Abstract
Aims: To evaluate the incidence, time course and predictors of late target lesion revascularisation (TLR) (between one and two years) as compared to early TLR (<1 year) following everolimus-eluting stent implantation in patients enrolled in the SPIRIT V study.
Methods and results: The SPIRIT V single-arm study enrolled a total of 2,700 patients (n=2,663 intent-to-treat) with de novo coronary artery lesions undergoing EES implantation. Patients were evaluated at 30 days, one year and two years following the index procedure. All patients who had a clinically driven TLR (not associated with stent thrombosis) were allocated to either the early or the late group. Clinical, angiographic and procedural data were recorded and predictors of early vs. late TLR were assessed by logistic regression analysis. There were no significant differences in baseline demographics and risk factors between the two groups with the exception that patients in the late TLR group were significantly older (68.5±8.5 years vs. 63.5±8.9 years, p=0.022). At two years, only 2.7% (70/2,562) experienced a TLR unrelated to a stent thrombosis event with 1.6% (43/2,627) occurring within one year of the index procedure and 1.1% (27/2,562) occurring between one and two years. There were no differences between the groups in terms of clinical outcomes. Age ≥70 years was the only variable which independently predicted late TLR (OR=4.80 [1.69-13.63], p=0.0032).
Conclusions: In this large registry without angiographic follow-up, early (<1 year) and late (>1 year) TLR rates were exceedingly low and thereby confirm the previous findings of randomised controlled studies. Age (>70 years) emerged as the only predictor of late TLR.
Introduction
Despite exciting results being reported during initial trials evaluating safety and efficacy of first-generation drug-eluting stents (DES), the real-world use of DES showed that in-stent restenosis (ISR) still occurred after DES implantation, with the temporal window of ISR presentation being wider when compared to that of bare metal stents (BMS). Some evidence did indeed raise the possibility of a late catch-up phenomenon1-4, suggesting that antiproliferative drugs might simply delay the occurrence of ISR. Recent findings derived from the SYRTAX-LATE study clearly showed that first-generation DES present a continuous increase in late lumen loss at five-year follow-up, suggesting an incomplete vascular healing5. Of interest, delayed late loss seems to occur mainly with polymeric DES rather than with non-polymeric DES4. However, other studies failed to demonstrate a significant occurrence of late restenosis after DES implantation when compared to BMS6,7, thus prompting further investigation.
Results from the COMPARE trial8 and SPIRIT IV study9 showed that newer-generation everolimus-eluting stents (EES) significantly reduced the occurrence of target lesion revascularisation (TLR) compared with paclitaxel-eluting stents (PES) up to two-year follow-up. EES also showed lower rates of TLR compared with sirolimus-eluting stents (SES) up to three-year follow-up10.
SPIRIT V is a prospective, single-arm, multicentre study evaluating the performance of the XIENCE V everolimus-eluting stent (Abbott Vascular, Redwood City, CA, USA) in real-world use. Clinical follow-up was performed up to two years and TLR were computed according to time of occurrence. In this report we evaluate the incidence, time course and predictors of late TLR (between one and two years) versus early TLR (<1 year) in the SPIRIT V single-arm study.
Materials and methods
PATIENT POPULATION AND STUDY DESIGN
Approximately 2,700 patients were enrolled in the SPIRIT V study at 93 clinical sites between November 2006 and November 2007. Inclusion and exclusion criteria as well as baseline characteristics of the study have been previously reported11. Briefly, the SPIRIT V patient population confirmed high numbers of complex patients including 30% of diabetic patients, 82% of lesions with class B2 or C classification according to the American College of Cardiology/American Heart Association, and 29% of patients with greater than one target lesion treated. The mean number of lesions treated per patient was 1.4. Adverse events were assessed in the hospital, and at one, 12 and 24 months following the index procedure. Study monitors collected data by visits, phone calls, and postal questionnaires. Source document verification was routinely performed in 20% of random cases and 100% of all reported adverse events at the time of the study conduct, resulting in an overall rate of 30%. In addition, sites with low rates of reported events received additional training and monitoring visits to confirm event reporting. Finally, data processing and adjudication of adverse events were done in a blinded fashion by an independent contract research organisation and core laboratory (BioClinica, Leiden, The Netherlands). The primary endpoint of the study was the adjudicated composite rate of all death, myocardial infarction (MI) and target vessel revascularisation (TVR) at 30 days. TLR was a secondary endpoint of the study and, due to lack of angiographic follow-up, all TLR were considered clinically indicated. TLR was defined as any repeat percutaneous intervention of the target lesion or bypass surgery of the target vessel performed for restenosis or other complication of the target lesion. The target lesion was defined as the treated segment, including 5 mm margins on both edges of the stent. Thirty-seven patient records were excluded from the intent-to-treat (ITT) population due to enrolment errors, informed consent not being obtained, and/or no attempt to implant EES during the index procedure, leaving an ITT population of 2,663. Of this ITT population, at the one-year time point, 2,627 patients had clinical follow-up data available. At the two-year time point, 2,562 patients had clinical follow-up data available. All patients enrolled were pretreated with a loading dose of ≥300 mg of clopidogrel. After the procedure, the protocol recommended that patients receive clopidogrel 75 mg daily for a minimum of three months with ≥75 mg of aspirin for a minimum of five years. Eighty-two percent of patients were on clopidogrel and 96% on aspirin at one-year follow-up. Forty-nine percent of patients were on clopidogrel and 94% on aspirin at two-year follow-up.
