
Abstract
Aims: Residual SYNTAX score (rSS) is known to be associated with cardiac events. We sought to investigate the association between rSS and greyscale and virtual histology (VH)-intravascular ultrasound (IVUS) plaque morphology, and the association between rSS and non-culprit-related major adverse cardiac events (MACE) using data from the PROSPECT study.
Methods and results: A total of 697 patients with acute coronary syndromes were enrolled in the PROSPECT study. Three-vessel greyscale and VH-IVUS were performed. Among them, 688 patients with paired baseline SS or SYNTAX score and rSS were identified and divided into three groups – rSS=0 (n=184), 0
Conclusions: Plaque morphology based on greyscale IVUS and VH-IVUS was significantly correlated with rSS, and rSS and plaque burden ≥70% independently predicted non-culprit-related MACE.
Abbreviations
ACS: acute coronary syndrome
IDI: integrated discrimination improvement
IVUS: intravascular ultrasound
MACE: major adverse cardiac events
NRI: net reclassification improvement
PCI: percutaneous coronary intervention
ROC: receiver-operating characteristic
rSS: residual SYNTAX score
SS: SYNTAX score
TCFA: thin-cap fibroatheroma
VH: virtual histology
Introduction
The Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) score (SS) is a well-established tool to quantify the extent and complexity of coronary artery disease pre revascularisation1,2. The residual SYNTAX score (rSS) is the SS remaining after completion of percutaneous coronary intervention (PCI). Généreux et al3 demonstrated that the rSS risk-stratified acute coronary syndrome (ACS) patients undergoing an early invasive strategy with PCI and that the rSS predicted cardiac events with similar accuracy to the baseline SS. Moreover, Farooq et al4 reported that rSS was an indicator of long-term mortality in patients with unprotected left main or de novo three-vessel coronary artery disease. Although previous studies have shown that rSS was an independent predictor of overall cardiac events3,4, the association between rSS and non-culprit lesion-related major adverse cardiac events (MACE) has not been investigated. In previous intravascular ultrasound (IVUS) studies5-7, greyscale and virtual histology (VH) IVUS findings (e.g., plaque burden, minimum lumen area, and VH thin-cap fibroatheroma [TCFA]) were significantly associated with non-culprit lesion-related MACE; however, the association between rSS and IVUS findings has not been investigated. We investigated: 1) the association between rSS and greyscale and VH-IVUS findings; 2) the association between rSS and non-culprit lesion-related MACE; and 3) additive prediction of IVUS findings to the rSS for non-culprit lesion-related MACE.
Methods
STUDY PROTOCOL
The Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study has been described in detail previously5. In brief, 697 ACS patients were enrolled after successful PCI of all lesions believed to be responsible for the clinical presentation and after completion of any other planned interventions. Three-vessel quantitative coronary angiography, greyscale IVUS, and VH-IVUS imaging were then performed. Patients were followed for a median of 3.4 years. The primary endpoint was MACE at three years, consisting of cardiac death, cardiac arrest, myocardial infarction, or rehospitalisation for unstable or progressive angina as adjudicated independently by a committee blinded to imaging results. Culprit lesions/vessels were defined as those believed to be responsible for the index event by the operator and treated during the index or planned procedure before enrolment into PROSPECT. Non-culprit lesions included all coronary segments outside of culprit lesions and defined as ≥30% visual diameter stenosis by angiography and in a segment in which ≥3 consecutive slices had ≥40% plaque burden by greyscale and VH-IVUS. Each adverse event was adjudicated to either an originally treated culprit lesion or to an untreated non-culprit lesion or was “indeterminate” in the absence of follow-up angiography. The study was approved by the institutional review board at each participating centre, and all patients signed written informed consent.
For the present study, the baseline SS was assessed visually by technicians of the Angiography Core Laboratory at the Cardiovascular Research Foundation (CRF, New York, NY, USA) who were trained for SS assessment and were blinded to clinical outcomes, as previously described8. Each lesion with visual diameter stenosis ≥50% in vessels ≥1.5 mm in diameter was scored using the SS algorithm9. The rSS was determined as the SS remaining after completion of planned PCI. Complete revascularisation was defined as rSS=0. Based on previous reports3,4, patients with incomplete revascularisation were divided into two groups: (i) 0
QUANTITATIVE CORONARY ANGIOGRAPHY
Quantitative coronary angiography measurements were performed over the entire length of the coronary tree (including side branches) in any vessel ≥1.5 mm in diameter using proprietary methods modified from QCA-CMS version 7.0 (Medis medical imaging systems bv, Leiden, the Netherlands). Reference vessel diameter, minimum lumen diameter, and diameter stenosis were calculated for each vessel. Analysis of all angiographic lesions with ≥30% visual diameter stenosis was pre-specified in the PROSPECT protocol5.
