Abstract
Aims: To evaluate clinical outcomes in patients receiving the next-generation, paclitaxel-eluting, platinum-chromium TAXUS Element stent in a real-world setting. The PERSEUS Workhorse and Small Vessel studies showed positive results with the TAXUS Element stent in a clinical trial setting.
Methods and results: TE-PROVE was a prospective, open-label, multicentre, “all-comers” study which enrolled 1,014 patients at 37 European sites. Follow-up was at 30 days, six months and one year, and will continue annually up to five years. The primary endpoint was overall and stent-related target vessel failure (TVF), defined as cardiac death, target vessel-related myocardial infarction (MI) and target vessel revascularisation (TVR) at one year post implantation. Secondary endpoints included the components of TVF, all-cause mortality, and ARC definite/probable stent thrombosis. Follow-up was available in 97.3% (987/1,014) of patients. Patients were 75.0% male (760/1,014), mean age was 65.1±10.8 years, 25.5% had medically treated diabetes (259/1,014), and 10.7% (109/1,014) were treated for STEMI. At baseline, mean lesion length among 1,299 treated lesions was 19.8±12.0 mm and mean reference vessel diameter was 3.1±0.5 mm. At one year, the rate of TVF (primary endpoint) was 6.0% (59/987) overall; 3.7% (37/987) of TVF events were stent-related. Cardiac death was 0.7% (7/987), target vessel-related MI was 1.1% (11/987), and TVR was 4.7% (46/987). All-cause death occurred in 1.2% (12/987) of patients and ARC definite/probable ST was 0.5% (5/987).
Conclusions: The primary endpoint results from the TE-PROVE registry demonstrate good performance and safety for the TAXUS Element paclitaxel-eluting stent at one year in everyday clinical practice. Clinical Trial Registration Information: NCT01242696.
Introduction
First-generation stainless steel paclitaxel-eluting stents (PES) are known to reduce angiographic restenosis and target vessel revascularisation (TVR) compared with bare metal stents1-6. However, repeat revascularisation with first-generation PES is still required in 7-10% of patients7. Strut thickness may impact on the ability of a stent to reduce restenosis: thinner struts are associated with less stent-induced arterial injury and inflammation, potentially resulting in better deliverability but at the price of reduced radiopacity and radial strength8-10.
The TAXUS® Element™ stent (Boston Scientific, Marlborough, MA, USA) uses the same polymer, antiproliferative agent and drug release kinetics as previous-generation PES, but incorporates a thin-strut platinum-chromium (PtCr) alloy platform intended to improve deliverability, radial strength and radiopacity while preserving safety and efficacy. In the PERSEUS (Prospective Evaluation in a Randomized Trial of the Safety and Efficacy of the Use of the TAXUS Element Paclitaxel-Eluting Coronary Stent System) Workhorse trial, the TAXUS Element PES showed comparable efficacy to the TAXUS Express PES (Boston Scientific), with no safety concerns related to the novel PtCr platform11. In this manuscript, we report the one-year results of all-comers patients undergoing implantation of TAXUS Element PtCr PES in a real-life setting.
Methods
STUDY DESIGN
The TE-PROVE (TAXUS® Element™ Paclitaxel-Eluting Coronary Stent System European Post-Approval Surveillance Study) was an international multicentre, prospective, open-label, single-arm, post-marketing, observational study (ClinicalTrials.gov identifier NCT01242696).
All candidates for percutaneous coronary intervention (PCI) with stent implantation were eligible for inclusion. PCI procedures and periprocedural adjunctive pharmacotherapy were performed according to local practice. Follow-up was conducted via standard practice or telephone. Additional methods are described in the Online Appendix.
The study was conducted according to the Declaration of Helsinki and was approved by local medical ethics committees at enrolling sites. Written informed consent was obtained from all patients.
DEVICE DESCRIPTION
The TAXUS Element stent system is a PtCr alloy stent with a nominal strut thickness of 81 μm, coated with paclitaxel incorporated at 1 μg/mm2 into a poly(styrene-b-isobutylene-b-styrene) polymer11.
STUDY ENDPOINTS
The primary endpoint was target vessel failure (TVF): cardiac death, myocardial infarction (MI) related to the target vessel, and TVR at 12 months post stent implantation. Additional endpoints are described in the Online Appendix.
STATISTICAL ANALYSIS
The analysis consisted of all enrolled patients in whom a TAXUS Element stent was attempted and/or implanted. A secondary analysis compared outcomes in the PERSEUS-like and non-PERSEUS-like patient subgroups. Additional statistical methods are described in the Online Appendix.
