Abstract
Aims: Recent studies have suggested that EES may reduce ST compared to PES, but no individual trial has been adequately powered for this endpoint. The incidence of stent thrombosis, as well as the impact of dual antiplatelet therapy (DAPT) discontinuation during the first two years following everolimus-eluting stent (EES) and paclitaxel-eluting stent (PES) deployment were therefore analysed from a pooled, patient-level database derived from four randomised clinical trials.
Methods and results: Data from the SPIRIT II, SPIRIT III, SPIRIT IV and COMPARE trials (n=6,789 patients) were analysed. Two-year ST rates were determined using time-to-event methods and compared with the log-rank test. ST rates were also determined after DAPT discontinuation. EES compared to PES significantly reduced the two-year rates of ST (0.7% versus 2.3%, p=0.0001), including the interval rates of ST up to 30 days (0.2% versus 1.0%, p<0.0001), between 31 days and one year (0.2% versus 0.6%, p=0.02), and after one year (0.3% versus 0.8%, p=0.001). EES also reduced the two-year composite rate of cardiac death or MI (4.0% versus 6.6%, p=0.0001). Increased rates of ST after DAPT discontinuation beyond six months were observed in the PES cohort, but not in the EES cohort.
Conclusion: In this large pooled analysis from four randomised trials, treatment with EES compared to PES significantly reduced the rates of ST through two years of follow-up, with a concomitant reduction in cardiac death or MI. DAPT discontinuation beyond six months may be safe with EES.
Abbreviations
ARC: Academic Research Consortium
BMS: bare metal stents
DAPT: dual antiplatelet therapy
DES: drug-eluting stent(s)
EES: everolimus-eluting stent(s)
HR: hazard ratio
MI: myocardial infarction
PES: paclitaxel-eluting stent(s)
PCI: percutaneous coronary intervention
ST: stent thrombosis
Introduction
Paclitaxel-eluting stents (PES) and sirolimus-eluting stents (SES) substantially reduce target lesion and vessel reintervention rates compared to bare metal stents1,2. However, increased rates of very late stent thrombosis (ST), particularly in high-risk patient populations3,4, remain a matter of concern with these first-generation drug-eluting stents5.
Second-generation drug-eluting stents have been designed to improve the procedural performance as well as the safety and efficacy of the first-generation devices. Specifically, a second-generation, thin-strut, cobalt-chromium, everolimus-eluting stent (EES) demonstrated significant improvements in angiographic and clinical outcomes when compared to PES in two small to moderate-sized randomised trials6,7. Thereafter two large clinical trials, SPIRIT IV8 and COMPARE9, tested the same hypothesis in greater numbers of patients with either non-complex coronary artery disease or all-comers, respectively. Both trials reported significant improvements in safety and efficacy endpoints as well as reductions in definite ST and definite/probable ST rates, in favour of EES. However, no individual trial was adequately powered to examine the incidence rates of ST, or to determine the impact of dual antiplatelet therapy (DAPT) interruption on ST outcomes in the EES and PES cohorts separately. For this purpose we pooled patient-level data from the four randomised trials in which the outcomes of EES compared to PES in 6,789 patients have been examined.
Methods
The designs of the SPIRIT II, SPIRIT III, SPIRIT IV and COMPARE trials have previously been described6-9. Briefly, each study was a prospective, single-blind, randomised controlled clinical trial in which patients were assigned to receive either EES (manufactured and distributed as Xience V® by Abbott Vascular, Santa Clara, CA, USA; also distributed as Promus™ by Boston Scientific, Natick, MA, USA) or PES (TAXUS™ Express2™, or TAXUS® Liberté®; Boston Scientific). The SPIRIT trials enrolled patients with simple and moderately complex coronary lesions, excluding those with acute or recent myocardial infarction and difficult or high-risk lesions such as chronic total occlusions, true bifurcations, thrombus, and lesions in the left main coronary artery or a saphenous vein graft. COMPARE was an “all-comers” trial, excluding from randomisation only patients unable to comply with DAPT for a period of 12 months, and those presenting in cardiogenic shock. Aspirin ≥75 mg daily was recommended for a minimum of one year in SPIRIT II and indefinitely in the other trials. Clopidogrel (75 mg daily) was prescribed by protocol for ≥6 months in SPIRIT II and III, and for ≥12 months in SPIRIT IV and COMPARE. Each study employed an independent, angiographic core laboratory as well as independent clinical event adjudication committees, blinded to stent assignment. Follow-up is planned for five years in each trial, and is currently complete up to at least two years in all four studies.
