Abstract
Aims: The SYNTAX score has been proposed as a valuable tool to characterise coronary anatomy prospectively based on its complexity. This study evaluated the prognostic value on adverse outcomes of the residual SYNTAX score (rSS) in patients with complex lesions treated with an everolimus-eluting stent (EES).
Methods and results: One thousand eight hundred and fifty-one patients with small vessel (reference diameter <2.75 mm), long lesion (length >25 mm), or multivessel (>2 target vessels) disease who underwent percutaneous coronary intervention (PCI) with EES in the prospective SEEDS (A Registry To Evaluate Safety And Effectiveness Of Everolimus Drug Eluting Stent For Coronary Revascularization) trial were categorised into low (<6), mid (>6-<12) and high (>12) baseline SYNTAX score (bSS) groups, and into low (=0), mid (>0-<5) and high (>5) rSS groups. Mean bSS and rSS were 10.87±7.26 and 2.18±3.97, respectively; 64% of patients had complete revascularisation (rSS=0). At 12 months the primary outcome of ischaemia-driven target vessel failure (TVF, composite of cardiac death, target vessel myocardial infarction and ischaemia-driven target vessel revascularisation) was significantly higher in the high bSS and rSS groups than in the respective lower groups (p<0.01 for both). In multivariable analysis, rSS was an independent predictor of TVF (hazard ratio: 1.403, 95% confidence interval: 1.081 to 1.820, p=0.01).
Conclusions: Twelve-month TVF was significantly higher in the highest rSS group; rSS with a cut-off of 5 might therefore allow the risk stratification of patients with complex lesions treated with a second-generation drug-eluting stent (ClinicalTrials.gov identifier: NCT 01157455).
Abbreviations
ARC: Academic Research Consortium
bSS: baseline SYNTAX score
CABG: coronary artery bypass graft
CI: confidence interval
EES: everolimus-eluting stent
MI: myocardial infarction
PCI: percutaneous coronary intervention
rSS: residual SYNTAX score
SS: SYNTAX score
ST: stent thrombosis
TVF: target vessel failure
TVMI: target vessel myocardial infarction
TVR: target vessel revascularisation
VIF: variance inflation factor
Introduction
The SYNTAX score (SS) has been examined as an angiographic tool to grade complexity of coronary lesions based on their location and characteristics1. Baseline SS (bSS), a surrogate marker of disease burden before revascularisation, has demonstrated value in the treatment selection for patients with complex coronary lesions and the prediction of clinical outcomes after percutaneous coronary intervention (PCI)2,3. Residual SS (rSS), recently proposed as an index of completeness of revascularisation after PCI, has additionally been validated as an independent predictor for adverse events4-6. However, studies examining the impact of rSS were primarily performed using first-generation drug-eluting stents (DES, namely paclitaxel- or sirolimus-eluting stents) while the use of second-generation DES has been more common in recent years.
Patients with lesions with complex anatomic characteristics (lesions in small vessels, long lesions, and multivessel disease), who experience worse clinical outcomes after PCI7-9, are very common in “real-world” practice, probably more so in China than in the West10. We therefore investigated the prognostic value of SS, particularly rSS, in Chinese patients with complex anatomic lesions treated with an everolimus-eluting stent (EES), a second-generation DES.
Methods
STUDY PROTOCOL, POPULATION AND PROCEDURE
The SEEDS was a prospective, multicentre, open-label registry trial that complied with the Declaration of Helsinki and whose protocol was approved by the institutional ethics committee at each participating site. From June 2010 to December, 2011, 1,900 eligible patients treated with EES (XIENCE V; Abbott Vascular, Santa Clara, CA, USA) were enrolled in 45 sites in mainland China, two in Taiwan and one in Macao. All patients signed a written informed consent. Inclusion criteria were: i) patients aged 18 to 75 years old with symptomatic ischaemic heart disease and/or objective evidence of myocardial ischaemia; and ii) by visual estimation, target vessel diameter <2.75 mm (small vessel disease), target lesion length >25 mm (long lesion), or number of target vessels >2 and at least one target lesion with >70% diameter stenosis in one epicardial target vessel (multivessel disease). Patients were assigned to each anatomic group based on the presence or absence of these anatomic characteristics, and therefore patients could belong to multiple groups. For example, if a patient’s lesion length was >25 mm and RVD <2.75 mm, this patient was assigned to both the long lesion and the small vessel groups. Exclusion criteria were: i) acute myocardial infarction (MI) within a week; ii) congenital heart disease, severe valve dysfunction, bypass graft lesions, severe heart failure (NYHA ≥III level) or left ventricular ejection fraction ≤30%; iii) previous stent implantation within one year; iv) renal dysfunction (serum creatinine >2.0 mg/dl); v) bleeding disorder contraindicating antiplatelet and/or anticoagulant therapy; vi) hypersensitivity or allergy to drugs and devices related to the PCI procedure; vii) illness limiting life expectancy; viii) participation in other clinical trials; ix) heart transplant; and x) incapacity to complete the study.