ESTIMATION OF COMPARATOR 12-MONTH OUTCOMES
The pooled rates of TVF, MACE, and death at 12 months from recent, published all-comers PCI studies were assessed. In keeping with established methodologies and the MOOSE (Meta-analysis Of Observational Studies in Epidemiology) group recommendations12,13, studies were included after a literature search if they satisfied the criteria included in the Online Appendix (Methods). No statistical comparison was performed.
Results
PATIENT POPULATION
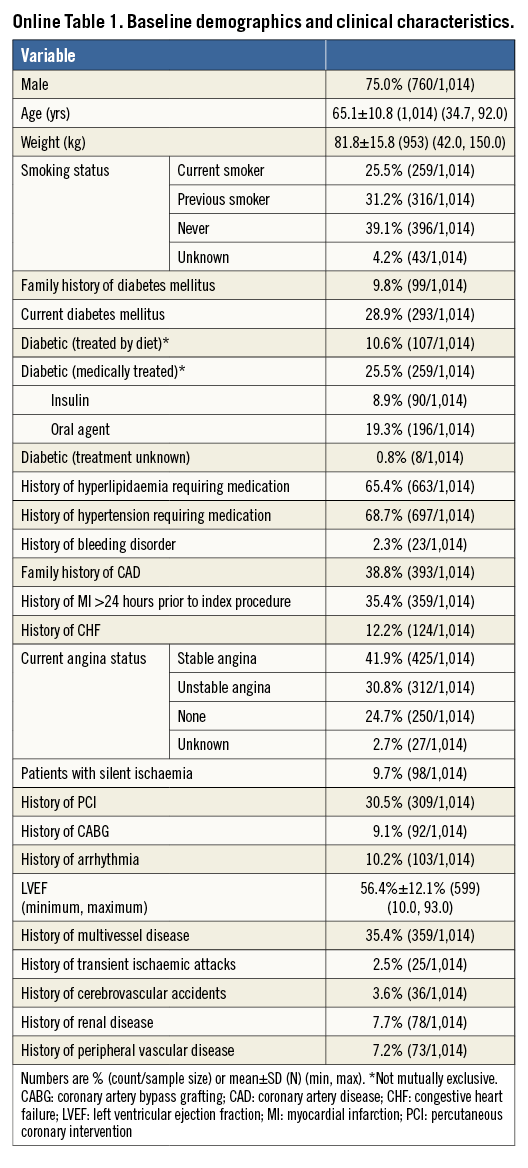
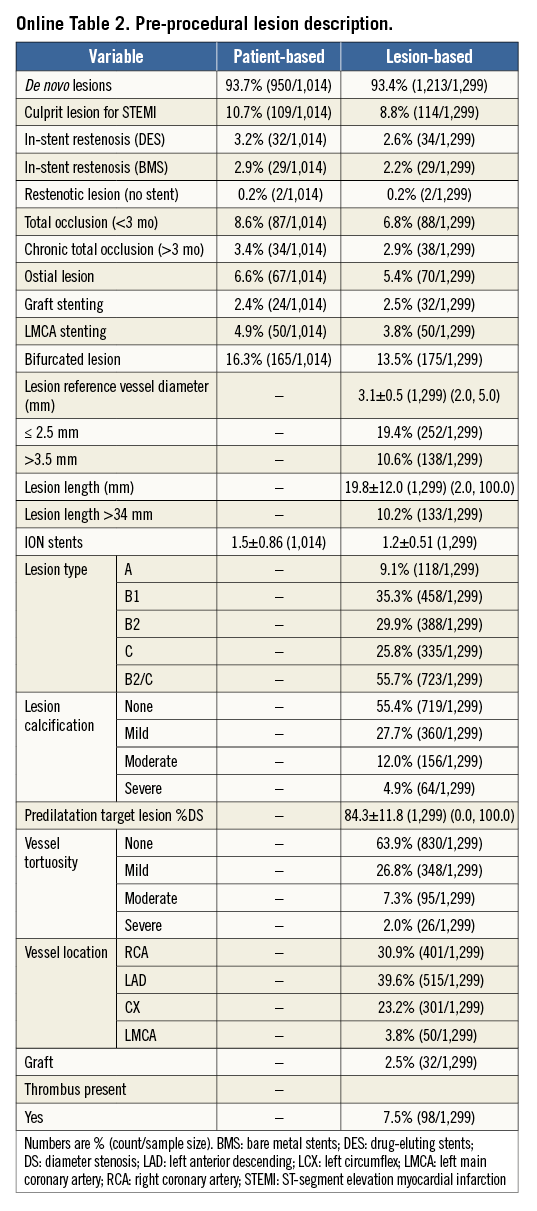
A total of 1,014 patients from 37 centres in 10 European countries were enrolled between November 2010 and October 2011. Clinical follow-up at 12 months was available in 987/1,014 (97.3%) patients. Baseline patient and lesion characteristics are listed in Online Table 1 and Online Table 2. The mean age was 65±11 years, and diabetes mellitus was present in 28.9% (8.9% were on insulin therapy). Mean lesion reference vessel diameter and length were 3.1±0.5 and 19.8±12.0 mm, respectively. More than half (56%) of lesions were of class B2/C.