PREDICTORS AND PROGNOSTIC ROLE OF EARLY VS. LATE TLR
For the present analysis all patients having a clinically driven TLR were allocated to either the early TLR group (<1 year) or to the late TLR group (between one and two years). However, TLR, when it was a result of stent thrombosis (n=8: seven at <1 year, and one between one and two years), was excluded from the analysis. For each patient, clinical, laboratory, angiographic and procedural data were recorded and predictors of early vs. late TLR were assessed among these variables. Clinical variables included demographics, risk factors and clinical presentation as well as rate of single or dual antiplatelet therapy at the time of follow-up. Among laboratory data cardiac enzymes were assessed, including pre- and post-procedure troponin levels and post-procedure CK and CK-MB ratio. In the periprocedural period an abnormal and clinically relevant troponin level was defined as >3 times the upper limit of normal. Angiographic variables included number of diseased vessels and data regarding location and characteristics of the target vessel including eccentricity, angulation, tortuosity and calcification, the presence of thrombus, bifurcation and ACC/AHA lesion classification. Pre-procedure reference vessel diameter (RVD) and pre- and post-procedure in-stent percent diameter stenosis were also collected by physician assessment. Finally, procedural variables were recorded and assessed among predictors of early vs. late TLR.
The prognostic role of early vs. late TLR was assessed by comparing the rate of the composite of death, MI, and TVR at two years between patients experiencing early TLR and those experiencing late TLR.
STATISTICAL ANALYSIS
Categorical variables are presented as counts and percentages. Continuous variables are presented as mean±SD. P-values were calculated with Fisher’s exact test for binary variables and t-test for continuous variables. All p-values reported are for descriptive purposes and were not from formal hypothesis testing.
Multiple logistic regression analysis was used to identify significant predictors of TLR before one year. Included in this analysis are the demographic, clinical, angiographic and procedural variables. The model was built using a stepwise (forward/backward) elimination procedure, with independent variables entered into the model at the 0.20 significance level and removed at the 0.10 level. For the logistic regression, odds ratios and 95% confidence intervals are reported.
Statistical analyses were performed using SAS version 9.1.3 (SAS Institute Inc., Cary, NC, USA).
Results
PATIENT POPULATION AND TIME COURSE OF TLR
At two-years, only 2.7% (70/2,562) of patients had experienced a TLR unrelated to stent thrombosis, with 1.6% (43/2,627) occurring within one year of the index procedure and 1.1% (27/2,562) occurring between one and two years. Figure 1 shows the temporal distribution of the TLR and Figure 2 and Figure 3 show Kaplan-Meier curves of TLR, throughout two years and with a landmark analysis at one year, respectively.
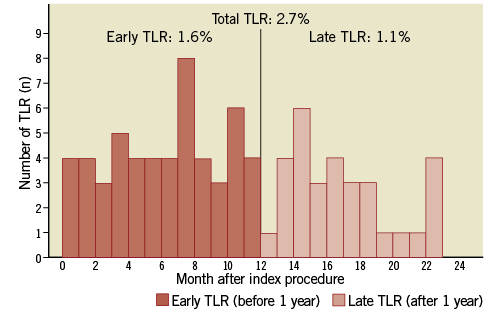
Figure 1. Time course of target lesion revascularisation (TLR) according to time of presentation from the index procedure. A sizeable proportion of TLR occurs after 1 year.
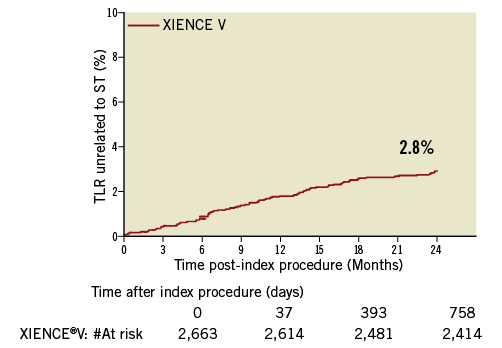
Figure 2. Kaplan-Meier curves of TLR throughout 2 years.