GREYSCALE IVUS AND VH-IVUS ANALYSIS
Greyscale IVUS and VH-IVUS of the left main coronary artery and proximal 6 to 8 cm of each major epicardial coronary artery were performed using motorised catheter pullback at 0.5 mm/s. Offline greyscale and VH-IVUS analysis was performed using: (i) QCU-CMS (Medis medical imaging systems bv) for contouring; (ii) pcVH version 2.1 (Volcano Corporation, Rancho Cordova, CA, USA) for contouring and VH data output; and (iii) qVH software (developed within CRF) for segmental qualitative assessment and data output. Methodologies of quantitative and qualitative IVUS measurements have been described previously10. Fibroatheroma was defined as >10% confluent necrotic core. If >30° of the necrotic core abutted the lumen in ≥3 consecutive frames, the fibroatheroma was classified as VH-TCFA; otherwise, it was classified as thick-cap fibroatheroma.
STATISTICAL ANALYSIS
All data were analysed at the patient level. Continuous variables are presented as median and first and third quartiles and compared using the Kruskal-Wallis test. Categorical variables are presented as percentage and count and compared using the χ2 test or Fisher’s exact test, as appropriate. Time-to-event data are presented as Kaplan-Meier estimates and compared using the log-rank test. Multivariable linear regression analysis was used to determine independent correlates of rSS. Multivariable Cox proportional hazards analysis was used to determine predictors for non-culprit-related MACE with one variable entered for 10 or more events to avoid overfitting. For each multivariable model, variables were chosen based on their historical and pathophysiologic relationship to each endpoint. The discriminatory capability of rSS to