Abstract
Aims: To assess the safety and efficacy of the XIENCE V everolimus-eluting stent (EES) compared to the TAXUS paclitaxel-eluting stent (PES) in women at two years.
Methods and results: In this pooled analysis, a cohort of 395 women and 906 men was studied by using patient level and lesion level clinical data from SPIRIT II and SPIRIT III studies. Women enrolled in these two studies had higher demographic and lesion risk characteristics than their male counterparts. In-stent and in-segment late loss (LL) was significantly less in the women in the EES group compared to the women in the PES group (in-stent 0.15±0.44 mm vs. 0.45±0.51 mm; P<0.01, in-segment 0.09±0.46 mm vs. 0.29±0.40 mm; P<0.01). In women, EES compared to PES resulted in significant reductions in major adverse cardiac events (MACE) (8.5% vs 16.4%; p=0.02) and in target vessel failure (TVF) (11.2% vs 19.5%; p=0.02) at two years. In men, a significant difference was seen in in-stent LL and in-stent % diameter stenosis (DS) favouring EES (in-stent LL 0.14±0.33 mm vs. 0.28±0.47 mm; P<0.01, in-stent %DS 9.28±13.86 vs. 13.64±18.31; P<0.01). MACE rates at two years were lower in males treated with EES compared to PES (6.7% vs. 10.9%; p=0.03). The interaction between gender and stent type was not found to be significant for MACE at two years.
Conclusions: In this pooled analysis of two randomised trials, at two years, EES compared to PES resulted in reduced angiographic LL, fewer MACE and TVF events in women and reduced angiographic LL and %DS and fewer MACE events in men.
Introduction
Coronary artery disease (CAD) remains the leading cause of morbidity and mortality in both men and women in developed countries1. Unfortunately, many women underestimate or do not recognise the threat coronary artery disease (CAD) poses to their health, and primary health care providers are not always alert in recognising the symptoms and referring women to see a cardiologist. There is a perception that women are protected against CAD but, in fact, the rate of CAD increases two to three times after menopause.2 This increase is not completely understood, but serum cholesterol, blood pressure, and fat around the abdomen also increase around this time.
In the past, medical research on heart disease was primarily focused on men. Now, researchers recognise that there are significant differences in coronary artery disease between women and men.4 For example, men usually have typical anginal symptoms: retrosternal chest pain that radiates to the shoulders, neck, and arms. Although women may have these symptoms too, many have less common symptoms such as breathlessness, heartburn, nausea, jaw pain, back pain, or fatigue. Myocardial infarctions (MI) in women are often brought on by anxiety or mental stress or even sleep, while in men they occur more often with exercise or exertion.4
Because women do not always have the classic symptoms of MI or typical onset of angina, they may delay seeking care, and when they do, the symptoms may not be recognised as cardiac, resulting in less aggressive treatment approaches. In response to these concerns, the American Heart Association published specific guidelines for preventing and treating coronary artery disease in women.3 These guidelines address lifestyle changes, medication and supplements, and, in menopausal women, hormone therapy.
In addition, many of the assumptions regarding cardiovascular disease in women are from studies where women have been under-represented, and in trials that were not designed to account for any gender differences. This is best demonstrated by the smaller representation of women in clinical studies4; the majority of percutaneous coronary intervention (PCI) trials to date have enrolled only 15-35% of women. Conflicting results from past studies, some of which demonstrate possible poorer long-term clinical outcomes in women when compared to men5-7, may play a role in the referral process resulting in fewer women being referred for PCI.
Stenting of de novo lesions in native coronary arteries has been shown to be a safe and effective treatment of occlusion due to atherosclerosis8,9. Additionally, the application of anti-proliferative drugs to stents decreases the need for patients to undergo repeat percutaneous transluminal coronary angioplasty (PTCA) or coronary artery bypass graft (CABG)10,11. Drug-eluting stents (DES) are currently widely used in PCI practice globally.
The XIENCE V everolimus-eluting stent (EES) has been designed to release the drug from a thin (7.8-µm), non-adhesive, durable, biocompatible fluorinated copolymer coated onto a low-profile (0.0032-in [0.0813-mm] strut thickness), flexible cobalt-chromium stent. Preclinical studies have shown more rapid endothelialisation with this stent compared to sirolimus-eluting and paclitaxel eluting stents.12 Following favourable results with this device in the SPIRIT FIRST randomised study,13 the SPIRIT II study in Europe, India and New Zealand14 and the SPIRIT III study15 in the United States were performed to evaluate the everolimus-eluting stent in comparison to a widely used paclitaxel-eluting stent in patients with coronary artery disease.
