
Intracoronary functional and anatomical tools to stratify residual risk
There is increasing interest in the functional and anatomical characterisation of non-culprit coronary lesions in patients treated with percutaneous coronary intervention (PCI) for an acute ischaemic event. Functional data (fractional flow reserve [FFR]) are key to investigating plaque propensity to cause ischaemic events and to identifying lesions that will benefit from PCI1-4. Cardiologists have taken on board these concepts and there is now widespread consensus on the effectiveness of FFR for studying intermediate lesions5. The study of plaque morphology to predict outcome is certainly less supported by the literature and, despite the milestone PROSPECT study6, intravascular imaging modalities are confined to research rather than being used as clinical tools. The anatomical characterisation of “non-culprit” coronary lesions and the evaluation of the impact of classic risk factors in patients treated for an acute coronary event are moving in the direction of personalised medicine. The potential risk of the development of future events can be approached in a new multi-parametric dimension of secondary prevention in which intravascular imaging is slowly gaining an increasing role and clinical significance.
The study by Fujino et al in this issue of EuroIntervention7 investigated the clinical role of plaque anatomy at non-culprit sites by merging angiographic data and IVUS findings.
The authors explored for the first time the association between residual SYNTAX score (rSS) and non-culprit-related major adverse cardiac events (MACE) and found a significant difference in the three-year non-culprit-related MACE rates based on the rSS. Using data from the PROSPECT study, they also found a correlation between rSS and intravascular ultrasound (IVUS) features of vulnerability (≥1 lesion with plaque burden ≥70% or ≥1 lesion with a minimum lumen area ≤4 mm2 and total dense calcium volume). Furthermore, in the multivariable analysis, insulin-treated diabetes mellitus, rSS, and the presence of ≥1 lesion with plaque burden ≥70% independently predicted non-culprit-related MACE.
The correlation between rSS and IVUS findings, including minimum lumen area ≤4 mm2 or plaque burden ≥70%, indicates that the IVUS criteria of vulnerability can be predicted, to some extent, using a simple coronary mapping approach.
INTRACORONARY IMAGING FOR STUDYING PLAQUE VULNERABILITY: IS IT WORTH IT?
Building clinical and anatomical models of plaque vulnerability is a complex approach that aims at stratifying the residual risk of MACE after intervention. More aggressive procedures may be employed in an effort to pursue complete revascularisations, sometimes with the adoption of complex interventions, either implanting multiple stents or using modalities to remove calcium and permit adequate stent expansion. The conclusions of Fujino et al7 suggest a new approach for coronary interventions, requiring a meticulous assessment of the whole coronary tree, in an attempt to reduce future coronary events that may be caused by non-culprit coronary lesions. Since transcatheter procedures for treating non-culprit sites have the drawback of increased complexity and costs, we have to comprehend the clinical benefit this approach may lead to. The authors defined MACE as the composite of cardiac death, cardiac arrest, myocardial infarction, or rehospitalisation for unstable or progressive angina. The significant difference in MACE was predominantly provided by rehospitalisation for unstable or progressive angina, which was about five times higher in the patient group with rSS >8 as compared to the group with rSS <8. The fact that there were no significant differences regarding the occurrence of cardiac death or myocardial infarction does not minimise the importance of the data but rather encourages the search for new intermediate secondary prevention indicators. Perhaps the rehospitalisation for unstable or progressive angina should be reclassified as an intermediate acute cardiac event (IACE) in a prospective view of less negative prognostic significance. Furthermore, the adoption of a more complex approach based on the model combining IVUS findings (patient with ≥1 lesion with virtual histology thin-cap fibroatheroma [VH-TCFA] or plaque burden ≥70%) with risk factors and rSS improved MACE prediction even if it did not seem to influence hard events, including cardiac death and myocardial infarction. This observation triggers new questions. How does one distinguish patients who will develop hard events from those who will develop IACE? What is the goal we want to pursue during an invasive angiogram enhanced with IVUS? Does the expectation of reducing hard clinical endpoints mean setting the bar too high? Does VH-IVUS have the potential for doing that? The PROSPECT study6 showed that three features of vulnerability at radiofrequency intravascular ultrasonography (plaque burden ≥70%, minimal luminal area ≤4.0 mm2 or TCFAs) were significantly related to MACE occurrence at a three-year follow-up (FU). Furthermore, 18.2% of non-culprit lesions associated with recurrent events had all three variables. Radiofrequency IVUS mainly identified lesions at risk of plaque progression instead of lesions causing cardiac death and/or MI (that were rare events during 3.4 years of FU). Consistently, in the paper by Fujino et al, IVUS criteria identified more aggressive atherosclerosis, but recurrent events seemed to be a logical consequence of plaque progression of more “mature” lesions, having a relatively small lumen area or a larger plaque burden.
