Abstract
Aims: Small reference vessel diameter predicts adverse outcomes following coronary stenting. TAXUS Express and TAXUS Liberté paclitaxel-eluting stents (PES) reduce restenosis compared to bare metal stents (BMS) in small diameter vessels. TAXUS Element is a novel thin-strut, platinum chromium stent designed to enhance visibility, conformability, and drug delivery in small diameter vessels.
Methods and results: The PERSEUS Small Vessel (SV) prospective, single-arm, superiority trial evaluates the TAXUS Element PES in 224 subjects with target lesion length ≤20 mm and vessel diameter ≥2.25 to <2.75 mm, compared to 125 lesion-matched historical Express BMS control subjects from the TAXUS V trial. The primary endpoint was nine-month in-stent late loss. The secondary endpoint was 12-month target lesion failure (TLF) compared to a pre-specified performance goal (PG). Outcomes were analysed with and without propensity-score adjustment. TAXUS Element was superior to the Express BMS for late loss (0.38±0.51 versus 0.80±0.53 mm respectively; P<0.001), and TLF (7.3%) was significantly less than the 19.5% PG (P<0.001). No differences in mortality, myocardial infarction, or stent thrombosis were observed through 12 months. Results were similar after adjustment.
Conclusions: PERSEUS SV supports the efficacy and safety of the platinum chromium, thin-strut TAXUS Element stent in small coronary vessels.
Introduction
Small vessel diameter is a predictor of adverse clinical and angiographic outcomes following coronary stenting, particularly with bare metal stents (BMS).1,2 Prior studies of the first generation TAXUS™ Express™ paclitaxel-eluting stents (PES) (Boston Scientific, Natick, MA, USA) have demonstrated reduced restenosis rates compared with BMS in subgroups of patients with small calibre vessels.3,4 In the prospective TAXUS ATLAS small vessel trial, the second-generation 2.25 mm TAXUS Liberté™ (Boston Scientific) stent reduced target vessel revascularisation at 12 months compared to historical controls treated with the TAXUS Express (Boston Scientific) (6.1% versus 16.9%, respectively) in vessels 2.2 to 2.5 mm in diameter.5 Interpretation of these studies has been limited by subgroup analyses involving small numbers of patients.
The third generation TAXUS Element™ (ION™)(1) PES incorporates a novel, thin-strut (81 µm; Figure 1A), platinum chromium alloy platform designed to enhance visibility, conformability, and drug delivery in small calibre vessels.6
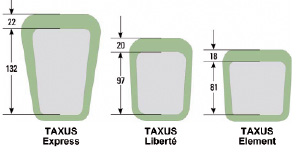
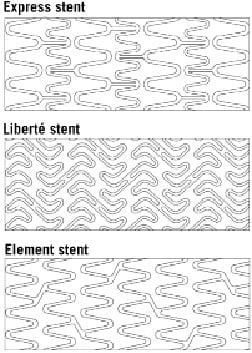
Figure 1. Stent characteristics. TAXUS Element stent architecture (A) and strut/polymer thickness (in microns) (B), in comparison with previous-generation PES (Reprinted with Permission from Trials7).
Both strut and polymer thickness of the TAXUS Element stent are reduced compared to prior generation TAXUS Express and TAXUS Liberté PES (Figure 1A) although polymer, drug, and drug release kinetics are similar. Bench testing has demonstrated the platinum chromium alloy to have enhanced radial strength relative to stainless steel and less stent recoil than cobalt chromium.7 Preclinical testing has supported the vascular compatibility of platinum chromium.8 In addition, the Element stent was associated with more rapid strut coverage and endothelialisation compared to the Express and Liberté stents in a rabbit model.9
The TAXUS PERSEUS Small Vessel (SV) study was designed to evaluate whether the TAXUS Element stent is superior in efficacy to the bare metal Express stent in small coronary vessels and to determine the relative safety of this novel PES platform.
(1) The TAXUS Element stent will be commercialised as the ION Stent in the United States.