For the present analysis, baseline clinical, angiographic and procedural data of these four trials were pooled to allow for a patient-level analysis. Events as adjudicated in each trial were utilised. The purpose of this analysis was to examine the incidence rates of ST as defined by the ARC definite and/or probable definition10, including examining the outcomes after DAPT discontinuation. The rates of definite ST and definite/probable ST were examined in the following periods: early (0-30 days); late (31 days to one year); cumulative to one year; very late (one to two years); and cumulative to two years.
To analyse the impact of DAPT interruption on subsequent, definite ST and definite/probable ST, four groups of patients were identified: patients who permanently discontinued DAPT (either aspirin and/or a thienopyridine) between one and six months, between six and 12 months, between 12 and 24 months, and those who were still taking DAPT at 24 months. DAPT compliance was ascertained for all studies at discharge, one, six, 12, and 24 months, as well as at the time of adverse events. Patients in whom a ST occurred while not on DAPT were included in the respective DAPT interruption group based on their DAPT status at the time of the event, regardless of DAPT usage afterwards. Moreover, only patients with definitely known DAPT status at each time point through the two-year follow-up were included in the present analysis. Thus, 183 patients (2.7%), including seven (0.1%) who had a ST (all between 0-30 days) were not included in this analysis due to unknown DAPT status after the event. Only 36 patients (0.5%) discontinued DAPT before one month; given the small size of this group these patients were excluded from the analysis. Only the first ST episode was considered; patients with ST were not included in the denominator of subsequent time periods. Finally, to test whether DAPT may be safely discontinued permanently beyond six months after DES implantation, definite ST and definite/probable ST rates at two years were compared in the following groups using a landmark analysis beyond six months: patients who permanently discontinued DAPT between six and 12 months, those who permanently discontinued DAPT between 12 and 24 months, and those who were still taking DAPT at 24 months.
All analyses were done by intention to treat. Categorical outcomes were compared by chi-square or Fisher’s exact test. Continuous variables are presented as mean±SD and were compared by t-test. The two-year event analyses were performed using time-to-event data. They are displayed using Kaplan-Meier plots, and were compared with the log-rank test. To adjust for slight differences in the baseline characteristics of the study population, a propensity score matching analysis was performed using the covariates in Table 1 and Table 2. The propensity score estimates were entered into the multivariable model, and adjusted Kaplan-Meier curves and HR were derived. A two-sided α=0.05 was used for all superiority testing. All statistical analyses were performed by SAS version 9.1.3 (SAS Institute, Cary, NC, USA).