TARGETING THE CLINICAL ENDPOINTS
Decreasing the risk of hard events is certainly a more difficult task. The use of an atherosclerotic burden score obtained with computed tomography (CT-adapted Leaman score), taking into account lesion localisation, plaque composition, and degree of stenosis, was found to be an independent long-term predictor of cardiac death and myocardial infarction in patients with suspected coronary artery disease8. Intravascular imaging modalities have the potential to explore further the relationship between plaque anatomy and hard cardiac events, and can probably accomplish this task better than FFR.
The ineffectiveness of IVUS to identify patients at risk of main hard cardiac events may be due to the fact that anatomy does not add to clinical and laboratory data for outcome prediction. Alternatively, radiofrequency IVUS might not be the unique ideal solution to study plaque components related to vulnerability due to its suboptimal resolution: in particular, it may misdiagnose thin fibrous cap, a well-known feature related to plaque rupture.
The ATHEROREMO study9, in a small population of 203 patients, addressed the long-term prognostic value of intracoronary NIRS, an imaging modality depicting plaque spectral characteristics indicative of lipid core. Patients with a lipid core burden index greater than or equal to the median value of 43.0 had a fourfold risk of adverse cardiovascular events during one-year FU. In 1,003 patients (1,776 lipid plaques located in untreated proximal left anterior descending coronary artery segments), the CLIMA registry addressed the predictive value of multiple vulnerable plaque features in the same plaque (minimum lumen area fibrous cap thickness, lipid arc circumferential extension, and presence of macrophages) (Prati F, Romagnoli E, Gatto L, et al; on behalf of CLIMA Investigators. Relationship between coronary plaque morphology of the left anterior descending artery and long term clinical outcome: the CLIMA study. Abstract submission to EuroPCR, Paris, France, May 2018). The coronary plaques were studied with optical coherence tomography, the intracoronary imaging modality with the highest resolution, in the range of 10-20 microns10. The simultaneous detection of four features of vulnerability (fibrous cap thickness <75 µm, presence of minimum lumen area <3.5 mm2, lipid arc circumferential extension >180° and macrophages) was found to be an independent predictor of hard events, including cardiac death and target segment myocardial infarction (HR 7.54).