PROCEDURAL DETAILS AND MEDICATIONS
A total of 1,566 stents in 1,029 procedures were implanted. Predilatation was performed in 61% of lesions. The mean numbers of treated lesions and vessels per patient were 1.3±0.57 and 1.1±0.37, respectively. Mean stent length per patient was 31.3±20.4 mm and placement of overlapping stents was required in 12.6% of lesions. Technical success was achieved in 1,010/1,014 (99.6%) of patients. Clinical procedural success was achieved in 997/1,014 (98.3%) of patients. Dual antiplatelet therapy was prescribed at discharge in 96.9% of patients and maintained at 12-month follow-up in 84.3%.
CARDIAC EVENTS AT FOLLOW-UP
Binary rates of cardiac events at 12 months are reported in Table 1. Corresponding Kaplan-Meier curves for TVF and cardiac death/MI are shown in Figure 1. At 12 months, TVF (primary endpoint) occurred in 59 (6.0%) patients. The clinical events committee adjudicated 37 TVRs as stent-related (3.7%). This figure was reasonably consistent across multiple subgroups (Figure 2). MACE occurred in 6.2% and death in 1.2% of patients over 12 months (Table 1).
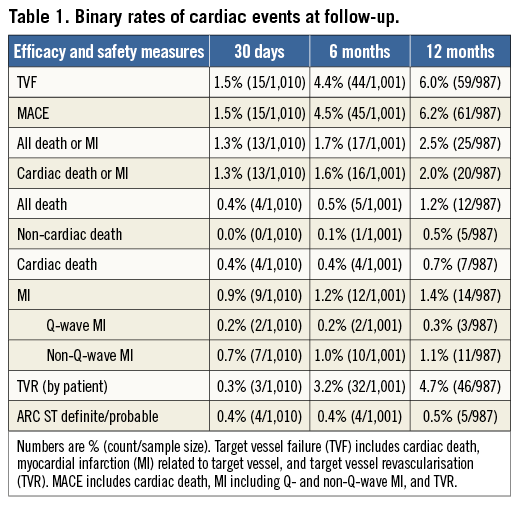
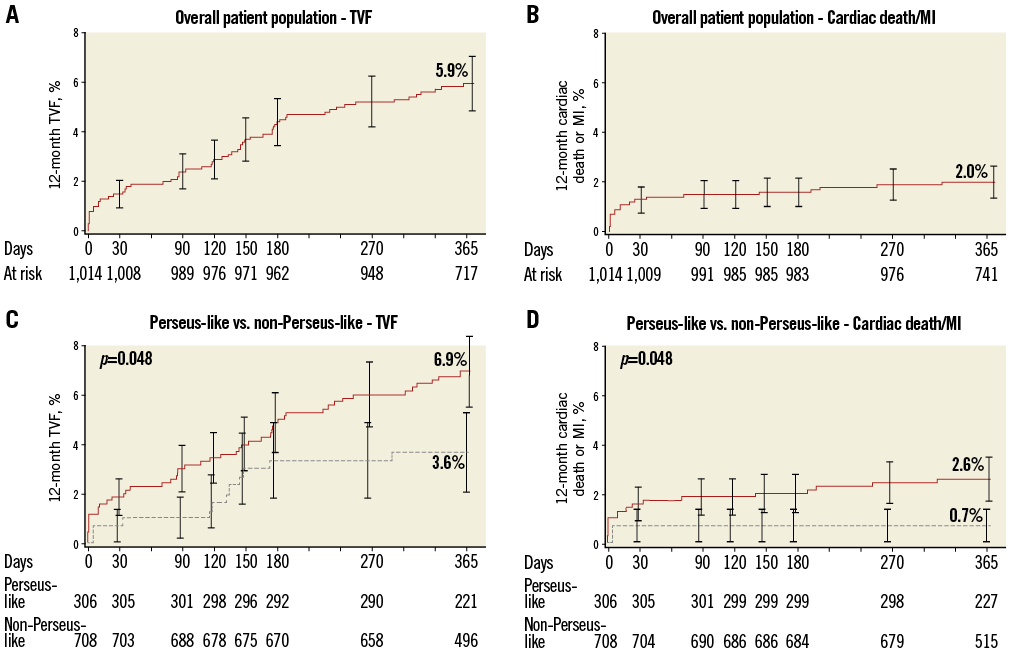
Figure 1. Cumulative 12-month incidences of target vessel failure (TVF: panels A and C), and the composite of cardiac death or myocardial infarction (MI: panels B and D) in the overall cohort (panels A and B) and in the non-PERSEUS-like (solid line) and PERSEUS-like (dotted line) subgroups (panels C and D).