Methods
Study design
The PERSEUS SV study design has been described previously.7 Briefly, PERSEUS SV is a prospective, single-arm, open-label trial. Eligible subjects with single, native vessel, de novo coronary atherosclerotic lesions of ≥50% to <100% diameter stenosis and ≤20 mm length with reference vessel diameter ≥2.25 to <2.75 mm were eligible for enrolment at 28 United States sites between July 13, 2007 and August 27, 2008.7 All subjects were required to undergo follow-up angiography at nine months post-enrolment and clinical follow-up at 30 days, nine months, 12 months, 18 months, and annually to five years.
The primary endpoint of nine-month in-stent late loss by quantitative coronary angiography (QCA) is designed to assess superiority of TAXUS Element compared with a historical bare metal (Express) stent control. The historical control is comprised of 125 intent-to-treat Express BMS subjects (108 of whom have nine-month QCA follow-up) with target vessel diameter ≥2.25 to <2.75 mm and target lesion length ≤20 mm who were previously enrolled into the TAXUS V de novo study between March 7, 2003 and March 5, 2004.3 The secondary endpoint of 12-month target lesion failure (TLF) compares the TAXUS Element to a pre-specified performance goal (see statistical methods below). TLF is defined as any ischaemia-driven revascularisation of the target lesion (TLR), myocardial infarction (MI) related to the target vessel, or cardiac death related to the target vessel.
Planned use of multiple stents within the target lesion was prohibited, and BMS patients from TAXUS V who had received planned multiple stent deployment during the TAXUS V index procedure were excluded from the PERSEUS historical BMS control group. Additional study stent deployment in the target lesion was allowed only when required for “bailout” indications. Treatment of one lesion in a non-target vessel during the index procedure was allowed prior to treatment of the target lesion, provided that non-target lesion treatment was successful angiographically and did not require additional unplanned stents. Per protocol, the non-target vessel lesion must have been treated with a commercially available TAXUS stent, if use of a drug-eluting stent was required (use of an approved BMS or balloon angioplasty were also allowed). Staged PCI procedures or subsequent planned coronary artery bypass graft surgery were not allowed. Myocardial infarction within 72 hours prior to the index PCI procedure was an exclusion criteria for enrolment. Other PERSEUS SV inclusion and exclusion criteria have been reported.7
Thienopyridine treatment was required per protocol for at least six months and preferably up to 12 months in subjects not at high risk of bleeding, consistent with the ACC/AHA/SCAI Science Advisory for Percutaneous Coronary Intervention.10 Aspirin therapy was required indefinitely.
The PERSEUS SV study protocol was approved by all participating ethics review committees and all patients provided written informed consent. An independent clinical events committee adjudicated all reported stent thromboses as well as major adverse cardiac events (MACE) and all angiographic studies were analysed by a central core laboratory (CardioVascular Institute at Beth Israel Deaconess Medical Center, Boston, MA, USA) using qualitative morphologic criteria similar to those used in the TAXUS Express and TAXUS Liberté clinical trials.4 Both the clinical events committee and angiographic core laboratory were blinded to stent type. An independent data monitoring committee provided oversight of aggregate safety data. Additional PERSEUS study organisation and oversight committee membership have been reported.7 The PERSEUS SV study is registered on the National Institute of Heath website (www.clinicaltrials.gov) as identifier NCT00489541.
Statistical methods
A two-sided t-test was used to determine if in-stent late loss at nine months was significantly less in the TAXUS Element group compared to the historical BMS control. For the secondary endpoint, the normal approximation to a 1-sided, single sample binomial test was used to compare the observed 12-month TLF rate in the TAXUS Element group to a pre-specified performance goal of 19.5%, which was based on the 12-month TLF rate from TAXUS Express-treated patients in the TAXUS IV and V clinical studies (approximately 13%) plus a 6.5% margin (preserving approximately half of the observed treatment difference between PES and BMS outcomes in those studies). The performance goal was utilised to provide a comparison between TAXUS Element and TAXUS Express PES outcomes, since a US Food and Drug Administration approved small vessel PES was not commercially available to be used as a comparator at the time of study inception.