Results
BASELINE CHARACTERISTICS
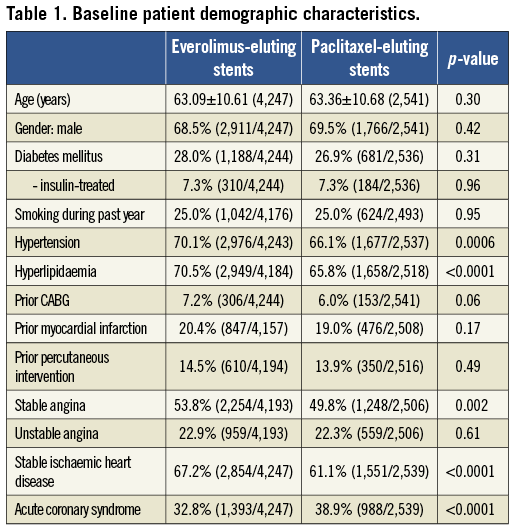
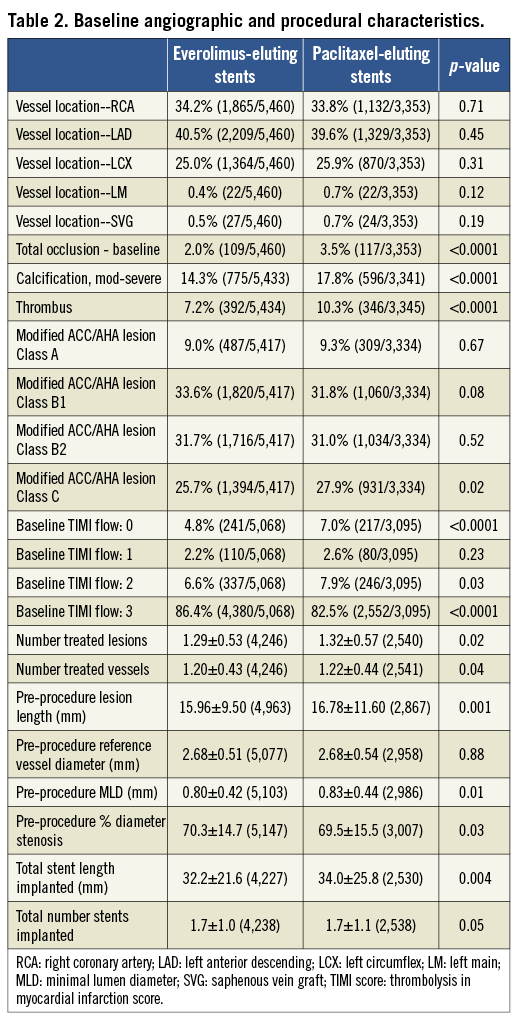
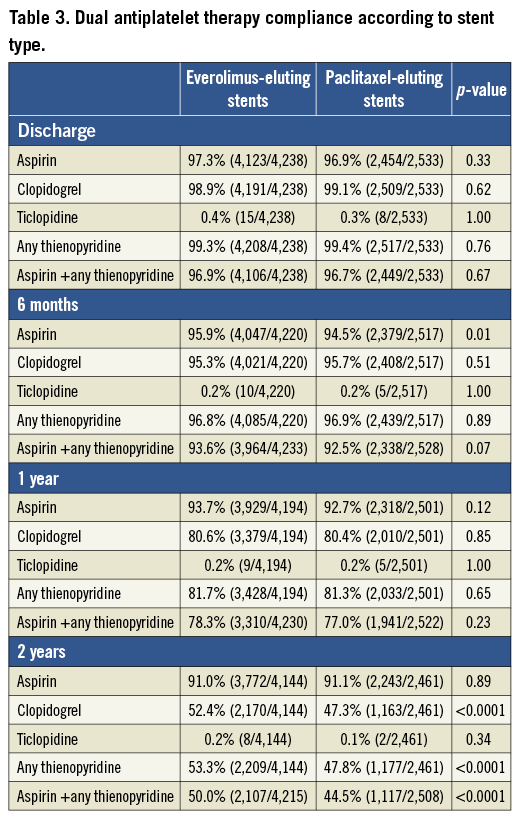
Pooling the four trials resulted in a total of 6,789 patients randomised to treatment with EES (n=4,247; 63%) or PES (n=2,542; 37%). Clinical follow-up at two years was available in 96.2% EES and 96.1% PES-treated patients. Baseline patient demographic characteristics as well as angiographic and procedural characteristics are shown in Table 1 and Table 2, respectively. Baseline coronary risk factors were similar between randomly assigned stent types except that EES patients had a slightly higher incidence of hyperlipidaemia and hypertension, while PES patients had a slightly higher rate of ACS at presentation. Minor imbalances in several angiographic variables, including the rates of total occlusion, presence of thrombus, moderate or severe calcification, modified ACC/AHA Class C lesion morphology, target lesion length and stented length were also present. Compliance with DAPT was similar between the randomly assigned stent types through one year of follow-up; at two years, aspirin usage was not significantly different between the groups, but thienopyridine usage was slightly more common in the EES group (Table 3).