All eligible patients underwent PCI with EES available at 2.25-4.0 mm in diameter and 8-28 mm in length. PCI procedures were completed in accordance with the established standard of care at each site, although predilatation of the target lesion was preferred. Aspirin 300 mg was given orally within 24 hours before the procedure. Clopidogrel was given at a dose of 300 mg six hours before or 75 mg/day three days before the procedure. After the procedure, dual antiplatelet therapy (aspirin 100 mg/day indefinitely and clopidogrel 75 mg/day for at least 12 months) was recommended.
ENDPOINTS AND DEFINITIONS
Clinical follow-up was scheduled at 1, 6, 12 and 24 months via clinical visit or phone contact. The primary endpoint was ischaemia-driven target vessel failure (TVF), defined as the composite of cardiac death, target vessel myocardial infarction (TVMI), and ischaemia-driven target vessel revascularisation (TVR), at 12 months post procedure. Secondary endpoints included: i) TVF at 1, 6 and 24 months; ii) each component of TVF at 1, 6, 12 and 24 months; and iii) Academic Research Consortium (ARC) defined definite/probable stent thrombosis (ST)11. Device success was defined as the attainment of <50% residual stenosis of the target lesion using only the assigned device. Lesion success was defined as the attainment of <30% residual stenosis, TIMI 3 flow, and no residual dissection and thrombosis of the target lesion using any percutaneous method. Clinical success was defined as attainment of lesion success in all target lesions and no in-hospital major adverse cardiac event. All major adverse events were adjudicated by an independent clinical events committee.
SYNTAX SCORE ASSESSMENT
The SS, bSS and rSS were assessed visually by three experienced imaging analysts from an independent core laboratory (CCRF, Beijing, China). Each lesion with >50% diameter stenosis in vessels ≥1.5 mm in diameter was scored using the SS algorithm described previously1. The rSS was calculated based on the remaining obstructive coronary disease after PCI.
STATISTICAL ANALYSIS
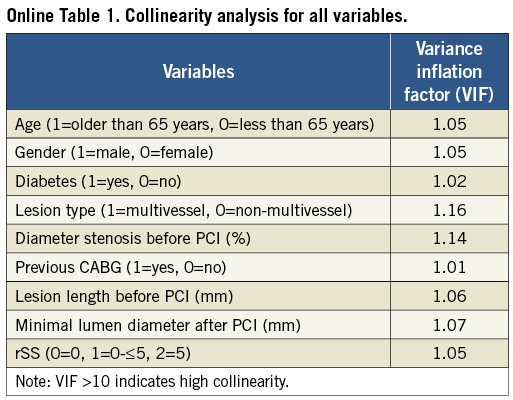
Continuous variables are expressed as mean±SD and were compared using the Student’s t-test or the Mann-Whitney test depending on data distribution. Categorical variables are presented as frequencies and were compared using the chi-square or Fisher’s exact test. Time-to-event variables were analysed using Kaplan-Meier methodology, and compared using the log-rank test and Cox proportional hazards regression. Multivariable analyses of covariates associated with TVF were conducted with a Cox regression model using a stepwise selection method, and a variance inflation factor (VIF) was used to detect collinearity. Two separate multivariable models were constructed because of the high correlation between bSS and rSS. The bSS was stratified into low (<6), mid (>6-<12), and high (>12) tertiles. Bootstrap methodology12 was used to evaluate different rSS thresholds (rSS>4, >5, >6 or >8) for the prediction of one-year TVF among patients without complete revascularisation. For each bootstrap run, 80% of the total population was sampled; a total of 1,000 bootstrap runs was performed to assess the performance of each rSS cut-off point. Comparison between bSS and rSS (when added to baseline covariates) was performed using the likelihood ratio test. All analyses were conducted using SAS system software, version 9.1.3 (SAS Institute, Cary, NC, USA).
Results
PATIENT DEMOGRAPHICS, LESION CHARACTERISTICS AND PROCEDURAL RESULTS
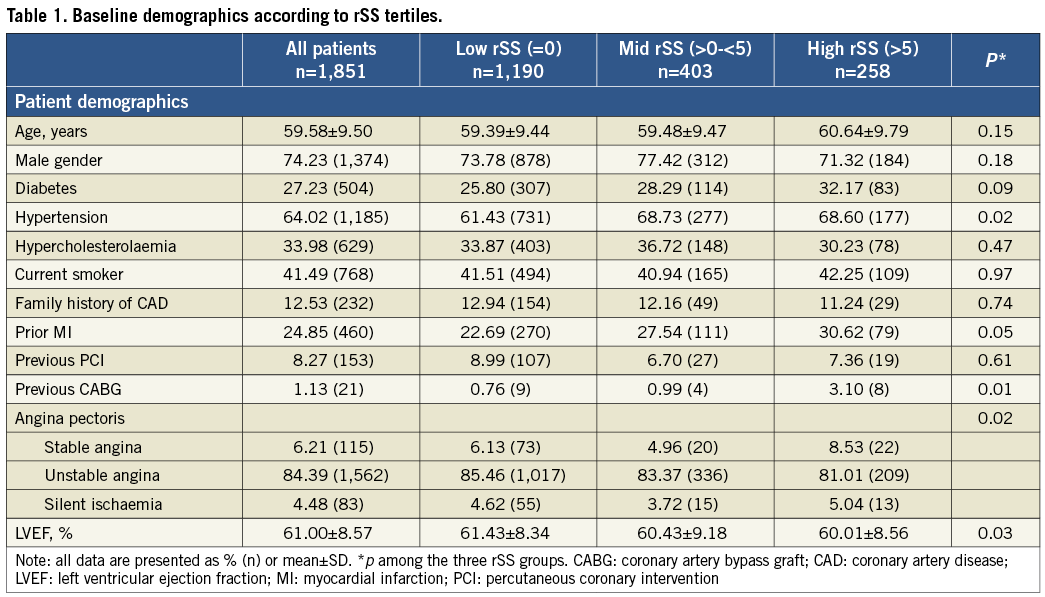
Among the 1,900 patients with coronary small vessel, long lesion or multivessel disease who were enrolled and implanted with EES in the SEEDS study, 1,851 patients (Table 1) had a residual SYNTAX score calculated (49 patients lacked bSS or rSS for technical reasons). As shown in Table 2, among the enrolled, 1,065, 1,291 and 708 patients had small vessel, long lesion and multivessel disease, respectively. Patients had a mean age of 60 years, 27% were diabetic and 84% presented with unstable angina. After PCI (Table 2), mean diameter stenosis was reduced from 69.60±15.57% to 13.30±8.26%. Device and clinical success rates were 99.95% and 96.76%, respectively. Mean bSS was 10.87±7.26 before PCI, ranging from 1 to 44.5 with a median of 9. Mean rSS following PCI was 2.18±3.97, ranging from 0 to 29.5 with a median of 0. Complete revascularisation (rSS=0) was achieved in 64% of patients.