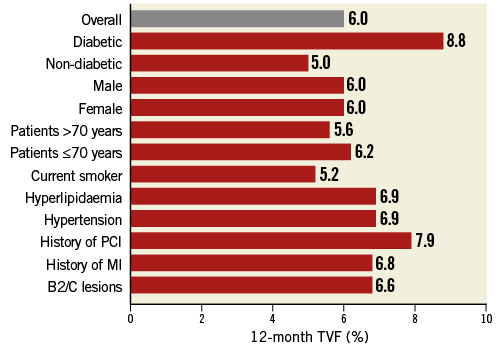
Figure 2. Incidence of 12-month target vessel failure in subgroups of interest.
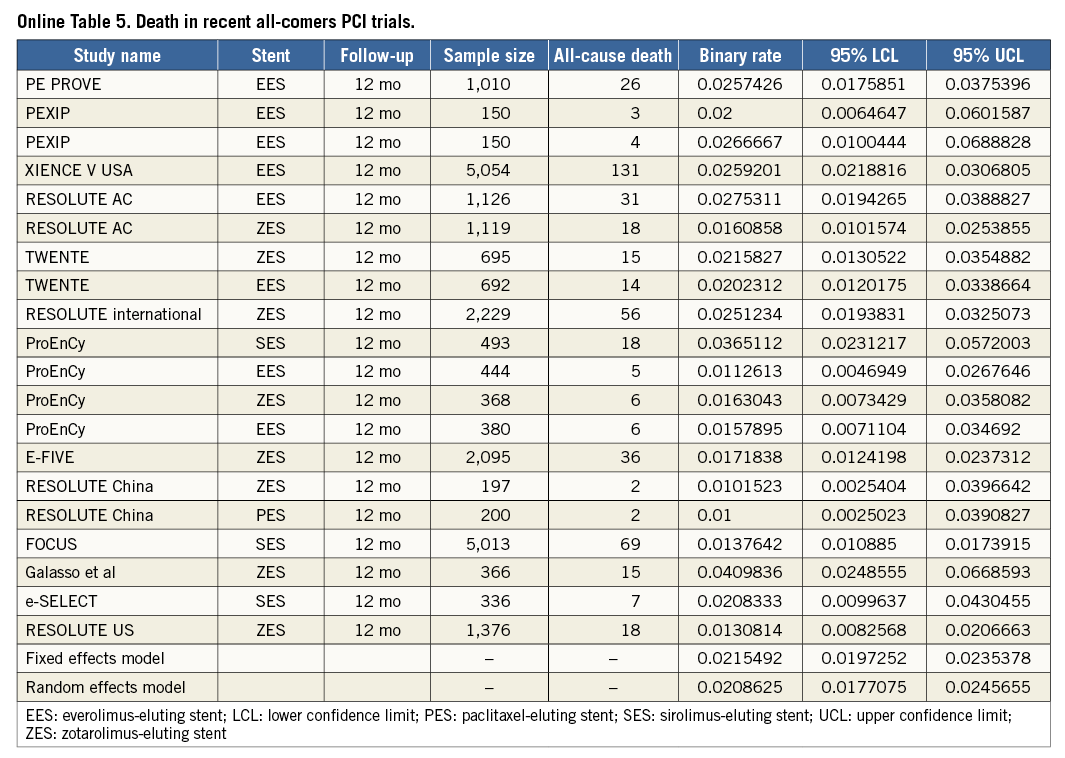
ESTIMATION OF COMPARATOR RATES OF TVF, MACE AND DEATH AT 12 MONTHS
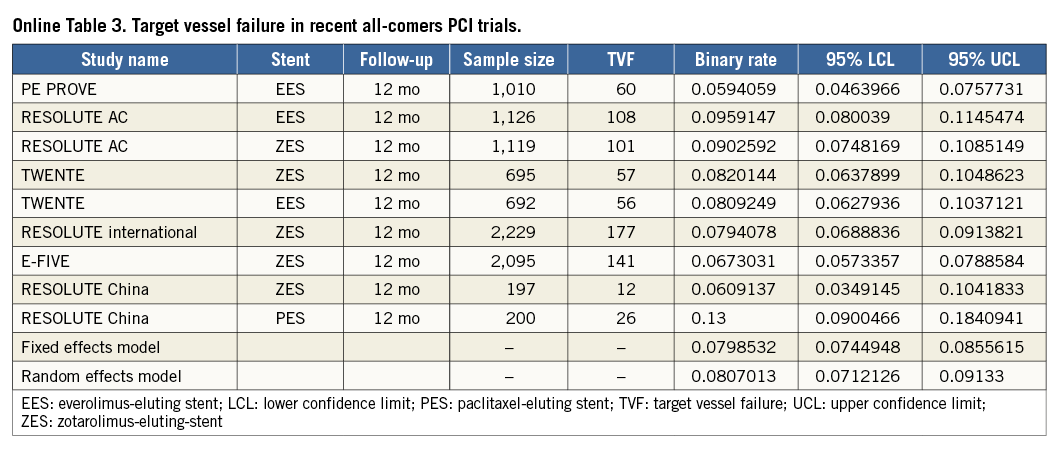
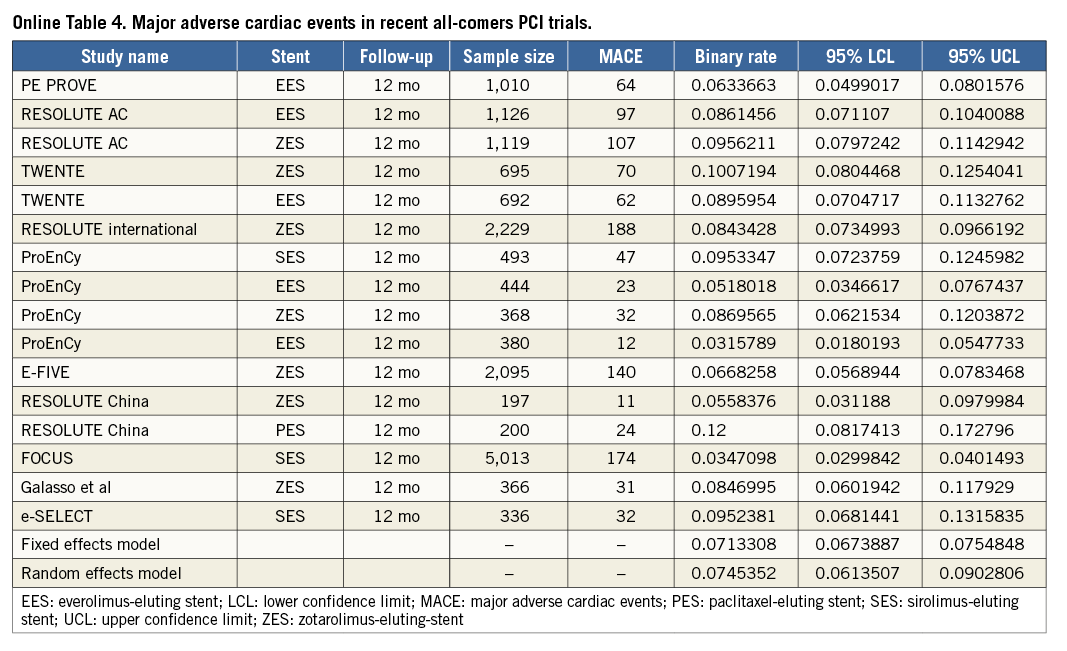
To put the results of the TE-PROVE registry into the perspective of recent all-comers PCI studies, we identified 13 stent studies reporting on TVF, MACE and/or death at 12 months (Online Table 3-Online Table 5, Online Figure 2). After weighting each rate by the number of patients per study, the overall weighted means for TVF, MACE and death at 12 months by a random effects model were 8.1%, 7.4% and 2.1%, respectively (Online Figure 1).
OUTCOMES OF NON-PERSEUS-LIKE VERSUS PERSEUS-LIKE PATIENTS
A total of 69.8% of patients presented with ≥1 clinical and/or angiographic characteristics of extended use over the eligibility criteria of the PERSEUS trial. At 12 months, PERSEUS-like patients had a lower incidence of MACE, the composite of cardiac death or MI, and non-Q-wave MI compared to those with more complex characteristics included in the non-PERSEUS-like group (Table 2, Figure 1). There was an overall trend towards lower event rates in the less complex group though these differences did not reach significance (Table 2).