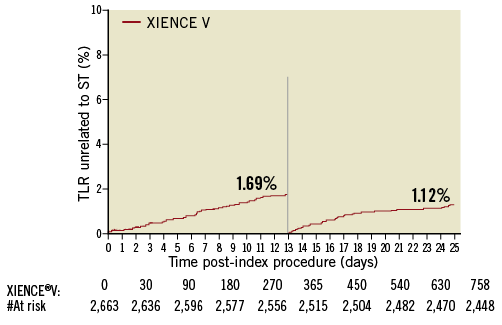
Figure 3. Kaplan-Meier curves of TLR with a landmark analysis at 1 year.
PREDICTORS AND PROGNOSTIC ROLE OF EARLY VS. LATE TLR
Clinical data from the overall group of patients with TLR as well as the clinical data according to the TLR time of presentation are shown in Table 1. Angiographic and procedural data are shown in Table 2.

Patients with late TLR were significantly older when compared to those having an early TLR (68.5±8.5 vs. 63.5±8.9, p=0.022, Table 1), and more frequently tended to be female (40.7% vs. 20.9%, p=0.104, Table 1). Figure 4 shows the absolute numbers of early and late TLR according to age. Neither clinical presentation on admission nor diabetic status was associated with late TLR (p=NS, Table 1). An abnormal pre-procedural troponin level tended to be more frequent in patients with early TLR when compared to those with late TLR (24.1% vs. 5.3%, p=0.123), as well as an elevated post-procedure CK-MB (5.3±11.0 vs. 1.3±2.6, p=0.108). Among angiographic data, moderate/severe angulation tended to be associated with late TLR (37.9% vs. 17.0%, p=0.057). None of the procedural variables was associated with late TLR (Table 2).
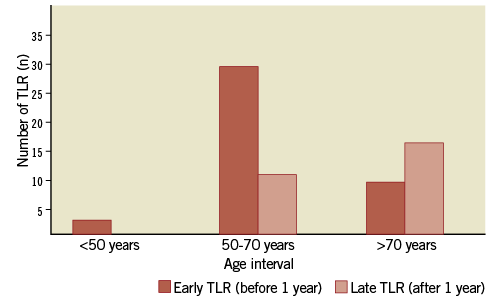
Figure 4. Distribution of target lesion revascularisation (TLR) according to age interval. The risk of late TLR increases with age, whereas the risk of early TLR is not affected by age.
Table 3 shows the results of the multiple logistic regression identifying multivariate predictors of late TLR. Age older than 70 years was the only variable that independently predicted late TLR (OR=4.80, p=0.0032). The rate of the composite of death, MI, and TVR was similar between patients with early vs. those with late TLR (28% vs. 21.4%, p=0.59).

Discussion
This study demonstrates that, in the context of a remarkably low TLR rate after EES implantation (2.7%), late TLR occurs in a sizeable proportion of patients (40.7%) with older age being associated with late TLR. However, there was no difference in the final MACE rate at two-year follow-up between patients having experienced an early or a late TLR. The clinical implication of our finding is that, for EES as well as for other types of DES, the window of follow-up should perhaps be extended past the standard one year used in most studies comparing DES and BMS, as symptom recurrence may be due to stent-related events rather than to native coronary artery disease progression.
Past observations demonstrated that the peak rate of BMS restenosis occurred between six and nine months12,13, and this time frame has been used to evaluate DES efficacy. Yet, data about the time course of DES restenosis are more limited. The occurrence of a late catch-up phenomenon has been reported for first-generation DES14,15. Recent findings of the SYRTAX-LATE study demonstrated a continuous increase in late lumen loss at five-year follow-up, suggesting an incomplete vascular healing5. Of note, the rate of late TLR was higher for SES (2.0%/year) when compared to PES (1.4%/year), suggesting that DES type could influence the rate and time course of late neointima formation. An increasing incidence of late TLR after SES but not after BMS implantation was recently shown in the j-Cypher registry16. These findings were not confirmed in another study by Applegate et al17 where the authors showed no evidence of late catch-up after utilisation of DES (either SES or PES) for off-label indications. Also PES exhibits a late catch-up phenomenon as demonstrated by Park et al in a study performed with serial quantitative angiography2. A recent study by Byrne et al4 investigated the notion of late catch-up restenosis, evaluating the time course of ISR following implantation of DES, with an angiographic follow-up at six to eight months and at two years. Interestingly, DES showed a delayed attenuation of antirestenotic efficacy, with an increasing late luminal loss (LLL) beyond six to eight months. In particular, polymer-free DES showed less delayed LLL (difference in in-stent LLL between six to eight months and two years) as compared with permanent-polymer DES, suggesting a critical role for a persistent inflammatory reaction triggered by the polymer in determining late DES restenosis. Mechanisms of late restenosis are probably multiple. Analysis of specimens derived from human coronary atherectomy revealed that DES restenosis was associated with a larger amount of old thrombus and fibrinoid as compared with BMS, possibly as a consequence of the delayed tissue healing induced by the eluted drug18. In addition, increased thrombotic burden in DES-treated patients might be related to a hypersensitivity reaction to the stent polymer19. Furthermore, a recent study showed different smooth muscle cell phenotypes in specimens of restenotic tissue obtained from BMS or DES ISR20. Optical coherence tomography (OCT) studies confirmed that DES restenosis may be composed of neointimal components with different optical properties (heterogeneous, homogeneous or layered tissues)21. In contrast, neointimal hyperplasia after BMS implantation is usually recognised by OCT as an homogeneous tissue22. However, a delayed healing after BMS implantation may occur, as the annualised TLR rate after the first year was about 1%6.