Due to the similarity of these two study protocols and their inclusion and exclusion criteria, an ad hoc independent pooled analysis was performed with individual patient data up to two year follow-up from SPIRIT II and SPIRIT III, to confirm that the positive results for the XIENCE V (EES) when compared to the TAXUS paclitaxel-eluting stent (PES) seen in the overall study populations could also be applicable to the separate female and male populations. In addition the demographic differences and outcomes in men compared to women are reported.
Methods
Patients and design
Details of the SPIRIT II and SPIRIT III trials have been previously described14,15. In brief, SPIRIT II was a prospective, randomised, single-blind, clinical study performed at 28 centres in Europe, India and New Zealand in which 300 patients were randomised in a 3:1 ratio to receive either the polymer-based EES (XIENCE V; Abbott Vascular, Santa Clara, CA, USA) or the polymer-based PES (TAXUS EXPRESS2 or TAXUS LIBERTE; Boston Scientific, Natick, MA, USA). All patients underwent angiographic follow-up at six months. A total of 81 patients (27%) were women.
SPIRIT III was a prospective, multicentre, randomised, single-blind, clinical study performed at 65 sites in the United States in which 1,002 patients were randomised in a 2:1 ratio to receive the EES or the PES stent. A subgroup of 564 patients underwent angiographic follow-up at eight months. A total of 314 patients (31%) were women. Clinical follow-up was performed in both studies annually up to five years.
For both studies, patients were eligible if they were older than 18 years and had evidence of myocardial ischaemia. The patient could have a maximum of two de novo native coronary artery lesions, which had to be located in different major epicardial vessels. The de novo target lesion(s) had to have a reference vessel diameter between 2.5 mm and 3.75 mm for SPIRIT III (4.25 mm for SPIRIT II) by visual estimation, a target lesion length ≤28 mm, a visually estimated stenosis between 50-99% of the luminal diameter, and a thrombolysis in myocardial infarction (TIMI) flow grade of one or more. Patients were not eligible for enrolment if they had a known diagnosis of acute myocardial infarction three days prior to the baseline procedure, a left ventricular ejection fraction of less than 30%, or were awaiting a heart transplant. Additionally, patients having target lesion(s) with an aorto-ostial or left main location, a lesion located within 2 mm of the origin of the left anterior descending or left circumflex, heavy calcification, or a visible thrombus within the target vessel were also excluded from the study.
All patients provided written informed consent before the procedure and the study protocols were approved by the review board of the participating institutions.
Major adverse cardiac events (MACE) and stent thrombosis events were adjudicated by an Independent Clinical Events Committee and a Data Safety Monitoring Board oversaw the safety of the trials.
The pooled, patient level analysis of combined SPIRIT II and SPIRIT III data was performed by The Cardiovascular Research Foundation, (New York, NY, USA). This pooled analysis included 1,302 patients, 892 in the EES group and 410 in the PES group.
Clinical endpoint definitions
Target vessel (or lesion) revascularisation was considered to be ischaemia-driven if associated with a positive functional study, a target vessel (or lesion) diameter stenosis ≥50% by core laboratory quantitative analysis with ischaemic symptoms, or a target vessel (or lesion) diameter stenosis ≥70% with or without documented ischaemia. TVF was defined as the occurrence of either cardiac death, MI, or ischaemia-driven TVR. MACE was defined as the occurrence of either cardiac death, MI, or ischaemia-driven TLR. MI was defined as either the development of new pathologic Q-waves ≥0.4 seconds in duration in ≥2 contiguous leads, or an elevation of creatine phosphokinase levels to ≥2.0 times normal with positive creatine phosphokinase-MB. Stent thrombosis was prospectively defined by the study protocols as an acute coronary syndrome with angiographic evidence of thrombus within or adjacent to a previously treated target lesion, or in the absence of angiography, any unexplained death or acute MI with ST-segment elevation or new Q-waves in the distribution of the target lesion occurring within 30-days post procedure. Definite or probable stent thrombosis was also adjudicated in a post hoc analysis using the Academic Research Consortium (ARC) definitions.16
Statistical analysis
All analyses were performed on the intent-to-treat population, consisting of all patients randomised in the study, regardless of the treatment actually received. However, patients lost to follow-up in whom no event had occurred prior to the follow-up windows were not included in the denominator for calculations of binary endpoints. Survival curves using all available follow-up data were also constructed for time to event variables using Kaplan-Meier estimates and compared by log-rank test. For binary variables, such as major adverse cardiac events (MACE), counts and percentages were calculated. Fisher’s exact test was used to compare binary variables between the two treatment arms. For continuous variables, such as in-segment late loss, means and standard deviations were calculated. A two sided t test was used to compare continuous variables between the two treatment arms. All statistical analyses were performed using SAS version 9.1.3 (SAS Institute Inc, Cary, NC, USA).