CLINICAL EVENTS
Outcomes of unadjusted early, late and very late definite ST and definite/probable ST as well as rates of cardiac death or MI with follow-up to two years are shown in Table 4 and Figure 1A and Figure 1B. EES resulted in a 70% reduction in the rate of definite/probable ST at two years compared to PES, with significant interval reductions in early, late and very late ST. Similarly, EES resulted in a 70% reduction in the rate of angiographically confirmed definite ST at two years compared to PES, with significant reductions in definite ST during each interval, including at 30 days and one year. After propensity adjustment for differences in the baseline clinical and angiographic characteristics, EES showed comparable reductions in rates of definite/probable ST at 30 days (0.3% [8] versus 1.0% [24], HR: 0.33, 95% CI [0.15,0.74], p=0.005), one year (0.6% [13] versus 1.5% [35]; HR: 0.37; 95% CI: [0.20, 0.70], p=0.0001) and two years (0.9% [19] versus 2.2% [51]; HR: 0.37; 95% CI [0.22,0.63], p=0.0001), (Figure 1C and Figure 1D).
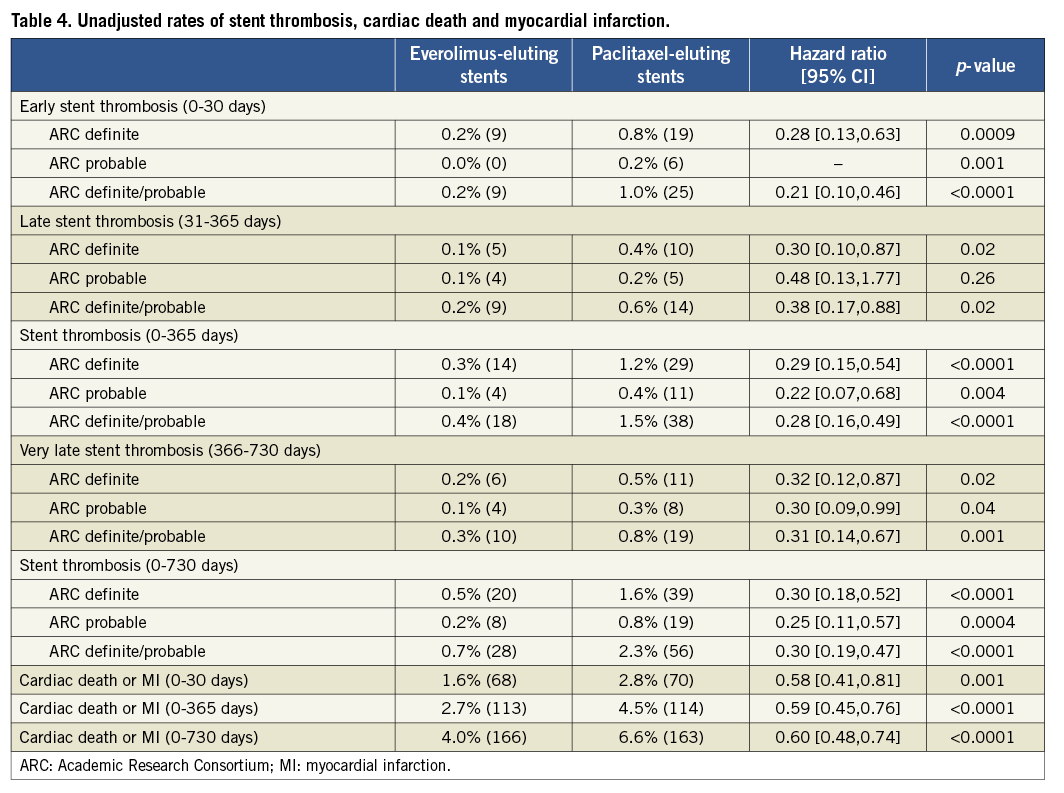
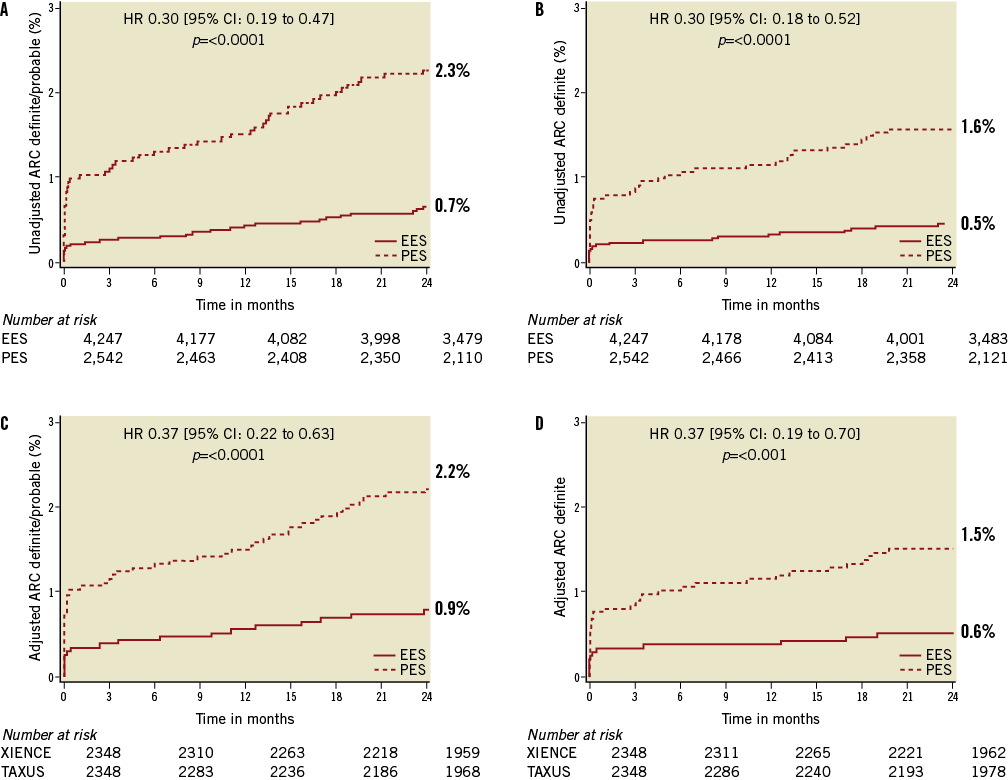
Figure 1. Time-to-event curves for ARC definite/probable stent thrombosis (A) and ARC definite stent thrombosis (B), propensity score adjusted ARC definite/probable stent thrombosis (C) and propensity score adjusted ARC definite stent thrombosis (D) through two-year follow-up. EES: everolimus-eluting stents; PES: paclitaxel-eluting stents
Reduced definite ST and definite/probable ST rates with EES paralleled a significant reduction in the composite rate of cardiac death or MI in each time period and at two years (Table 4).
STENT THROMBOSIS ACCORDING TO DUAL ANTIPLATELET THERAPY USE
The two-year ST rates from the four groups according to DAPT compliance in the EES and PES cohorts are presented in Table 5. No significant differences in two-year definite ST and definite/probable rates according to early DAPT discontinuation were observed among the four groups in the EES cohort. In contrast, a significant increase in the rate of definite/probable ST was observed in the PES patients who interrupted DAPT between one and six months. After excluding the ST events of the first six months, the overall two-year definite/probable ST rates in the groups of patients who permanently discontinued DAPT between six and 12 months, between 12 and 24 months and who were still on DAPT at 24 months were 0.3% (n=2), 0.4% (n=5) and 0.4% (n=8), respectively, in the EES cohort (p for trend=0.97), and 1.2% (n=4), 1.7% (n=14) and 0.5% (n=5) in the PES cohort (p for trend=0.04) (Figure 2).