Patients were stratified into low, mid and high tertile groups based on bSS (≤6, >6-≤12, and >12, respectively). In bootstrap analyses, the threshold of rSS >5 among incomplete revascularisation patients was the most predictive for one-year TVF, with the highest mean hazard ratio (HR) of 1.53±0.21 (HR of rSS threshold >4, >6 and >8 was 1.47±0.22, 1.19±0.17 and 1.01±0.18, respectively). Accordingly, patients were stratified by rSS into low (=0), mid (>0-≤5) and high (>5) groups. There was a strong correlation between bSS and rSS (correlation coefficient=0.533, p<0.001) (Figure 1).
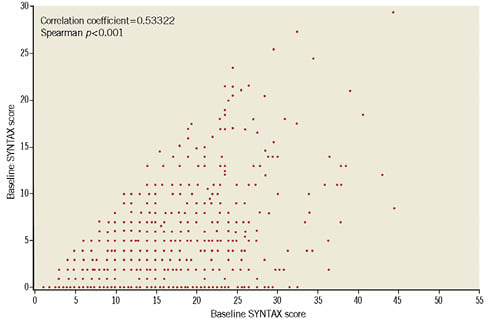
Figure 1. Correlations between baseline and residual SYNTAX scores. Residual SYNTAX score (rSS) correlated strongly with baseline SYNTAX score (bSS). However, rSS varied within a considerable range for each level of bSS.
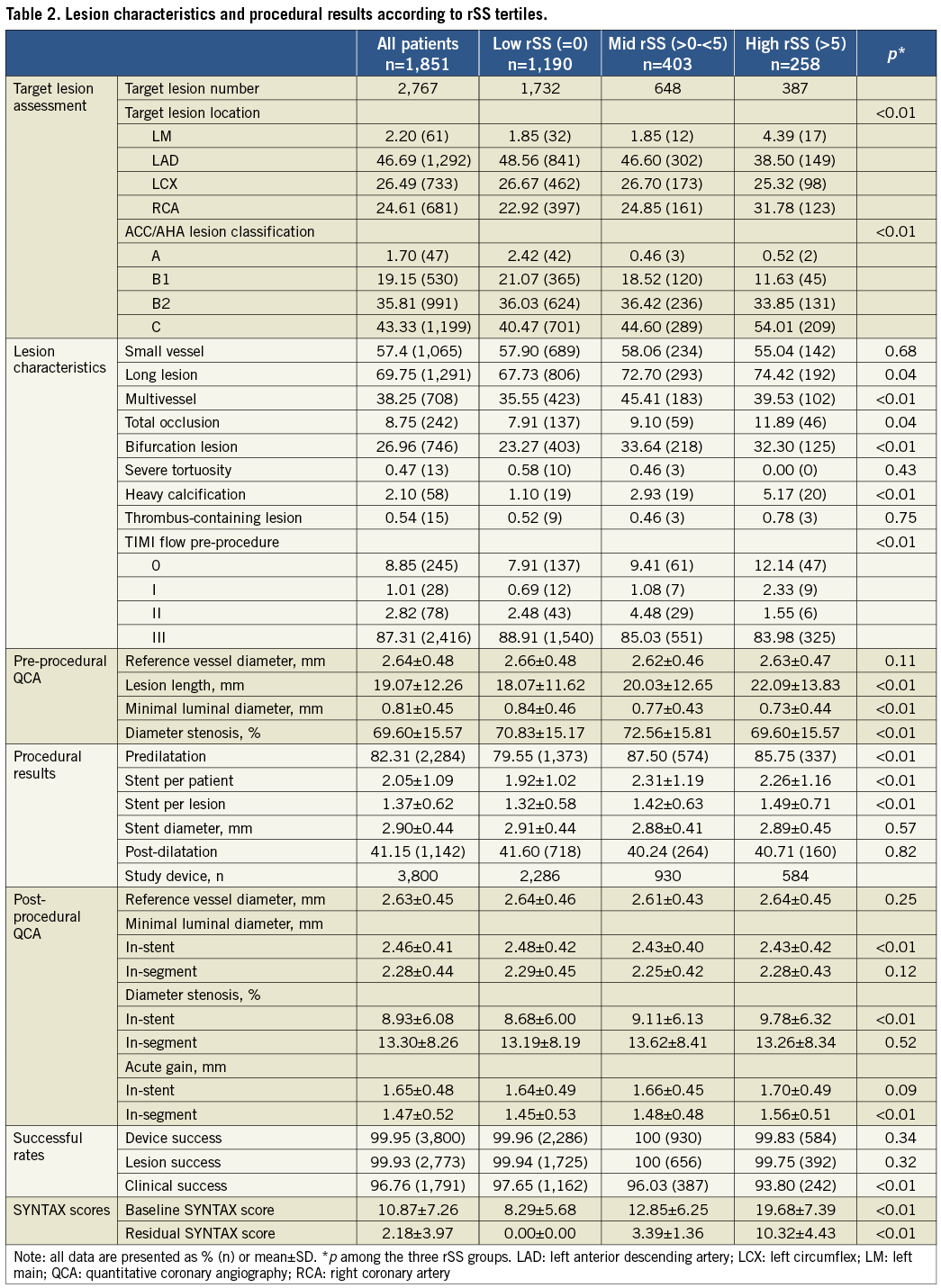
The clinical and angiographic characteristics of patients stratified by rSS are shown in Table 1 and Table 2. Compared with patients with complete revascularisation (rSS=0), those in the highest rSS tertile (rSS >5) were more likely to have had previous CABG, stable angina, lower TIMI flow, and more complex coronary lesions resulting in a higher SS at baseline (p<0.01), including higher proportions of longer lesions, total occlusions, severe calcified lesions, type C lesions and left main disease.
CLINICAL OUTCOMES