Results
Patient characteristics
Of the overall pooled analysis study population, a total of 395 patients (30%) were women; 265 patients (67%) in the EES group and 130 (33%) in the PES group. Of these women, 161 patients (41%) in the EES group and 68 patients (17%) in the PES group underwent angiographic follow-up at six or eight months. Among men, there was a total of 906 patients included, 627 (69%) in the EES group and 279 (31%) in the PES group. Of these men, 419 patients (46%) in the EES group and 176 patients (19%) in the PES group underwent angiographic follow-up at six or eight months.
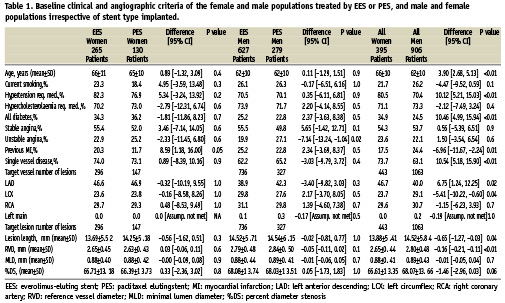
When comparing the overall female and male populations, women were older, had more hypertension, more diabetes, less prior MI, more single vessel disease and more often a lesion treated in the left anterior descending artery (LAD) but less often in the left circumflex artery (LCX). (Table 1)
Baseline characteristics of the patient groups according to stent type implanted were comparable except for more prior MI in the female EES group compared to the female PES group and more unstable angina in the male PES group compared to the male EES group. (Table 1)
For lesion characteristics as measured by quantitative coronary angiography of the overall female population compared to the male population the females had shorter lesions and a smaller reference vessel diameter (RVD). (Table 1)
The patient groups according to stent type implanted were similar between the two stent type groups for both males and females.
Post procedural results and angiographic outcomes
As shown in Table 2, post procedure angiographic measures were not significantly different between the two stent type groups except for a greater post procedure in-stent % diameter stenosis (%DS) in the EES female group and a smaller in-stent minimal lumen diameter (MLD) in the EES male group. When comparing the overall female and male populations, the females had a smaller RVD, a smaller in-stent MLD and a smaller in-stent % DS when compared to the men.
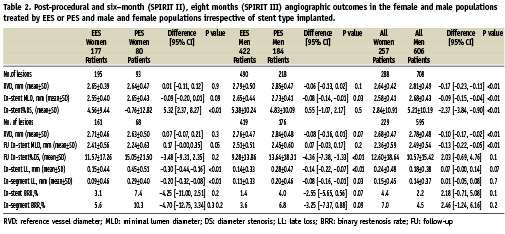
Angiographic outcome measures at six months (SPIRIT II) and eight months (SPIRIT III) follow-up are shown in Table 2. In the female patients in the two stent groups, the in-stent late loss (LL) was 0.15±0.44 mm in the EES group versus 0.45±0.51 mm in the PES group (difference: –0.30 mm [95% CI= -0.44 to -0.16]; p<0.01). In-segment LL was also less in the EES group (0.09±0.46 mm versus 0.29±0.40 mm; difference: –0.20 mm [95% CI= –0.32 to –0.08]; p<0.01). As a result, strong trends were present towards a reduction in binary in-stent and in-segment restenosis rates (BRR) with the female EES group compared to the female PES group, 3.1% and 5.6% for the EES group vs. 7.4% and 10.3% for the PES group respectively.