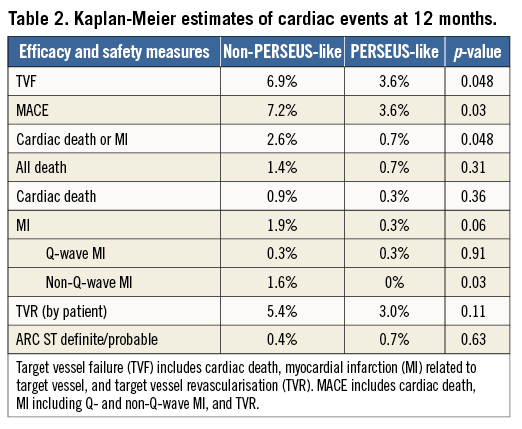
Discussion
The TE-PROVE study demonstrates that, in unselected patients with coronary artery disease undergoing PCI with implantation of a novel PtCr PES, TVF occurs at the relatively low rate of 6.0% at 12 months. These findings compare favourably with recent all-comers PCI trials and registries of DES. In particular, from studies between 2010 and 2013, we calculated a weighted mean of 8.1% for TVF (versus 6.0% in TE-PROVE), 7.4% for MACE (versus 6.2%), and 2.1% for death (versus 1.2%). Although useful to put the TE-PROVE results into perspective, and despite the baseline characteristics of those studies being largely similar to those of TE-PROVE, this comparison is intended for descriptive purposes only. Interestingly, the TVF rate of the overall population at 12 months was reasonably consistent in multiple subgroups of interest (with 8.8% and 6.6% estimates for patients with diabetes mellitus and B2/C lesions, respectively).
Strut thickness is known to impact on angiographic and clinical outcomes after coronary artery stenting8,9. Newer-generation 316L stainless steel stent designs have reduced strut thickness while preserving radial strength and minimising recoil, but with significantly less radiopacity14. The TAXUS Element PES incorporates a new PtCr alloy with high radial strength and high radiopacity which enabled the design of a thin-strut, flexible, trackable and conformable stent platform, while improving stent visibility15.
The TAXUS PERSEUS clinical trial programme evaluated the TAXUS Element PES in two parallel studies10. In the PERSEUS Workhorse trial, target lesion failure (TLF) at 12 months was reported in 5.6% of patients treated with the TAXUS Element PES and in 6.1% of patients treated with the TAXUS Express PES, meeting the criteria for non-inferiority (posterior probability p=0.9996)11. In the PERSEUS Small Vessel trial, the TAXUS Element PES was superior to the Express BMS in terms of late loss (0.38±0.51 vs. 0.80±0.53 mm, respectively, p<0.001), and TLF (7.3% vs. 19.5%, p<0.001)16. TE-PROVE provides further assurance of the safety and efficacy of the PtCr PES in a larger real-world population than those included in the PERSEUS trials. No safety concerns were observed, with low incidences of death and MI at 12 months.
The incidence of ARC definite and probable ST was 0.5% overall, with 0.4% occurring early (0 to 30 days) and 0.1% occurring late (31 to 365 days). These figures are reassuring for a PES17, and may suggest a role of the PtCr platform in reducing thrombogenicity and promoting vascular healing18. Additionally, increased radiopacity of PtCr platforms may help to identify underexpanded stents and, potentially, aid in reducing the rate of ST. The optimal length of dual antiplatelet therapy after placement of a stent is a matter of ongoing investigation. In this registry, about 84% of patients were taking dual antiplatelet therapy at 12 months, a high rate and in keeping with the guidelines available at the time of the study19.
Limitations
This study carries some obvious limitations. First, the TE-PROVE registry lacked a concurrent comparator, but the objectives were to extend the results of the PERSEUS trials in a broader patient population and more precise detection of low-frequency events. Second, although random monitoring of source data from 20% of patients enrolled at each site was employed, the potential for under-reporting of adverse events cannot be entirely ruled out. Third, quantitative coronary angiography data were based on site-reported data with no collection by a core laboratory reflecting the “real-world” approach of the study. Fourth, patients were not consecutively enrolled and there may have been some selection bias. Lastly, we do not know the total number of DES patients treated at these sites during the enrolment.
Conclusions
The post-marketing surveillance TE-PROVE study showed favourable early and 12-month safety profiles and high-level efficacy of the novel PtCr PES in the treatment of coronary lesions in a “real-world” patient population. The 12-month rate of TVF was comparable to rates reported across other contemporary studies.
| Impact on daily practice The introduction of a thin-strut platinum-chromium alloy platform with increased radiopacity is intended to address multiple technical conundrums of stent-based percutaneous coronary intervention, including stent deliverability, radial strength and visibility. The TE-PROVE, a large post-marketing surveillance real-world registry of a novel platinum-chromium paclitaxel-eluting stent, demonstrated low 12-month rates of target vessel failure, in the range of those reported across other contemporary stent studies, and low rates of stent thrombosis. |
Acknowledgements
The authors thank Kristine Roy (Boston Scientific Corporation) for assistance in manuscript preparation and Songtao Jiang (Boston Scientific Corporation) for statistical analysis.