Among all 1,900 enrolled patients, the primary endpoint of TVF rate at one year was 5.95%. All-cause death and components of TVF, namely cardiac death, TVMI, and TVR occurred in 0.68%, 0.47%, 3.42% and 2.47% of patients at 12 months after PCI, respectively. The rate of ARC-defined definite/probable ST was 0.58%.
One-year rates of TVF were significantly higher among patients in the highest bSS or rSS tertile. Cumulative TVF rates at one year were 9.14%, 4.31% and 2.69% in patients in the high, mid and low bSS groups, respectively (log-rank p<0.01). After stratification by rSS, TVF rates were 10.08%, 6.95% and 4.37% in patients in the high, mid and low rSS groups, respectively (log-rank p<0.01). In terms of individual TVF components, patients in the highest tertiles of bSS and rSS were more likely to suffer cardiac death, TVMI and/or TVR (Table 3, Figure 2, Figure 3).
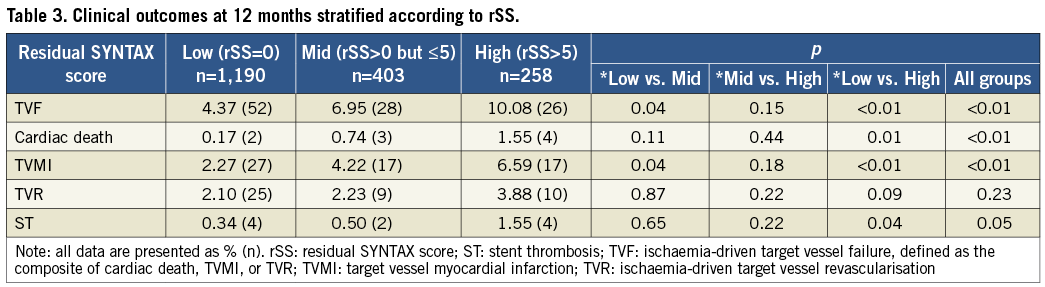
Figure 2. Kaplan-Meier estimates of cumulative event rates up to one year stratified by bSS. A) TVF; B) cardiac death; C) TVMI; and D) TVR, stratified by tertiles of baseline SYNTAX score (bSS). Patients were categorised based on bSS into low (≤6), mid (>6-≤12) and high (>12) bSS groups. The high bSS group had the worst clinical outcomes. TVF: ischaemia-driven target vessel failure, defined as the composite of cardiac death, TVMI, and TVR; TVMI: target vessel myocardial infarction; TVR: ischaemia-driven target vessel revascularisation