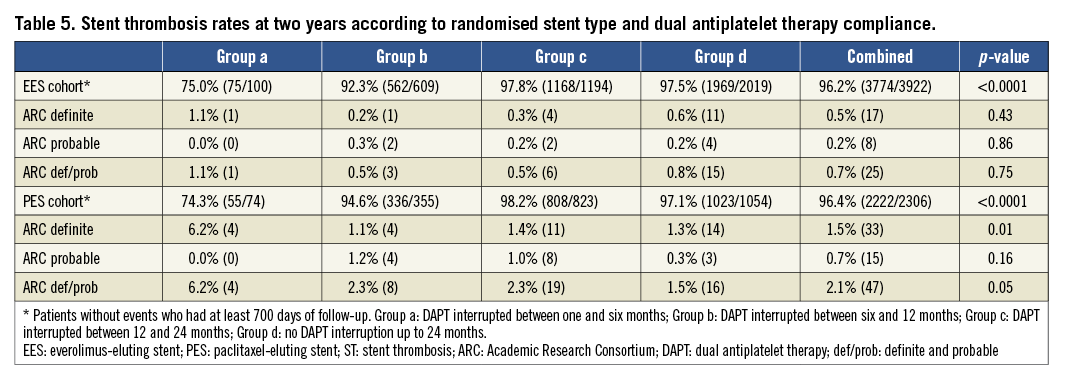
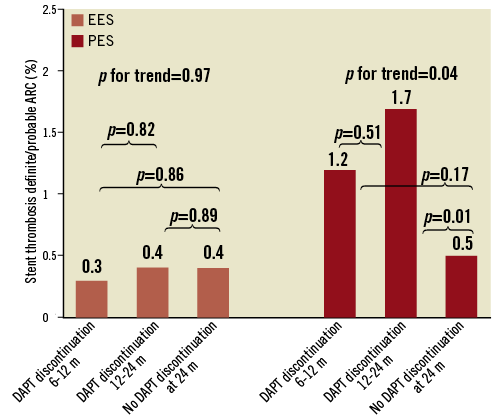
Figure 2. Cumulative two-year rates of definite or probable stent thrombosis after excluding events from the first six months in subjects who discontinued dual antiplatelet therapy (DAPT) between six and 12 months, between 12 and 24 months, and subjects still on DAPT at 24 months in the everolimus-eluting stent (EES) and paclitaxel-eluting stent cohorts, respectively. EES: everolimus-eluting stents; PES; paclitaxel-eluting stents
Discussion
The major finding of the present pooled analysis from four randomised trials is that EES markedly reduced the two-year rates of definite ST and definite/probable ST compared to PES. A significant difference in ST frequency was present at every interval examined through the two-year follow-up period. The reduction in definite ST and definite/probable ST was accompanied by a reduction in the composite occurrence of cardiac death or MI. Finally, DAPT discontinuation beyond six months (and any time before two years) was associated with an increased risk of ST with PES, but not with EES.
The benefits of reduced clinical restenosis achieved with first-generation DES compared to BMS were offset in many studies by higher rates of very late ST4,5. Furthermore, the on-going propensity for ST with first-generation DES has generated reluctance regarding unrestricted use of DES, and recommendations for long-term use of DAPT, despite the lack of evidence that such an approach improves clinical outcomes post PCI. The present study establishes that gains in safety as well as efficacy11,12 may be achieved by a single stent platform (EES). Of note, however, the present pooled analysis refers only to the results of EES vs. PES; further study is required to determine whether these results apply to other second and third-generation DES.
Stent thrombosis
Both unadjusted and propensity score adjusted multivariable analysis showed significant and important reductions in rates of definite ST and definite/probable ST. The pathophysiologic mechanisms underlying the marked reduction in ST following EES are speculative, but may relate to specific stent design features. The combination of thin fracture-resistant struts with everolimus released from a thromboresistant non-inflammatory fluorinated polymer13,14 may contribute to the lower rates of early ST with EES. In preclinical animal models more rapid and complete stent re-endothelialisation has been seen with EES compared to other DES15. Reduction in inflammation along with the faster and more complete re-endothelialisation may result in lower rates of early, late and very late ST. Moreover, despite the focus of the physician and public on very late ST (beyond one year) with first-generation DES when compared with BMS3,16, early ST accounts for a substantial proportion of total ST events. While low rates of late and very late ST have been reported with other new DES17-19, the EES is the only second-generation DES which has repeatedly shown a reduced rate of definite ST events, especially in the early period9,18.