Because a non-randomised historical control group was employed, propensity adjustment was performed to correct for differences in baseline characteristics. The propensity score for each patient was estimated using a logistic regression model, with all covariates included as predictors and the treatment as the outcome. Covariates are listed in Online Appendix A. An analysis of covariance (ANCOVA) model was used to compare in-stent late loss (the primary endpoint) between intent-to-treat groups after adjusting for propensity score using a two-sided P value. For additional clinical and angiographic outcomes, the entire study analysis set was divided into propensity score quintiles (five equal-size subclasses). Comparisons of the primary and pre-specified endpoints stratified by propensity score subclass were then carried out. SAS System Version 8.2 software or higher (SAS Institute Inc., Cary, NC, USA) was used to develop the model.
Analyses were performed using data pooled across different clinical sites as described in the Online Appendix B.
Results
A total of 349 patients (125 historical BMS and 224 TAXUS Element) were included in the intent-to-treat analysis set, with 97.4% complete clinical follow-up and 87.4% complete angiographic follow-up (Figure 2).
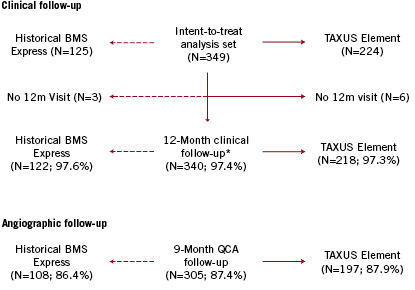
Figure 2. PERSEUS small vessel study flow. Patients included in the PERSEUS SV clinical and angiographic analysis. *Includes two BMS Express patients and three TAXUS Element patients who died prior to the 12-month follow-up visit.
Baseline differences between treatment groups included a more frequent history of congestive heart failure and a less frequent history of unstable angina or current smoking among TAXUS Element patients (Table 1).
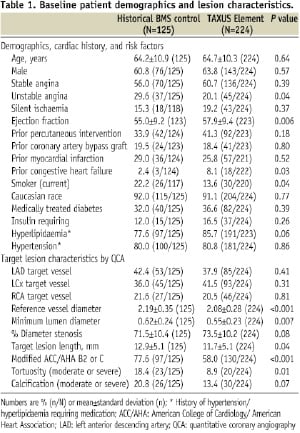
The TAXUS Element cohort had more severe % diameter stenosis, shorter lesion length, and smaller RVD than the BMS-treated group. However, ACC/AHA B2/C lesions and tortuosity were more frequent in the BMS control.
Periprocedural platelet GPIIb/IIIa inhibitors were more frequently administered in the BMS versus TAXUS Element groups (35.2% vs. 13.8% respectively; P<0.001). Both periprocedural and discharge treatment with aspirin and/or clopidogrel were similar between BMS and TAXUS Element groups as was the administration of statin therapy at hospital discharge. Technical success (successful delivery and deployment of the study stent without balloon rupture or stent embolisation) was comparable between treatment groups (Table 2).
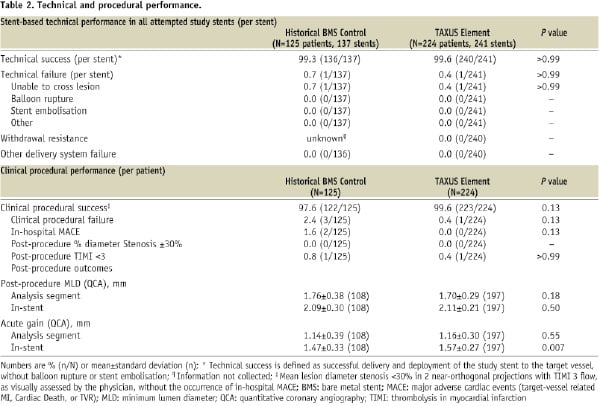
Deployment balloon withdrawal resistance was not observed in the TAXUS Element group. There were no stent fractures11 observed by the angiographic core laboratory in TAXUS Element treated patients. Stent fractures were not assessed in the TAXUS V historical control group.