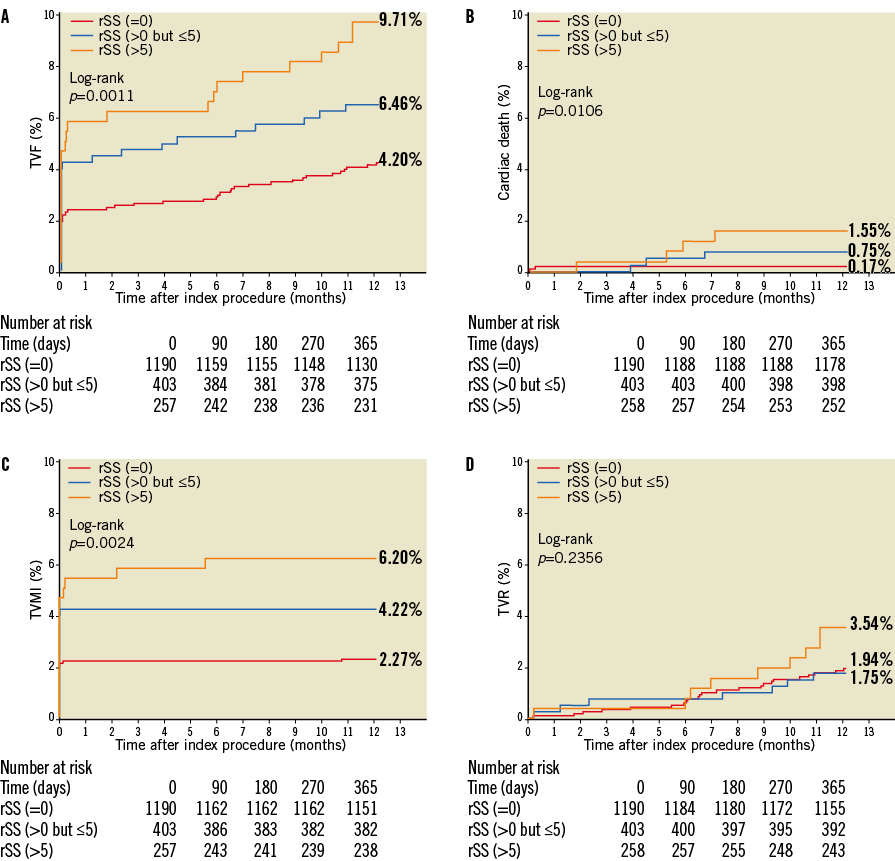
Figure 3. Kaplan-Meier estimates of cumulative event rates up to one year stratified by rSS. A) TVF; B) cardiac death; C) TVMI; and D) TVR, stratified by tertiles of residual SYNTAX score (rSS). Patients were categorised based on rSS into low (=0), mid (>0-≤5) and high (>5) bSS groups. The high rSS group had the worst clinical outcomes. TVF: ischaemia-driven target vessel failure, defined as the composite of cardiac death, TVMI, and TVR; TVMI: target vessel myocardial infarction; TVR: ischaemia-driven target vessel revascularisation
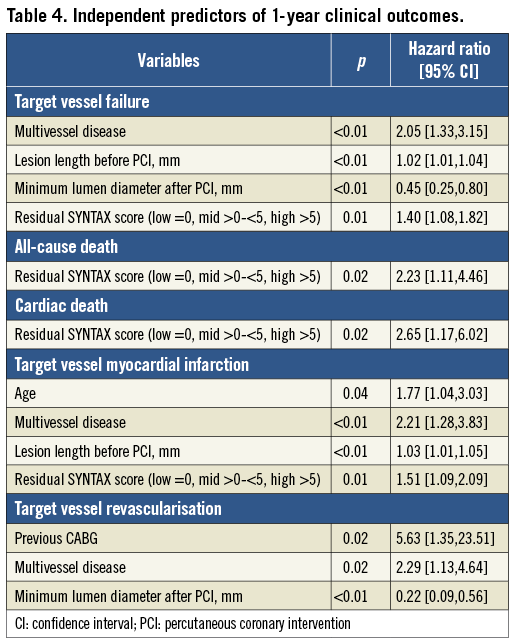
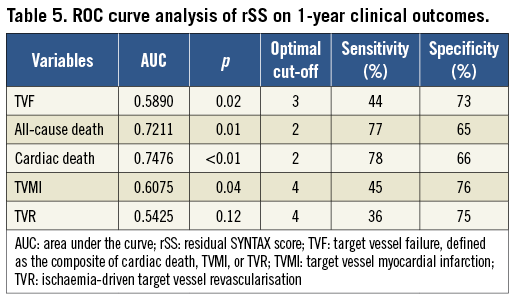
MULTIVARIABLE ANALYSIS AND ROC ANALYSIS
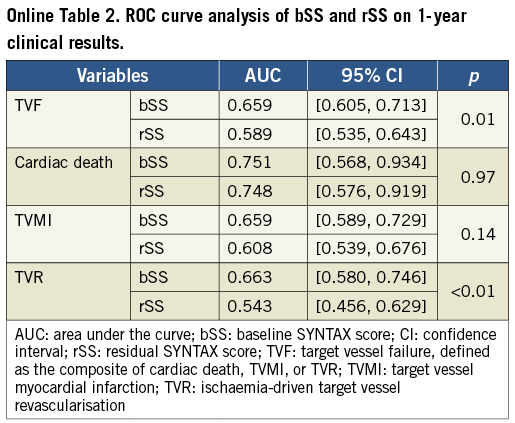
In Cox multivariable analyses, rSS was a strong independent predictor of TVF, all-cause death, cardiac death and TVMI, but not of TVR, at one year (Table 4). VIF contributes to collinearity diagnosis in multivariate analysis and showed low collinearity for all variables (all VIF <2) (Online Table 1). ROC curve analyses also demonstrated a significant association between rSS and one-year TVF (p=0.02), all-cause death (p=0.01), cardiac death (p<0.01) and TVMI (p=0.04) (Table 5). Compared with bSS, rSS had a similar predictive value and discrimination for cardiac death (p=0.97) and TVMI (p=0.14) at one year, whereas bSS was a slightly stronger predictor of TVF and TVR (both p<0.01) (Online Table 2).