identify patients with non-culprit-related MACE was assessed using the area under the receiver operating characteristic (ROC) curve. The incremental predictive value was assessed using net reclassification improvement (NRI) and integrated discrimination improvement (IDI)11. On ROC analysis, NRI, and IDI, the following models were used: model 1 – insulin-treated diabetes mellitus and prior PCI; model 2 – insulin-treated diabetes mellitus, prior PCI, and rSS; model 3 – insulin-treated diabetes mellitus, prior PCI, rSS, and patient with ≥1 VH-TCFA; and model 4 – insulin-treated diabetes mellitus, prior PCI, rSS, and patient with ≥1 lesion with plaque burden ≥70%. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Two-sided p-values <0.05 were considered significant.
Results
BASELINE CHARACTERISTICS
Among 697 patients enrolled in PROSPECT, 688 with paired baseline SS and rSS were identified. Almost all patients (n=629, 91.4%) had a baseline low SS <23, and complete revascularisation (rSS=0) was achieved in 184 (26.7%). Patients with incomplete revascularisation were divided into two groups based on previous reports: 0
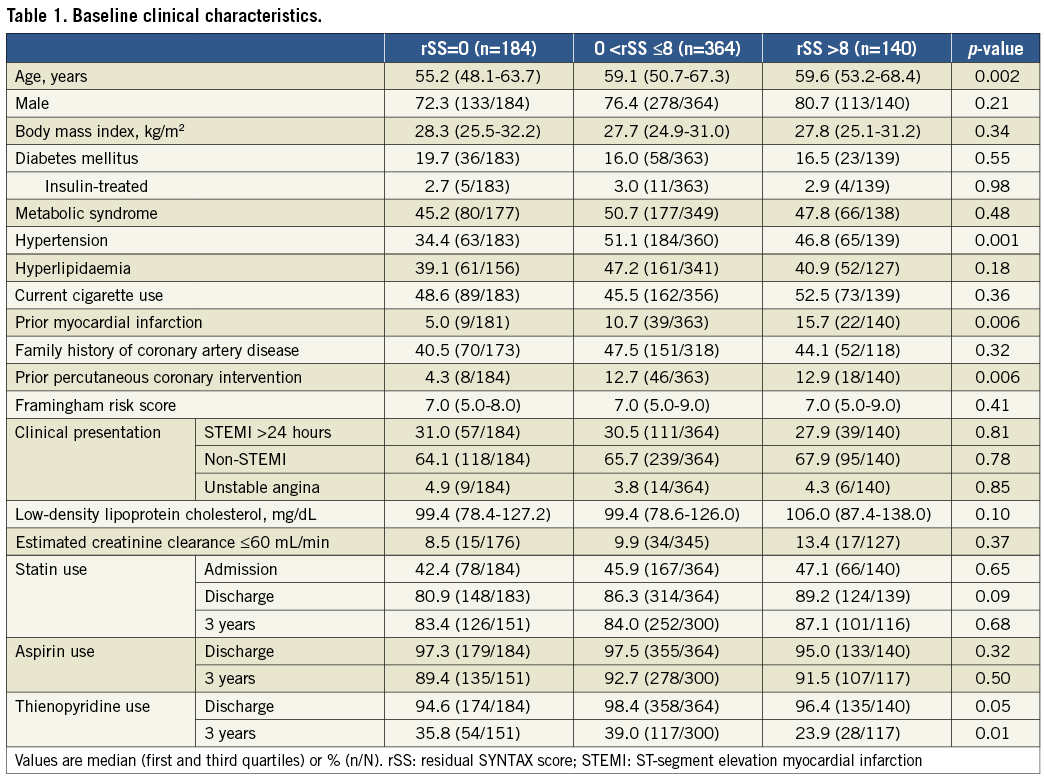
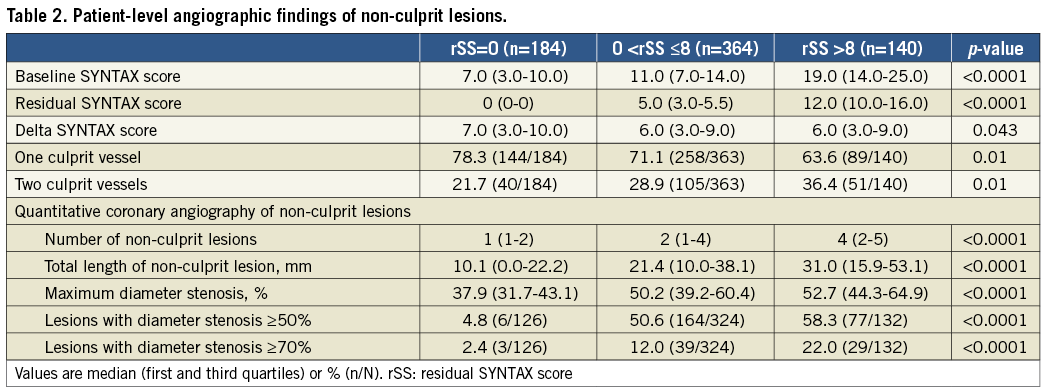
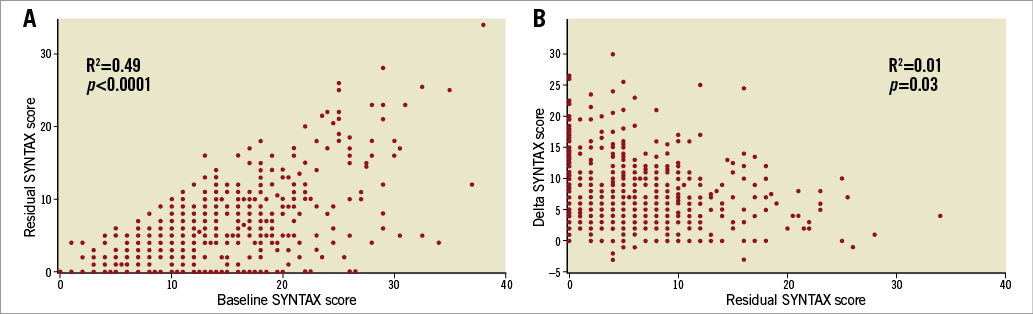
Figure 1. Correlation between baseline SYNTAX score and residual SYNTAX score, and residual SYNTAX score and delta SYNTAX score. A) Baseline SYNTAX score and residual SYNTAX score. B) Residual SYNTAX score and delta SYNTAX score. The range of residual SYNTAX score varied considerably in patients with high baseline SYNTAX score. There was no correlation between the residual SYNTAX score and the delta SYNTAX score (R2=0.01, p=0.03). Each point may represent more than one patient.