Although the planned use of multiple stents was prohibited in both treatment arms, multiple stents were implanted for bailout purposes in 8.0% (10/125) of the historical BMS group and 8.0% (18/224) of the TAXUS Element group.
Although aspirin compliance at one year was similar between treatment groups (98.3% BMS control versus 96.7% TAXUS Element; P=0.50), thienopyridine compliance was more frequent in TAXUS Element (93.5%) compared with the Express BMS historical control (65.0%, P<0.001). Based on guidelines at the time of subject enrolment, the TAXUS V protocol mandated only six months of thienopyridine use.
In-stent late loss by QCA at nine months, the study primary endpoint, was significantly reduced by TAXUS Element compared with the Express BMS control (Figure 3A).
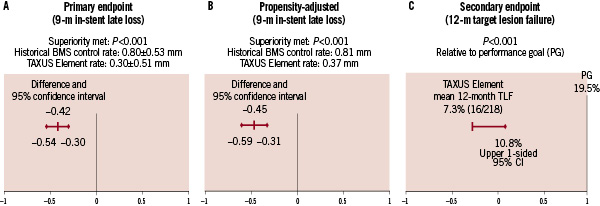
Figure 3. PERSEUS SV primary and secondary endpoints and propensity adjustment. (A) PERSEUS SV primary endpoint was met: unadjusted nine-month in-stent late loss was significantly lower (P<0.001) in the TAXUS Element group than in the historical BMS control group. (B) Propensity-adjusted nine-month in-stent late loss using an analysis of covariance (ANCOVA) model to compare the nine-month in-stent late loss between the treatment groups based on the intent-to-treat analysis set after adjusting for the propensity score using a 2-sided P value. (C) PERSEUS SV secondary endpoint was met: the unadjusted 12-month TLF rate in the TAXUS Element group was significantly lower (P<0.001) than the pre-specified performance goal of 19.5%.
The superiority of TAXUS Element (versus bare metal Express) was maintained after propensity adjustment for differences in baseline clinical and angiographic characteristics between the two non-randomised treatment groups (Figure 3B). The key secondary endpoint of 12-month TLF was significantly lower in the TAXUS Element cohort when compared to the pre-specified 19.5% performance goal (Figure 3C). Cumulative frequency of TLF to 12 months versus the BMS control group is shown in Figure 4.
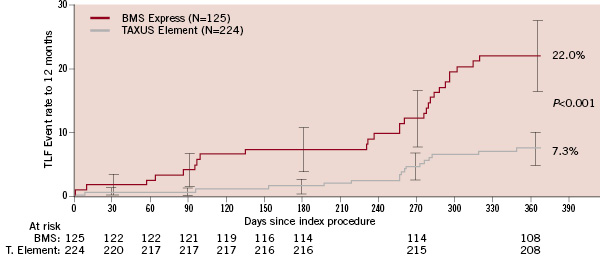
Figure 4. Target lesion failure to 12 months, unadjusted outcomes. Cumulative rate of target lesion failure to 12 months, event rate ±1.5 standard error, all intent-to-treat patients (N=349).
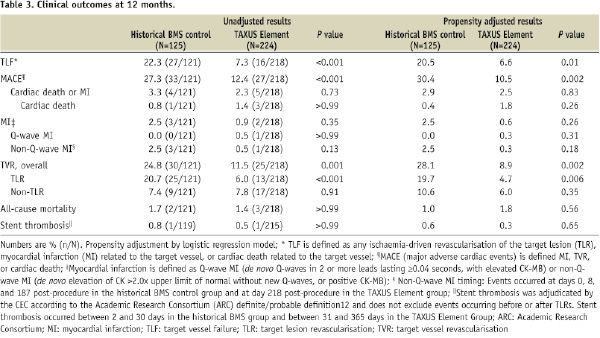
Following propensity adjustment, TLF was significantly reduced in the TAXUS Element group compared to the historical BMS control group (Table 3).
Significant predictors of increased TLF to 12 months by multivariate analysis included LAD target lesion and non-Caucasian race (Online Appendix C).