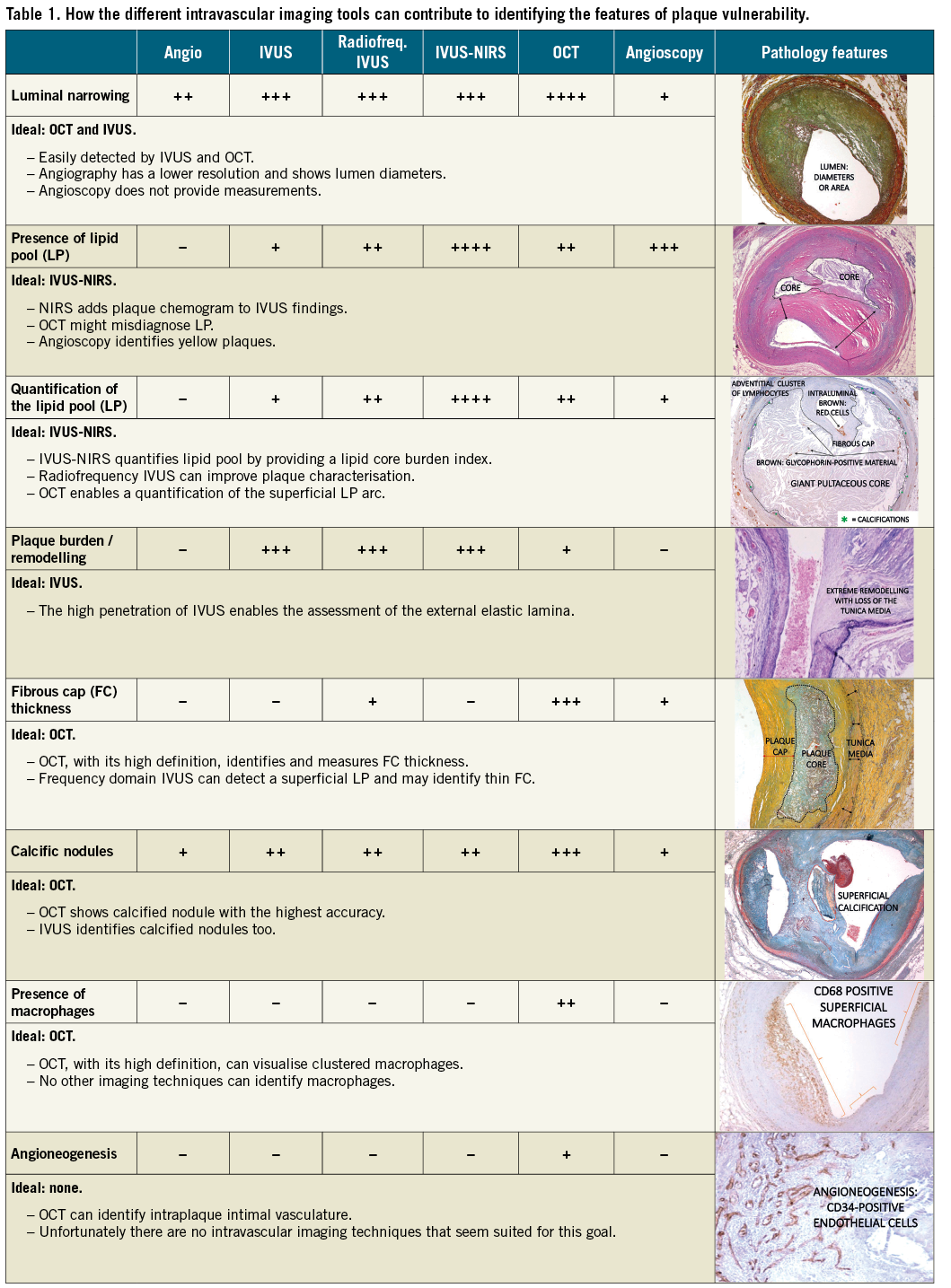
The implementation of intravascular imaging during invasive procedures offers a good opportunity to improve patient outcome. The assessment of coronary anatomy by means of imaging modalities is certainly worth doing, especially if plaque anatomy detection serves to reduce hard clinical endpoints. To accomplish this goal, the selection of the most appropriate technique is crucial (Table 1). In the more than welcome era of personalised medicine, operators should tailor interventional procedures with a more extensive use of intracoronary tools to see the coronaries from the inside and provide complex solutions to treat them (balloon, scaffolds, drills or shock waves), keeping in mind the clinical endpoints they are aiming at.
Funding
Research on cardiovascular imaging and pathology is supported by the CLI Foundation (F. Prati, L. Gatto) and the IRCCS Cardiology Network, supported by the Italian Ministry of Health (E. Arbustini).
Conflict of interest statement
F. Prati served as consultant for Amgen and Abbott-St. Jude. The other authors have no conflicts of interest to declare relevant to the contents of this paper.