GREYSCALE AND VH-IVUS ANALYSIS
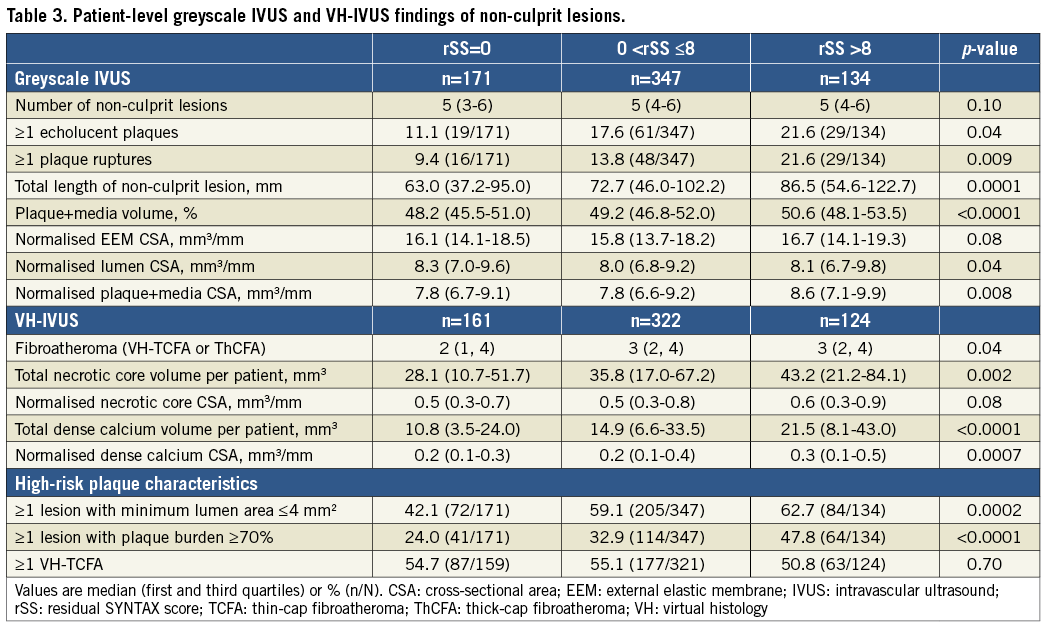
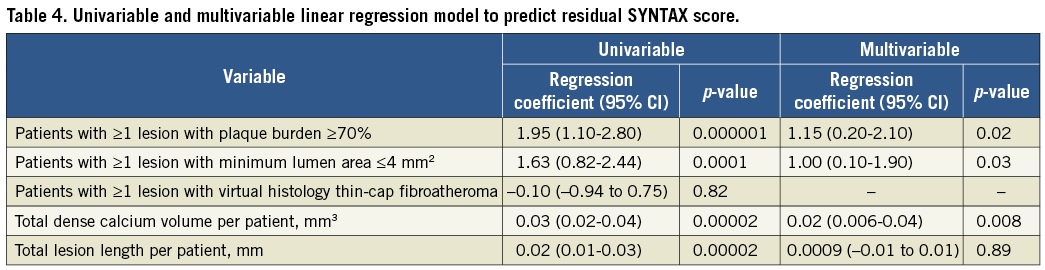
Patients with higher rSS had longer IVUS non-culprit lesions and greater percent plaque+media (plaque+media divided by external elastic membrane) volumes (Table 3). Patients with rSS >8 had a higher prevalence of ≥1 echolucent plaque and ≥1 plaque rupture and more fibroatheromas versus rSS=0; they also had greater normalised necrotic core and dense calcium area than the other groups. As the rSS increased, a greater proportion of the patients had high-risk plaque characteristics such as ≥1 lesion with minimum lumen area ≤4 mm2 (42.1% versus 59.1% versus 62.7%; p=0.0002) or ≥1 lesion with plaque burden ≥70% (24.0% versus 32.9% versus 47.8%; p<0.0001). On multivariable analysis, ≥1 lesion with plaque burden ≥70%, ≥1 lesion with minimum lumen area ≤4 mm2, and total dense calcium volume per patient were significantly associated with rSS (Table 4).
