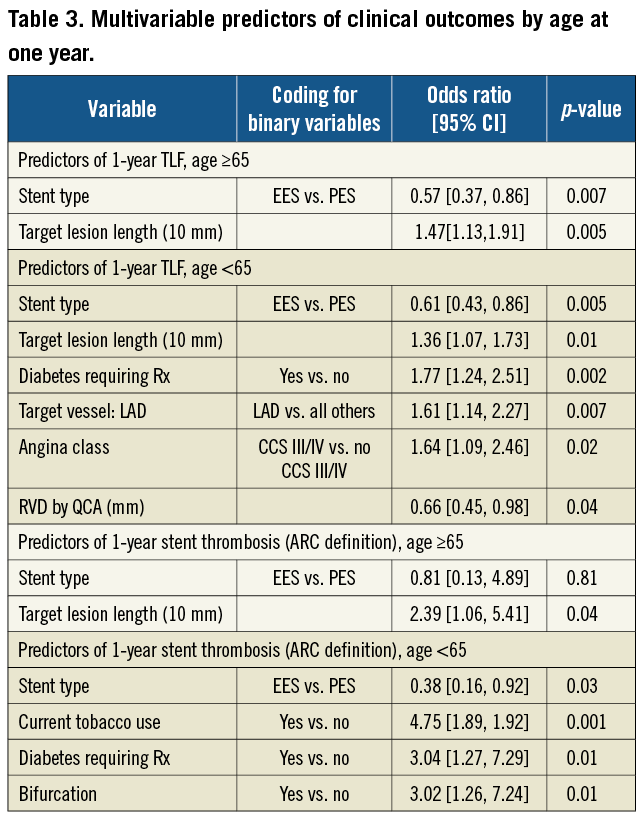
In older patients, multivariable predictors of TLF were stent type (EES vs. PES, OR [95%CI] = 0.57 [0.37, 0.86], p=0.007) and target lesion length. Only target lesion length was a predictor of ARC defined stent thrombosis in older patients (Table 3). In younger patients, multivariable predictors of TLF were stent type (EES vs. PES, OR [95%CI] = 0.61 [0.43, 0.86], p=0.005), diabetes, angina class, left anterior descending coronary artery (LAD) target vessel, reference vessel diameter and target lesion length. Multivariable predictors of stent thrombosis in younger patients were stent type (EES vs. PES, OR [95%CI] = 0.38 [0.16, 0.92], p=0.03), current tobacco use, diabetes and bifurcation lesions (Table 3).
Discussion
The present analysis represents the largest systematic age-based comparison of two DES, and demonstrates that in both older and younger patients, TLF, MACE and ID-TLR at one year were significantly lower in patients treated with EES compared to PES. Target-vessel MI and stent thrombosis rates were significantly lower with EES compared to PES in younger patients.
A previous substudy8 from the SPIRIT III trial with follow-up to three years showed that: a) implantation of both EES and PES in elderly patients was safe, without increased rates of cardiac death or MI; b) implantation of EES vs. PES in the elderly resulted in a significant reduction in late loss and a lower incidence of binary angiographic restenosis translating into a significant reduction in rates of TLR and TVR, as well as enhanced three-year survival free from MACE and TVF; c) three-year clinical and eight-month angiographic outcomes did not differ significantly as a function of age after the implantation of EES, while implantation of PES in the elderly as opposed to younger patients was associated with significantly increased late loss and rates of binary angiographic restenosis within the stent and within the segment.
In the present analysis, patients ≥65 years of age represented 44% of the study population. Despite a higher prevalence of prior MI in older patients receiving an EES, and a higher number of stents per lesion, total stent length per lesion and total stent:lesion length ratio in the EES compared to PES subgroup in both older and younger patients, the clinical outcomes of TLF, MACE and TLR were all improved with EES compared to PES irrespective of age. Stent type was a significant predictor of TLF in both younger and older cohorts, with the use of EES being a predictor of improved event-free survival in both groups. These findings are consistent with the one-year outcomes in the overall population in SPIRIT IV9, and may be a result of thinner stent struts (EES vs. PES: 81 vs. 132 μm strut thickness and 7.8 vs. 19.6 μm coating thickness10) and improved efficacy of the antiproliferative drug (everolimus) resulting in less neointimal proliferation and restenosis than with paclitaxel11. In addition, reduced MI rates with EES in SPIRIT III patients with jailed side branches has been previously reported12. The enhanced performance of EES compared to PES in jailed side branches may account for the lower periprocedural MI rate with EES in younger patients in this study.