When comparing males implanted with either an EES or PES a significant difference was seen in the rates of in-stent LL and in-stent % Diameter Stenosis (DS) favouring EES (in-stent LL: mean, 0.14 [SD, 0.33] mm vs 0.28 [SD, 0.47] mm; difference, –0.14 [95% CI, –0.22 to –0.07]; P<0.01, in-stent %DS: mean, 9.28 [SD, 13.86] vs. 13.64 [SD, 18.31]; difference, –4.36 [95% CI, –7.38 to –1.33]; <0.01). As with women, a trend was present towards a reduction in binary in-stent and in-segment restenosis rates (BRR) with the male EES group compared to the male PES group, 1.4% and 3.6% for the EES group vs. 4.0% and 6.8% for the PES group respectively.
When comparing the overall populations irrespective of stent type, women had a smaller MLD at follow-up (2.36±0.59 mm) compared to the men (2.49±0.54). However, although the in-stent %DS, in-stent LL and in-stent BRR were numerically higher in the women this did not reach statistical significance. (%DS 12.6±18.6 vs. 10.6±15.4; LL 0.24±0.48 mm vs. 0.18±0.38 mm; %BRR 4.4% vs. 2.2%).
Clinical outcomes
Use of the EES compared to the PES in women resulted in a reduction in the composite endpoint of MACE at two years (8.5% vs. 16.4%; relative risk 0.52 [95% CI= 0.42 to 0.65]; p= 0.02). (Table 3) Likewise, the rate of TVF in the female population at two years was lower in the EES group compared to the PES group (11.2% vs. 19.5%; relative risk 0.57 [95% CI= 0.46 to 0.71]; p=0.02).
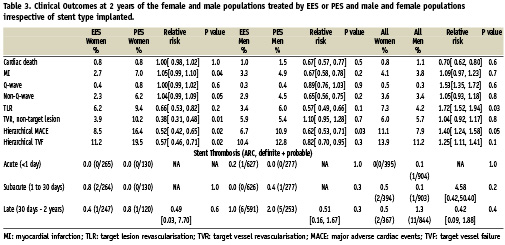
As shown in Table 3, there were no differences between the EES and the PES female population groups in the two year rates of cardiac death, myocardial infarction (all, Q-wave, or non-Q-wave) and target lesion revascularisation (all, treated with percutaneous coronary intervention PCI, or treated with coronary artery bypass graft CABG). The rates of target vessel, non-target lesion revascularisation at two years were lower in the EES group compared to the PES group (3.9% vs. 10.2%; relative risk 0.38 [95% CI= 0.31 to 0.48]; p=0.01). As shown in Figure 1, the difference between the curves for MACE became apparent in the early postprocedural period due to fewer myocardial infarctions with the EES, and then diverges further between six months and 12 months due to fewer target lesion revascularisation procedures with the EES (Long-Rank P value = 0.02).
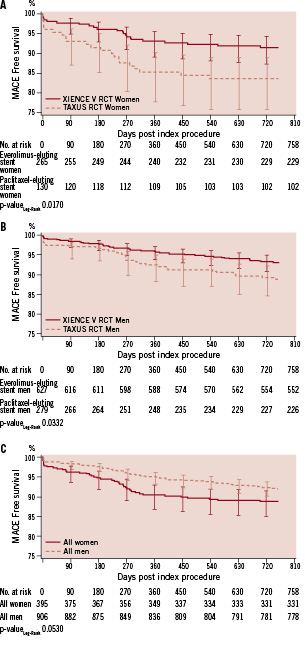
Figure 1. Kaplan-Meier survival curves for major adverse cardiac events among male and female patients randomised to receive EES or PES.
In the male population MACE rates in males treated with an EES were lower when compared to those treated with a PES (6.7% vs. 10.9%; relative risk 0.62 [95% CI= 0.53 to 0.71]; p=0.03). (Table 3)
In both the male and female EES vs. PES populations, there were no differences between the two devices in the rates of stent thrombosis, either early (≤30 days) or late or very late (>30 days), when analysed by the Academic Research Consortium definitions16.