Additional unadjusted and propensity adjusted clinical outcomes (12 months) are shown in Table 3 and angiographic outcomes (nine months) are shown in Table 4.
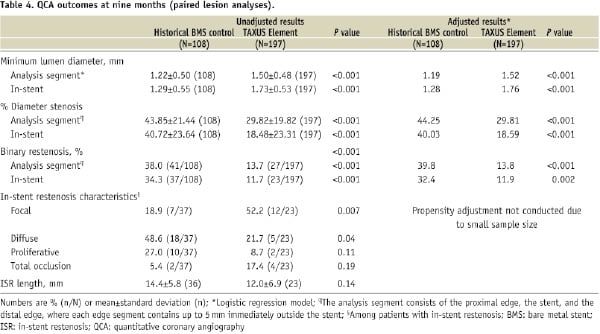
Compared with the historical BMS control, TAXUS Element-treated patients had a significant reduction in MACE, primarily driven by less frequent TLR, which was accompanied by reductions in angiographic measures of restenosis. The relative improvement in outcomes following TAXUS Element deployment was maintained after propensity adjustment. Predictors of increased in-stent late loss at nine months by multivariate analysis included hyperlipidaemia requiring medication, smaller baseline RVD, and longer target lesion length (Online Appendix C).
Non-Q-wave MI was numerically less frequent in the TAXUS Element group. A single stent thrombosis (Academic Research Consortium [ARC] definitions)12 event was observed in each treatment arm (subacute thrombosis [eight days post-index procedure] in the historical BMS arm and late stent thrombosis [152 days post-index procedure] in the TAXUS Element arm).
Discussion
The TAXUS PERSEUS SV study was designed to evaluate the relative efficacy of the TAXUS Element PES when compared with a historical Express BMS treated control. The principal finding of this study was that TAXUS Element is superior to Express BMS with respect to late lumen loss in-stent as measured by QCA nine months following stent deployment. The relative benefit of TAXUS Element (for reduction in late loss) remains superior to Express BMS even after propensity adjustment for 28 clinical and angiographic variables. Similarly, clinical endpoints reflective of restenosis including TLF and MACE were also significantly reduced following TAXUS Element (versus Express BMS) after propensity adjustment. The secondary endpoint, 12-month TLF, was lower (7.3%, P<0.001) compared with a pre-specified performance goal (19.5%) derived from a historical cohort of paclitaxel-eluting TAXUS Express-treated patients.
Furthermore, no safety concerns were observed with respect to the occurrence of death (cardiac or all-cause), MI, or stent thrombosis through 1-year follow-up. Although risk for stent thrombosis may be increased following small vessel stenting, the low incidence of propensity-adjusted or unadjusted definite or probable stent thrombosis (ARC definition)12 observed following TAXUS Element PES deployment in the present study (0.3%) is noteworthy. Finally, although no stent strut fractures were observed by QCA following TAXUS Element treatment, neither intravascular ultrasound nor optical coherence tomography (more sensitive techniques for detection) were performed.
Although the PERSEUS SV trial demonstrates reduced revascularisation rates following the TAXUS Element compared to the Express stent, the relative impact of strut thickness (81 µm versus 132 µm respectively) when comparing the PES to a BMS cannot be determined.
Study limitations
A limitation of PERSEUS SV is that the TAXUS Element PES is compared with a non-randomised Express BMS historical control group as there were no commercially available small vessel PES in the US at the time of study inception. After the completion of PERSEUS SV enrolment, two dedicated small vessel PES (TAXUS Express Atom and TAXUS Liberté Atom) were approved for use by the US Food and Drug Administration.3,5 Although propensity score adjustment was performed using both clinical and angiographic variables, unmeasured covariate imbalance may still confound the conclusions drawn. Furthermore, practice patterns and adjunctive pharmacotherapies (including statin use, oral clopidogrel loading, and compliance with dual antiplatelet therapy) may differ between the study time frames analysed (TAXUS V versus PERSEUS SV) and may not be accounted for.