Previous studies from the bare-metal stent era have shown mixed age-dependent results. Higher rates of angiographic restenosis after implantation of a bare-metal stent were observed in elderly patients (≥75 years) compared with younger patients in one report13, but others have shown that rates of angiographic restenosis did not differ significantly between these age groups14. A clinically significant restenosis rate of >14% in the first year after PCI was reported in Medicare beneficiaries ≥65 years of age treated with PCI in 199815. In most studies that reported the effects of age on restenosis, rates of TLR were not found to be related to age13,14,16,17. In the present study, elderly patients had generally higher rates of bleeding complications compared to younger patients, consistent with previous studies assessing bleeding risk from antiplatelet therapy18,19. However, bleeding rates were not significantly different between stent types in either age group.
In our study, low rates of stent thrombosis in both younger and older patients (0.5% and 0.3%, respectively) receiving EES were observed. This is consistent with rates from the entire SPIRIT IV cohort20, as well as the “standard risk” population from the XIENCE V® USA Post-Approval Study21. Two additional real world cohorts from the COMPARE trial22 and the RESOLUTE AC trial23 have also shown low rates of stent thrombosis (0.7%) for EES in the first year, even when complex patients are included. These observations are consistent with the hypothesis that the fluorocopolymer coating on the EES confers protection against thrombosis, a feature referred to as “fluoropassivation24”. By contrast, stent thrombosis rates were higher in younger compared to older patients treated with PES (1.6% vs. 0.3%), which is consistent with the observation from a retrospective study of PES outcomes in elderly patients25. An intravascular ultrasound study of subacute stent thrombosis found that subacute closure occurred predominantly in plaques with high lipid and cellular content and the lesions were rarely calcified26. The lower stent thrombosis rates in older patients treated with PES might be related to the plaque composition as higher rates of lesion calcification were seen in the elderly patients for both treatments, although further studies are needed to confirm these findings. In addition, our observation of overall lower rates of stent thrombosis while higher rates of bleeding complications in older versus younger patients is worth further investigation with a larger population to help guide the usage of dual antiplatelet therapy after DES in elderly patients.
In patients <65 years, rates of stent thrombosis with EES were significantly lower than with PES, suggesting that the safety benefit of EES is particularly accentuated in this younger cohort. Treatment with EES was also a significant independent predictor of freedom from stent thrombosis in younger subjects, while tobacco use, diabetes and bifurcations were positive predictors in the model. Conflicting data have been reported regarding the impact of smoking on the clinical outcomes of PCI27-30. In this analysis of 4,689 randomised patients from the pooled SPIRIT III-IV results, the percentage of current smokers is similar between the two stent types in patients <65 years of age (EES vs. PES: 30.8% vs. 30.9%, p=0.96). Current tobacco use was shown to be an independent risk factor for stent thrombosis but not for TLF in the younger cohort with PES. The outcomes with EES at one year were not associated with current tobacco use in this study.
Limitations
This post hoc analysis was not prespecified in either of the original trial designs, and thus has inherent limitations. Patients with complex clinical and angiographic characteristics such as ST-segment elevation MI, bifurcations with side branches >2 mm in diameter, and chronic total occlusions were largely excluded from the SPIRIT III and SPIRIT IV trials, therefore data from this analysis cannot necessarily be extrapolated to all “real world” patients with coronary artery disease requiring coronary revascularisation. Additionally, sample sizes in individual subgroups in this analysis may have been insufficient to derive reliable estimates of stent thrombosis, recognised as a low frequency event requiring several thousand patients to determine accurate rates of incidence31.
In conclusion, the current analysis suggests that PCI using EES or PES is safe in patients ≥65 years of age. In this report, EES appears to be safer than PES in younger patients as shown by a reduction in the rates of MI and stent thrombosis with EES. Everolimus-eluting stents were more effective than PES in both the young and elderly as measured by reduced rates of TLR, as well as the composite safety and efficacy measures of TLF and MACE.
Acknowledgements
The authors thank Abbott Vascular, Santa Clara, CA, USA for funding.
Conflict of interest statement
J. Hermiller reports serving as a consultant for Abbott Vascular and Boston Scientific Corp. R. Applegate reports serving on scientific advisory boards and honoraria/consulting for Abbott Vascular and receiving research funding from Abbott Vascular. J. Wang reports serving on the medical advisory board for Boston Scientific Corp. and as a speaker for Abbott Vascular and Boston Scientific Corp. P. Gordon reports having received research support from Abbott Vascular and Boston Scientific Corp. K. Sudhir, M. Yaqub, S. Cao, J. Ferguson, R. Smith and P. Sood are employees of Abbott Vascular. G. Stone reports serving on scientific advisory boards for and receiving honoraria from Abbott Vascular and Boston Scientific Corp, and as a consultant to Medtronic. All other authors have no conflict of interest to declare.