In the overall population, although the TLR, TVF and MACE rates were numerically higher in women compared to men, (TLR; 7.3% vs. 4.2%; TVF; 13.9% vs. 11.2%; MACE; 11.1% vs. 7.9%), these numbers did not reach statistical significance. (Table 3)
Discussion
This pooled analysis confirms other reports17-19 that women included in stent studies generally have higher demographic risk profiles than their male counterparts. The women patients in our studies were older than the men, had higher incidence of hypertension, were more often diabetic, had more prior MI, more single vessel disease and more often a target lesion in the LAD. Men had more often a target lesion in the LCX. In addition in the women patients the reference vessel diameter of their treated vessels was significantly lower and the length of the lesions shorter.
When comparing men and women with regards to stent type implanted, women included in the EES group had more often a history of prior MI that reached a statistically significant difference. Men in the PES group however had more frequently unstable angina.
Clinical outcomes - EES vs. PES in both genders
Despite EES and PES groups being very similar with regards to patient inclusion, improved outcomes were obtained in both the male and female EES groups when compared to the male and female PES group, with statistically significant reductions in the EES group in rates of MACE and TVF at two years for women and in MACE rates for men.
The interaction between gender and stent type was also tested by using a multivariate logistic regression model, and the interaction term was not found to be significant for MACE at two years (p=0.32). This result indicates that comparing with the PES group; the improved outcomes in two year MACE obtained in the EES group are similar for both males and females. However, this result does not exclude the possibility that there is indeed a gender effect, since this study was not powered for such an analysis.
In addition, in these two groups according to stent type implanted, although statistical significance was not reached, the numbers for myocardial infarctions, and target lesion revascularisations were consistently lower in the EES groups when compared to the PES groups.
This is in line with the previous reports of the SPIRIT II14 and SPIRIT III15 studies which showed benefit of the XIENCE V stent over the TAXUS stent in treating coronary artery lesions in the combined male and female population.
Clinical outcomes - women vs. men
When the overall populations of female vs. male, irrespective of stent type implanted were considered, there was a numerically higher rate of TLR, TVF and MACE events at two years observed in women compared to men, which indicates that women patients do have a worse outcome than men when undergoing PCI procedures. However TVF and MACE differences did not reach statistical significance. This could correlate with the fact that women have a higher risk profile than men, undergo procedures in smaller vessels, have shorter lesions treated and possibly come later for treatment but further studies will be required to demonstrate a real difference.
A multivariate logistic regression analysis was also conducted, and gender was not found to be a significant predictor for MACE at two years. This result indicates that no evidence was found to support the assumption that being a male or female will affect the patient’s probability of having a MACE at two years after adjusting for the patient and lesion level characteristics. However, this result does not exclude the possibility that there is indeed a gender effect, since this study was not powered for such an analysis.
Angiographic outcomes
With regards to angiographic outcomes there was a statistically significant difference in in-stent late loss in favour of male and female patients receiving an EES compared to a PES (male; 0.14 mm vs. 0.28 mm, p<0.01; female; 0.15 mm vs. 0.45 mm, p<0.01). Although there was no difference in in-stent LL between males and females receiving an EES (0.15 mm vs. 0.14 mm) the in-stent late loss was higher for females than males in patients receiving a PES (0.45 mm vs. 0.28 mm, difference (95% CI) = 0.18 (0.04, 0.32) p=0.02).
Limitations
The limitation of this pooled analysis is that the studies were not designed to look at specific differences between the male and female populations, either as a pooled gender based analysis or with regards to gender and type of stent implanted. The analysis was performed retrospectively and other relevant data that could have been of importance for this subject, such as menopausal status, body mass index, time for referral, etc. was not gathered. The subgroups analysed were small, so any observations should be interpreted cautiously. In addition the inclusion criteria of both studies were limited to less complex lesions in more stable settings, so it is unclear if these results would be replicated in a more complex patient population with, for example, bifurcation lesions, the setting of acute myocardial infarctions, etc. Analysis of patient data with different drug eluting stents included in one group should also be viewed with caution, as EES and PES have different drugs that elute at different rates.
Specific studies to address the outcome of women undergoing PCI are warranted. The first study designed to examine this in detail is currently underway. The Spirit Women study18 will include 2,000 patients worldwide (excluding the USA) and will collect additional demographic data and report on both clinical and angiographic outcomes.
Conclusion
Women included in PCI studies have higher risk profiles than their male counterparts and show trends to worse outcomes. The clinical and angiographic benefits seen and previously reported for XIENCE V when compared to TAXUS in the SPIRIT II and SPIRIT III studies can be translated into a similar benefit when gender specific populations are assessed.