DAPT interruption analysis
The premise that EES-treated patients may require shorter durations of DAPT is supported by the findings of the current analyses. Interruption of DAPT at any time point after the first month did not influence the rate of ST at two years in the EES cohort. After excluding events within the first six months, no difference was observed with regard to definite ST and definite/probable ST rates in the EES cohort regardless of DAPT compliance, suggesting that permanent DAPT interruption beyond six months following EES implantation may be safe. This observation is consistent with the XIENCE V USA registry where DAPT interruption at any time point beyond 180 days following EES implantation was safe in an unselected (all-comers) population20. Continued exposure to DAPT after six months may expose EES-treated patients to increased bleeding risk without efficacy benefit21. However, large-scale randomised trials are required to confirm the safety of permanent DAPT discontinuation at six months (or earlier) in EES-treated patients before this practice can be routinely recommended.
In contradistinction, we found that discontinuation of DAPT following PES deployment any time before 24 months (but especially between one and six months) was associated with increased rates of definite/probable ST. Similarly, Eisenstein and colleagues reported increased rates of death or myocardial infarction when DAPT was discontinued after six months or after 12 months with first-generation DES22. In contrast, Airoldi et al reported that ST rates with first-generation DES were increased when DAPT discontinuation occurred prior to six months (compared with continued DAPT therapy), but not after six months23, and Park and colleagues did not find that extended clopidogrel use after one year prevented ST24. Additional large-scale randomised trials are underway to clarify the relative risks and benefits of prolonged DAPT use after first-generation DES25.
Limitations
This study has several limitations. Despite the randomised design of the four component trials, slight imbalances in baseline covariates between the two randomly assigned stents were observed after pooling. However, after propensity score adjustment, the HRs for definite ST and definite/probable ST did not directionally differ from the unadjusted HRs. Additional confounding variables (either measured or unmeasured) not incorporated in this analysis may have influenced the study observations as well. Angiographic measures of reference vessel diameter and lesion length were assessed by an independent core laboratory in the SPIRIT trials, and by operator assessment in COMPARE. Outcomes of ST in high-risk categories of patients presenting with acute coronary syndromes or diabetes mellitus were not the focus of this analysis, as they have been previously26,27. Subjects in the present analysis were not randomised by duration of DAPT compliance. A small percentage of patients with definite ST and definite/probable ST were excluded from this analysis due to unknown DAPT status at multiple time points after the event; however, all patients in whom ST occurred between 0-30 days were taking DAPT at the time of the event. Finally, bleeding was not a focus of these trials, and long-term DAPT may entail haemorrhagic risks which may offset their advantages. For all of these reasons the findings of the present study should be considered hypothesis-generating, requiring confirmation in appropriately designed and powered randomised trials.
Conclusion
The present large-scale patient-level pooled database from four randomised trials has shown that EES significantly reduces definite ST and definite/probable ST rates compared to PES. This major safety benefit was evident early (within 30 days) and increased in magnitude during the two-year follow-up. The reduction in ST was paralleled by a reduction in the combined rate of cardiac death or MI with EES compared to PES. Finally, the present analysis suggests that permanent DAPT discontinuation beyond six months may be safe following EES implantation.
Conflict of interest statement
E. Kedhi reports receiving lecture fees from Abbott Vascular and Saint Jude Medical. P. C. Smits reports receiving lecture fees from Abbott Vascular. D. J. Kereiakes reports serving on the advisory boards for Abbott Vascular and Boston Scientific. G. W. Stone reports having served as a consultant for Boston Scientific, Abbott Vascular, The Medicines Company, Merck, Eli Lilly, AstraZeneca, Bristol-Meyers-Squibb and Medtronic. C. A. Simonton, K. Sudhir and P. Sood are employees of Abbott Vascular. The other authors have no conflicts of interest to declare.