Discussion
The current study, drawn from a large cohort of patients with complex anatomic characteristics, is the first to investigate the predictive value on clinical outcomes of rSS in EES-treated patients with complex disease (small vessels, long lesions, and multivessel disease). The major findings of this analysis are as follows: 1) the 12-month rates of TVF and its individual components (cardiac death, TVMI and TVR) as well as those of ARC-defined definite/probable ST were low in patients with complex lesions after PCI with EES; and 2) rSS >5 were associated with increased rates of adverse clinical outcomes after EES implantation, including TVF, all-cause death and TVMI.
Recently, rSS has been proposed as an index of revascularisation completeness with rSS=0 defined as complete revascularisation4. Indeed, since its first introduction1, SS has been evaluated for treatment selection and prediction of clinical outcomes after coronary revascularisation2-6,13-15. However, most previous assessments of SS, particularly rSS, were conducted with early-generation DES. Moreover, no studies have been performed to determine whether the rSS is meaningful in patients with complex anatomic characteristics (lesions in small vessels, long lesions, and multivessel disease), who are known to experience worse clinical outcomes after PCI7-9. In the present study, we have shown that quantification of the extent and complexity of coronary stenosis with bSS and rSS provide substantial prognostic information among patients undergoing PCI with EES.
In this study, median bSS was 9 (mean±SD: 10.87±7.26), which is comparable to that of patients with moderate-risk and high-risk acute coronary syndrome from the ACUITY trial (mean±SD: 12.8±6.7)4. At one year after EES implantation, patients in the high bSS and rSS tertiles had greater rates of TVF, cardiac death, TVMI and TVR.
Residual SYNTAX score was correlated with bSS, which is consistent with previous studies4,6 (Figure 1). Because complete coronary revascularisation has been shown to result in improved clinical outcomes16,17, rSS, an index of completeness of coronary revascularisation after PCI, may provide better prognostic information. Indeed, the highest rSS tertile (rSS >5) had higher rates of TVF, TVMI and cardiac death at one year post PCI (Figure 3). Also, rSS was found by multivariate analysis in this study to be an independent predictor of TVF, all-cause death, cardiac death and TVMI, but not TVR, at one year (Table 4). Not surprisingly, a strong correlation was observed between baseline severity of coronary artery disease and revascularisation completeness. Compared with patients with complete revascularisation (rSS=0), those with incomplete revascularisation in the upper rSS tertile were more likely to have had previous CABG, stable angina, lower TIMI flow, and more complex coronary disease with higher proportions of longer lesions, total occlusions, calcified lesions, type C lesions and left main disease (Table 1, Table 2). These findings suggest that patients with the latter characteristics are least likely to benefit from PCI.
In the past, there have been no standard thresholds to stratify rSS. Généreux et al stratified rSS into 0, 0-2, 2-8 and >84, and Farooq et al stratified rSS into 0, 0-4, 4-8 and >86. In the present study, bootstrap methodology was used to determine an optimal rSS cut-off value; rSS >5 among patients with incomplete revascularisation was the best predictor of one-year TVF (HR for threshold of 5 and 8 was 1.53±0.23 and 1.01±0.18, respectively, p<0.01). The fact that the best threshold of 5 in this study is different from that in other studies might be due to the lower post-PCI mean rSS of 2.18 in this study, in contrast to that between 4 and 6 in others. Moreover, the rate of complete revascularisation (rSS=0) was higher in our study (64%) than in other real-world studies (40 to 43%)4-6. Therefore, it is possible that the lower cut-off point determined in this study is a result of the greater degree of complete revascularisation in this patient cohort (Table 2). This study suggests that a cut-off of 5 appears reasonable to stratify rSS.
Study limitations
Several limitations of the present study should be discussed. First, there was no “conventional” control group for head-to-head comparisons of stent efficacy or safety. However, it is worth noting that this study was aimed to investigate the safety and efficacy of EES among patients with complex anatomic characteristics in China, a largely unstudied patient population. Second, as in other studies4-6, cut-off values of bSS and rSS were generated within the present dataset, and as such remain exploratory. Future prospective studies are needed to assess the predictive value of rSS at pre-set cut-off values.