While caution must be exercised when comparing results across separate studies, the outcomes observed from the PERSEUS SV TAXUS Element cohort appear favourable in the context of previously published event rates following deployment of either the TAXUS Express or TAXUS Liberté PES in small calibre vessels.3,5
In addition, in lieu of a prospective PES comparator for PERSEUS SV, we performed a post-hoc patient-level analysis of 12-month clinical and angiographic outcomes from 555 TAXUS Liberté and 437 TAXUS Element treated subjects with baseline RVD <3.0 mm from the TAXUS ATLAS5,13 and PERSEUS trials (including both PERSEUS SV and PERSEUS Workhorse studies14) respectively and used multivariable regression to adjust for baseline differences between these non-randomised groups. TAXUS Element subjects had numerically less frequent TLR (5.7% versus 7.9 TAXUS Liberté, P=0.39), and significantly fewer non-Q-wave MI events (0.6% versus 1.9% TAXUS Liberté, P=0.03), suggesting improvement in clinical outcomes following TAXUS Element deployment (unpublished results). Furthermore, PERSEUS SV demonstrated a reduction in 12-month TLF following TAXUS Element treatment compared to a performance goal based on TAXUS Express PES outcomes. Although these observations suggest that the TAXUS Element PES may be associated with improved clinical outcomes (reduction in revascularisation and non-Q-wave MI) in small calibre vessels when compared to past generation PES, an adequately powered, randomised trial would be required to prove this hypothesis.
Conclusions
The PERSEUS SV study supports the efficacy and safety of the novel thin-strut, platinum chromium TAXUS Element stent when compared to the Express BMS in small calibre coronary vessels. Further studies are required to determine the relative advantages provided by this novel stent design when compared to more contemporary PES following deployment in small calibre vessels and more complex lesions.
Acknowledgements
The authors thank the following Boston Scientific employees for their contribution: Amy Britt, Andrey Nersesov, Manu Sondhi, Thomas Christen, and Stephen Mascioli for assistance with study design, management, and safety monitoring; Scott Wehrenberg for statistical design and analysis; Peggy Pereda for statistical support and manuscript review; Kristin Hood for medical writing support. The authors also humbly thank the PERSEUS SV clinical staff, committees, and investigational sites for their support (Online Appendix D). The PERSEUS SV study was funded by Boston Scientific Corporation, Natick, MA, USA.
Conflict of interest
Dr. Cannon serves on the Advisory Board or Speakers Bureau for Medtronic, Abbott, Boston Scientific, and holds equity in Boston Scientific, Medtronic, and BioStar Ventures. Dr. Kereiakes received research grants from Boston Scientific, Cordis, Medtronic, and Abbott Vascular, and serves on the Advisory Boards for Boston Scientific and Abbott Vascular. Dr. Popma has received personal and institutional research grants from Cordis Corporation, and institutional research grants from Boston Scientific and Abbott Vascular. Dr. Mooney serves on the Medical Advisory Boards for Medtronic, Boston Scientific, and Cordis and has received research grants (institutional) and honoraria from Medivance. Dr. Mishkel serves on the Medical Advisory Board for Boston Scientific, has received honoraria from Boston Scientific, and has received travel funds for Boston Scientific Advisory Board meetings. Dr. Lee serves as a consultant to and has received grants, travel funds, and honoraria from Boston Scientific. Dr. Wilson serves as a consultant to Boston Scientific and serves on the Speaker’s Bureau for Medtronic. Dr. Stuckey serves on the Medical Advisory Board and Speaker’s Bureau for Boston Scientific, and has received travel funds for educational presentations. Dr. Ring serves on the Advisory Boards for Boston Scientific and Abbott Vascular. Dr. Kellett has received research grant support from Boston Scientific. Dr. Underwood and Dr. Dawkins are full-time employees and stockholders of Boston Scientific Corporation. Drs. Orlow, Mann, and McGarry report no financial conflicts of interest. The PERSEUS Small Vessel study is funded by Boston Scientific Corporation, Natick, MA.