Funding
The TE-PROVE study was supported by Boston Scientific Corporation.
Conflict of interest statement
D. Capodanno reports receiving speaker’s bureau/advisory board fees from Eli Lilly, AstraZeneca and The Medicines Company. A. Erglis reports receiving consulting/speaker’s bureau fees from Boston Scientific, Abbott Vascular, Cordis J&J, Medtronic, Biosensors. I. Menown reports receiving institutional grants/conference sponsorship from Boston Scientific, Biosensors, Biotronik, Eurocor, and OrbusNeich. R. Moreno reports receiving consulting fees from Cordis, Biotronik, Medtronic, Boston Scientific, Abbott Vascular, Eli Lilly, Iroko, Covidien, General Electric, Ferrer, and St. Jude Medical. T. Gilbert reports receiving travel and accommodation support to attend educational meetings from BSC and was a member of the BSC Advisory Board in the development stages of the Element platform. D. Allocco and K. Dawkins are full-time employees and stockholders of BSC. C. Tamburino, J. Crowley, I. Horváth, and P. Calabria have no conflicts of interest to declare.
Appendix. Methods
STUDY DESIGN
No exclusion criteria were applied beyond contraindications to TAXUS Element implantation according to the “Instructions for Use”. The non-PERSEUS-like group was defined as patients with acute myocardial infarction (AMI), graft stenting, left main disease, chronic total occlusion, in-stent restenosis, failed brachytherapy, a bifurcation lesion, an ostial lesion, severe tortuosity, moderate or severe calcification by visual estimate in target lesion or target vessel proximal to target lesion, multivessel stenting, RVD <2.25 mm, RVD >4.00 mm, lesion length >28 mm, cardiogenic shock, or acute or chronic renal dysfunction (serum creatinine >3.0 mg/dL or subject on dialysis). Twelve-month follow-up was conducted via standard practice (n=56) or telephone (n=917).
DEVICE DESCRIPTION
The TAXUS Element stent system is a PtCr alloy stent with a nominal strut thickness of 81 μm, coated with a slow release formulation of paclitaxel incorporated at 1 μg/mm2 into a poly(styrene-b-isobutylene-b-styrene) polymer11. The stent was available in seven sizes between 2.25 and 4.5 mm and eight lengths ranging from 8 to 38 mm. At the time of the study, the TAXUS Element stent had a CE mark and was available for use in routine clinical practice in the participating hospitals.
ENDPOINTS
Secondary endpoints included technical success (defined as successful delivery or deployment of the stent to the target lesion without device malfunction), clinical procedural success (defined as mean lesion diameter stenosis <30%, a TIMI flow of 3 as visually assessed by the physician, and no occurrence of in-hospital cardiac events), the components of TVF, major adverse cardiac events (MACE: including cardiac death, MI and TVR), the composite of cardiac death or MI, all-cause death and definite/probable stent thrombosis using the Academic Research Consortium (ARC) definition at 30 days, six months and 12 months20. MI was categorised as either Q-wave MI, defined as the development of new pathological Q-waves in two or more leads lasting ≥0.04 seconds with post-procedure CK-MB levels elevated above normal, or non-Q-wave MI, defined as de novo elevation of CK total levels >2.0 × ULN without the presence of new Q-waves. Clinical follow-up was at 30 days, six months and one year, and will continue annually up to five years. Source data verification was carried out for 20% of subjects randomly selected at each site and with a risk-based monitoring approach, and on all subjects with reported death, MACE, ST and device malfunction. Overall and stent-related TVF, MACE, cardiac death, MI, TVR and ARC ST definite/probable rates were calculated. A clinical events committee (CEC) adjudicated all deaths, MACE and stent thrombosis events. The CEC classified an event as ‘‘stent-related’’ if the event occurred at the stented segment or relationship to the TAXUS stent could not be excluded based upon available information. Blood samples were taken as per local practice.
STATISTICAL METHODS
The pre-specified plan was to collect outcome data for approximately 1,000 patients. All statistical analyses were performed using SAS Version 9.2 (SAS Institute Inc., Cary, NC, USA). Subject baseline characteristics, lesion characteristics, procedure characteristics, and outcome variables were summarised using descriptive statistics for continuous variables and frequency tables for discrete variables. Continuous variables following a normal distribution were compared using the Student’s unpaired t-test, while continuous variables not following a normal distribution were compared with the Mann-Whitney rank-sum test. Categorical variables were compared by means of the chi-square test or Fisher’s exact test when at least 25% of values showed an expected cell frequency below five. Kaplan-Meier plots of time-to-event variables were constructed with 95% confidence intervals at appropriate time points. The log-rank test was used for comparison of Kaplan-Meier estimates.