Conclusions
EES is safe and effective in the treatment of Chinese patients with complex coronary small vessels, long lesions, and multivessel disease. The rSS has a good discriminatory power for risk prediction of one-year TVF in this population, with an rSS >5 being associated with an increased risk of one-year TVF.
| Impact on daily practice Residual SS (rSS) has been validated as an independent predictor for adverse events. However, studies examining the impact of rSS were primarily performed using first-generation drug-eluting stents (DES) while the use of second-generation DES has been more common in recent years. Moreover, patients with lesions with complex anatomic characteristics (small vessels, long lesions, and multivessel disease), who experience worse clinical outcomes after PCI, are very common in “real-world” practice, probably more in China than in the West. Our study revealed that everolimus-eluting stents are safe and effective in the treatment of Chinese patients with those complex anatomic characteristics. Furthermore, rSS >5, which is associated with an increased risk of one-year target vessel failure, could be considered as a good risk predictor in this population. |
Acknowledgements
The authors appreciate the dedicated efforts of the clinical research collaborators in the SEEDS study organisation and the contributions of the participating centres listed in the Online Appendix. We would also like to acknowledge Dr Xiaofeng Guo for data analysis, and Dr Ming Yu and Dr Roberto Patarca for linguistic advice in preparation of this manuscript.
Funding
This was an investigator-sponsored study supported by a research grant from Abbott Vascular.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix. SEEDS study organisation
Co-Principal Investigators: Yue-Jin Yang, Ya-Ling Han, Shu-Zheng Lu
Director of Executive Committee: Bo Xu
Clinical Events Committee: Wei-Min Wang (Chair), Zhu-Jun Shen, Hong-Wei Li
Angiographic Core Lab: CCRF, Beijing, China
Data Coordination, Management and Statistics: Bo Liu, Xiaofeng Guo, CCRF, Beijing, China
COLLABORATORS
Fu Wai Hospital, National Center for Cardiovascular Diseases (Yue-Jin Yang, Chao-Wei Mu, Jue Chen, Ke-Fei Dou, Li-Jian Gao, Chong-Jian Li, Hai-Bo Liu, Jie Qian, Shu-Bin Qiao, Xue-Wen Qin, Hong Qiu, Yong-Jian Wu, Liang Xu, Hong-Bing Yan, Shi-Jie You, Zhong-Wei Sun, Fang Wang, Yan Liu)
Shanxi Provincial Cardiovascular Institute (Bao Li, Jing-Ping Wang, Bin Yang, Jin Dong)
Shenzhen Sun Yat-sen Cardiovascular Hospital (Qiang Liu, Jian-Xin Weng, Yi Wei, Xiu-Hua Cai, Li-Li Wang)
Shenyang Northern Hospital (Ya-Ling Han, Ying-Yan Ma, Quan-Min Jing, Hai-Wei Liu, Geng Wang, Xiao-Zeng Wang, Kai Xu, Bin Wang, Jian Zhang, Fei Li)
Wuhan Asia Heart Hospital (Guo-Ying Zhu, Xi Su, Hua-Yun Liu, Lei Li, Ying Wang, Jin Zhang, Dan Song, Jian Peng, Hua Yan, Guo-Hong Chen)
Beijing Military General Hospital (Jun-Xia Li, Jun-Yu Cui, Yu-Jie Shi, Jian Zhang)
1st Affiliated Hospital of Guangxi Medical University (Lang Li, Xiang-Hong Wu, Ge Xu, Qiao-Lan Jiang, Guo-Qiong Ma)
1st Affiliated Hospital of Xi’an Jiaotong University School of Medicine (Zu-Yi Yuan, Ning Guo, Jian-Hua Huo, Ying Xiong, Yin-Hui Liu, Ke Han)
Tangshan Workers Hospital (Zheng Ji, Li Li, Ya-Li Di, Jing-Yi Zhang, Qing-Xia Zhao, Xue-Bin Geng, Jing-Fang Gao)
Henan Hongda Cardiovascular Hospital (Huai-Lin Liu, Yi-Bo Yang, Xin-Tao Chen, Hong-Jun Yan, Jie Zhang, Hong-Xing Song, Xiao-Guang Mu, Ying Zhang)
Bethune Peace Hospital (Dong-Mei Wang, Jun-Xia Li, Li Zhang,Yu-Ying Zhao, Yu-Hong Peng, Cui Wang, Jia-An Sun, Dong-Mei Wang)
1st Yunnan Provincial People’s Hospital (Hong Zhang, Yan Zhao, Yun-Mei Zhang, Li-Wen Liang, Qiong-Jie Yang)
Tianjin Chest Hospital (Yin Liu, Xiu-Jun Zhou, Li Yang, Wei Ma, Lu Cao, Jin-Xia Zhang)