In this study older age is associated with a higher rate of late TLR compared to early TLR. DES are currently used in older patients with favourable clinical outcomes despite the increased clinical and angiographic complexity of these patients. The role of ageing in late TLR may, however, be due to the interaction between a chronic inflammatory response to the polymer and smooth muscle cell (SMC) proliferation which is affected by age. Indeed, in the animal model, ageing increases SMC proliferation and neointima formation23, whereas senescent endothelial progenitor cells (EPC) have recently been associated with a higher rate of in-stent restenosis24. Whether these factors exist following XIENCE V implantation is unknown.
However, another explanation may be a more conservative management of older patients, which prolongs the time period between first angina and catheterisation. In addition, the threshold for a clinical manifestation of a restenosis may be higher in older people due to a reduced level of exercise.
A gender difference in the clinical outcome after DES implantation is currently a matter of debate, with conflicting data published in various reports25-29. The clustering of unfavourable features in women is often advocated as a possible explanation of gender difference in the outcome and may also explain our findings as women are usually older and present with more tortuous coronary arteries as compared to men. Of note, due to the small sample size, this finding should be assessed in other specifically designed studies. In a recent gender-specific evaluation of the SPIRIT III trial data at three-year follow-up30, women had higher rates of major adverse cardiac events (16.0% vs. 10.0%, p=0.01) and target lesion revascularisation (10.2% vs. 5.3%, p=0.008) compared to men. However, in women, those with XIENCE V continued to have lower major adverse cardiac event rates than those with TAXUS (Boston Scientific, Natick, MA, USA) at two years (9.5% vs. 18.3%, p=0.03) and three years (12.2% vs. 22.6%, p=0.03).
Although angulated lesions were not shown to be associated with late loss after DES implantation in a previous study31, our data suggest that a chronic stretch at the level of the angulation may promote local proliferation32 for the induction of late events. Troponin elevation on admission in our study is associated with early events, probably reflecting a more unstable and complex patient population prone to early complications33.
The overall TLR rate in this study was exceedingly low. However, similar findings were found in the COMPARE trial, an all-comers randomised open-label trial comparing EES (XIENCE V) and PES, showing that occurrence of TLR in EES patients was 2.0% at one year and 2.9% at two years8. In the SPIRIT IV trial, a randomised controlled trial comparing EES (XIENCE V) and PES, the occurrence of TLR in the EES group was slightly higher both at one year (2.4%) and at two years (4.5%) (9). Improvements in stent design, strut thickness, delivery platform, polymer coating, drug and drug release profile may explain these results.
STUDY LIMITATIONS
This study was limited by the single-arm nature of the design and the inherent lack of a control arm for direct comparison. Lesion characteristics were assessed and reported by the investigator at the time of the procedure without core lab evaluation of lesion morphology. Moreover, data about the rate of angiographic restenosis were not available, and analysis of the pattern of restenosis according to Mehran’s classification, known to have prognostic utility, have not been performed due to lack of angiographic follow-up.
Although this study was designed as an all-comers registry and patients were enrolled according to relatively broad inclusion/exclusion criteria, there were some limitations to the inclusions based on the IFU and device availability.
Moreover, the limited time period to evaluate late TLR should be acknowledged, as SPIRIT V was designed with a follow-up of two years.
Finally, TLR rates occur at low frequency and SPIRIT V was not powered to analyse within-study differences. In addition, post hoc analyses do not correct for multiplicity errors so the potential for a Type 1 error exists.
Conclusions
In this large registry without angiographic follow-up, early (<1 year) and late (>1 year) TLR rates were exceedingly low and thereby confirm the previous findings of randomised controlled studies. Age (>70 years) emerged as the only predictor of late TLR.
Conflict of interest statement
K. Sudhir, M. Stuteville and D. Li are employees of Abbott Vascular. E. Grube is a member of Abbott Vascular advisory board. The other authors have no conflicts of interest to declare.