ESTIMATION OF COMPARATOR 12-MONTH OUTCOMES
To put the results of the TE-PROVE into clinical perspective, we assessed the rates of TVF, MACE, and death at 12 months from published results of recent large, all-comers PCI studies. In keeping with established methodologies and the MOOSE (Meta-analysis Of Observational Studies in Epidemiology) group12,13, the studies were included after a literature search if they satisfied all of the following criteria: 1) 100 or more patients; 2) published in the English language in peer-reviewed journals, or presented at major cardiology conferences; 3) published between January 2010 and August 2013; and 4) provided the rate of TVF, MACE, and/or death at 12 months. The TVF, MACE rates reported were consistent with each individual study definition and death was defined as death from any cause, unless only cardiac death was available. Pooled incidence rates of individual outcomes were estimated (as detailed in single-arm studies or in each arm of comparative studies) with associated 95% confidence interval (95% CI), by using both fixed and random effects (DerSimonian-Laird method) models to address heterogeneity, if any, among reported rates. Such evaluation was descriptive only and did not lead to any statistical comparison.
Online results
Of all patients in the study population, 12 (1.2%) died, seven of a cardiac cause (0.7%). MACE and the composite of cardiac death or MI occurred in 61 (6.2%) and 20 (2.0%) patients, respectively. Forty-six (4.7%) patients underwent a TVR. Definite/probable ST according to the ARC definitions were adjudicated in five (0.5%) patients (four occurred within 30 days). There were four definite ST and one probable ST.
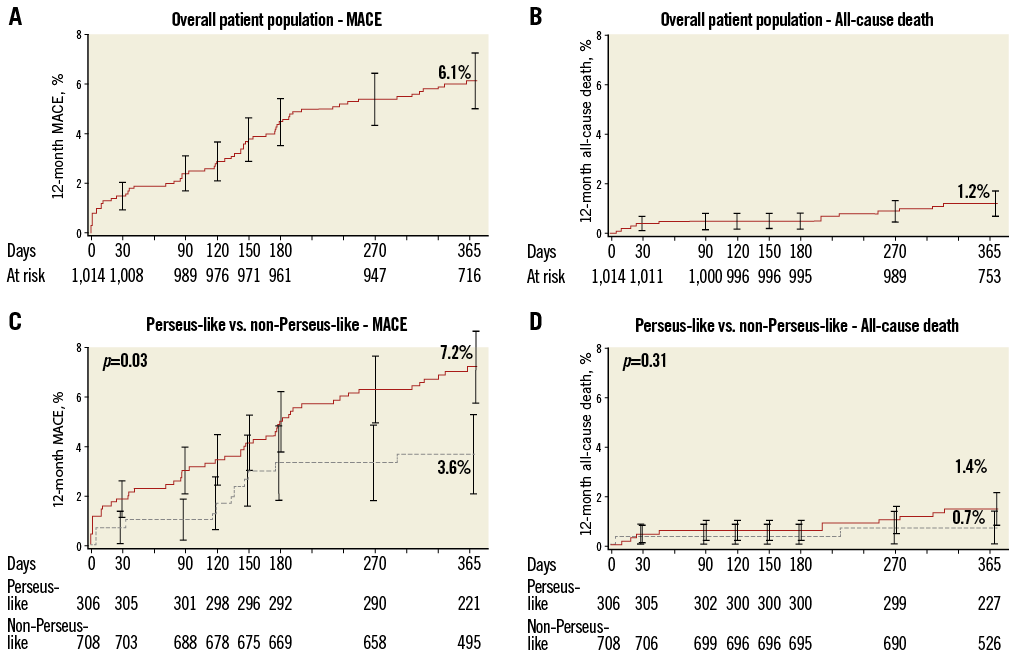
Online Figure 1. Cumulative 12-month incidences of major adverse cardiac events (MACE; panels A and C) and all-cause death (panels B and D) in the overall cohort (panels A and B) and in the non-PERSEUS-like (solid line) and PERSEUS-like (dotted line) subgroups (panels C and D).
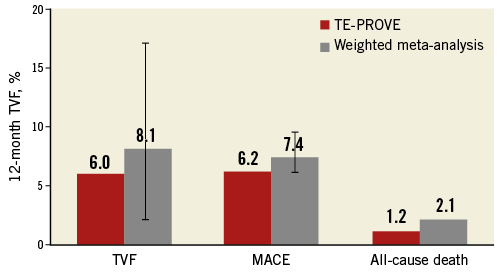
Online Figure 2. Cardiac outcomes at 12 months in the TE-PROVE cohort in the weighted meta-analyses of large contemporary trials of percutaneous coronary intervention.