Hubei Union Hospital (Qiu-Tang Zeng, Xiao-Bo Mao, Hui Zhu, Gui-Wen Yi)
1st Affiliated Hospital of Zhengzhou University (Hai-Bo Yang, Jun Zhang)
Affiliated Hospital of Ningxia Medical College (Shao-Bin Jia, Lin He, Hong-Rui Shi, Yu-Min Chou, Da-Peng Chen, Ning Wei, Hui Huang, Qing-Bin Xu, Hua Chen)
Chengdu Military General Hospital (Yong-Jian Yang, Da-Chun Yang, Shuang-Tao Ma, Wei-Hong Wang, Jin-Song Chen, Bing Tang, Gang Li, Jun Zhu, De Li, Xin Zhang, Xiao-Hua Su)
Affiliated An Zhen Hospital of Capital Medical University (Shu-Zheng Lu, Feng Xu, Xin Chen, Fei Yuan, Bao-Yan Wan)
Daqing Oilfield General Hospital (Shang-Yu Wen, Sheng-Quan Liu, Yan-Hong Li, Li-Jun Ma)
Shanghai 1st People’s Hospital (Shao-Wen Liu, Guo-Bing Zhang, Wei-Guo Zhou, Wen-Yi Yang, Hao Xu, Wei-Zhen Li, Heng Cao, Jie Chu, Yang Wang)
Peking University Shenzhen Hospital (Chun Wu, Hui Xu, Sai-Yong Chen)
2nd Affiliated Hospital of Zhejiang University School of Medicine (Yong Sun, Jian’an Wang, Jian-Jing Lin, Cheng Li)
Yulin 1st People’s Hospital (Ping Li, Jian Chen, Zheng-Dong Wang, Shao-Fu Zhang, Jian-Ting Gan, Zhong-Wu Xu, Zhi-Hai Lin, Rong Chen)
Hubei Provincial People’s Hospital (Hong Jiang, Jing Bai, Bo Yang, Xue-Jun Jiang, Hao Xia, Xiao-Hong Wang)
1st Affiliated Hospital of Kunming Medical College, Yunnan (Jia-Hua Pan, Jing-Fa Tian, Yun Wen, Jia Liu)
Liaoning Provincial People’s Hospital (Zhan-Quan Li, Li Liu, Yun-Qi Shi, Cheng-Yang Li, Na Zhang)
Weifang People’s Hospital (Yan-Zhen Zhang, Hai-Bin Song, Yong-Guang Li)
1st Affiliated Hospital of Lanzhou University (Zheng Zhang, Dong Wang, Ming Bai, Bo Zhang, Ming Pan, Su-Yu You)
Tianjin Medical University General Hospital (Zheng Wan, Bo Bian, Meng-Meng Li, Wen-Nan Liu, Yi Wang)
2nd Affiliated Hospital of Jilin University (Bin Liu, Zhi-Hui Wang, Xiao-Hao Zhang)
Shanghai Tonjin Hospital (Jin-Fa Jiang, Wen-Jun Xu, Hao-Ming Song, Ru-Hui Liu, Jia-Hong Xu, Yang Liu, Chao-Hui Hu, Xiao-Yi Qu)
Shanghai 9th People’s Hospital (Chang-Qian Wang, Yu-Qi Fan, Kan Chen, Jing-Chao Hu)
Foshan 1st People’s Hospital (Zhao-Yan Xu, Jian-Min Li, Xi-Li Yang, Yu-An Li, Zhao-Lun Zhou)
General Hospital of Armed Police Forces (Hui-Liang Liu, Wei Han, Lei Wang, Ji-Hua Sheng, Yu-Jie Wei, Ying Liu, Jiao Zhang)
1st Affiliated Hospital of Harbin Medical University (Wei-Min Li, Ling Jing, Guo Dong, Yong-Tai Gong, Shu-Sen Yang, Li-Yun Song, Yue Li, Li-Jun Zhou, Dang-Hui Sun, Lin Yuan)
Affiliated Shanghai East Hospital of Tongji University School of Medicine (Xue-Bo Liu, Dai-Fu Zhang, Yu-Lu Liang, Wei-Gang Qi, Yan Lai, Yi-An Yao, Ye Gu)
Zhengzhou 7th People’s Hospital (Yi-Qiang Yuan, Jian-Li Wang, Yu-Jie Zhao, Li Yu, Xiao-Ting Yin, Hong-Jie Ning, Si-Quan Niu)
Jiangmen Central Hospital (Jun-Xing Lai, Qiang Ren, An-Jian Song)
Fujian Union Hospital (Liang-Long Chen, Xing-Chun Zheng, Yu-Chen)
Guangzhou Military General Hospital (Jian Qiu, Chang-Jiang Hong, Guang-Cheng Xiang, Zhi-Hua Gong, Yun-Jun Ruan, Shao-Ying Pan, Xiao-Long Gu, Rui Li, Hua Xiao, Jia-Yong Liang, Dan-Dan Peng)
Affiliated Chaoyang Hospital of Capital Medical University (Le-Feng Wang, Li Xu, Hao Sun, Sheng-Li Du)
Guangxi Provincial People’s Hospital (Ying-Zhong Lin, Shao-Ming Qin, Ling Liu, Guang-Ma Xu, Guang-Wei Wu, Hong Lin, Xu-Ping Li, Chun-Hua Mo)
Linyi People’s Hospital (Zi-Shan Hou, Yan-Jin Wei, Qiu-Lin Zhang, Kai Liu)
Cangzhou Central Hospital (Ze-Sheng Xu, Shi-Peng Dai)
Beijing Huaxin Hospital (Li-Fu Miao, Yan-Ping Yin)
Jinghu Hospital, Macao (Xi-Wei Deng, Guan-Xu Tan, Wen-Jian Liang, Hong Liu)
National Cheng Kung University Hospital, Taiwan (Zheng-Han Lee, Yi-Heng Li, Shih-Huang Chan, Ping-Yen Liu, Chih-Chan Lin, Ju-Yi Chen, Wen-Hui Tseng)
Cathay General Hospital, Taiwan (Rong-Tian Wang, Chi-Hung Huang, Po-Ching Chou)