THREE-YEAR CLINICAL OUTCOMES
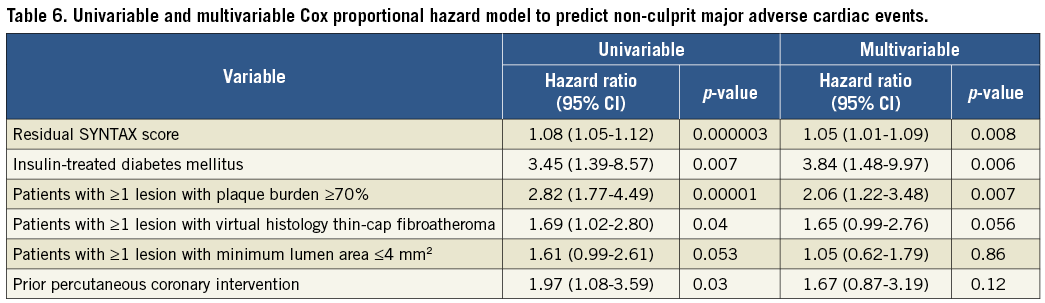
As shown in Table 5 and Figure 2A, there was a significant difference in three-year cumulative non-culprit-related MACE rates (5.7% versus 11.9% versus 19.7%, lowest to highest rSS, p=0.004). The difference was mainly due to rehospitalisation for unstable or progressive angina. There were no significant differences in culprit-related MACE (Figure 2B) or MACE combining non-culprit, culprit, and indeterminate events (Figure 2C) among the three groups. On multivariable analysis, rSS, ≥1 lesion with plaque burden ≥70%, and insulin-treated diabetes mellitus were independent predictors of non-culprit-related MACE (Table 6).
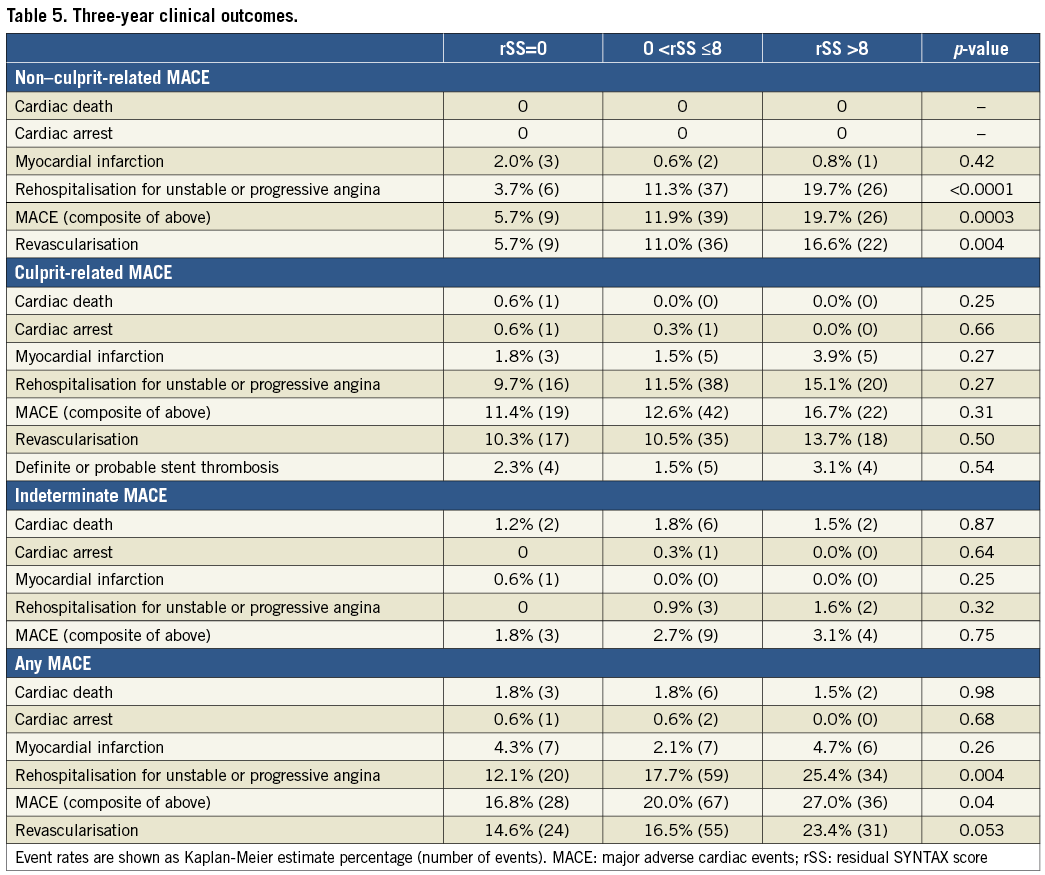
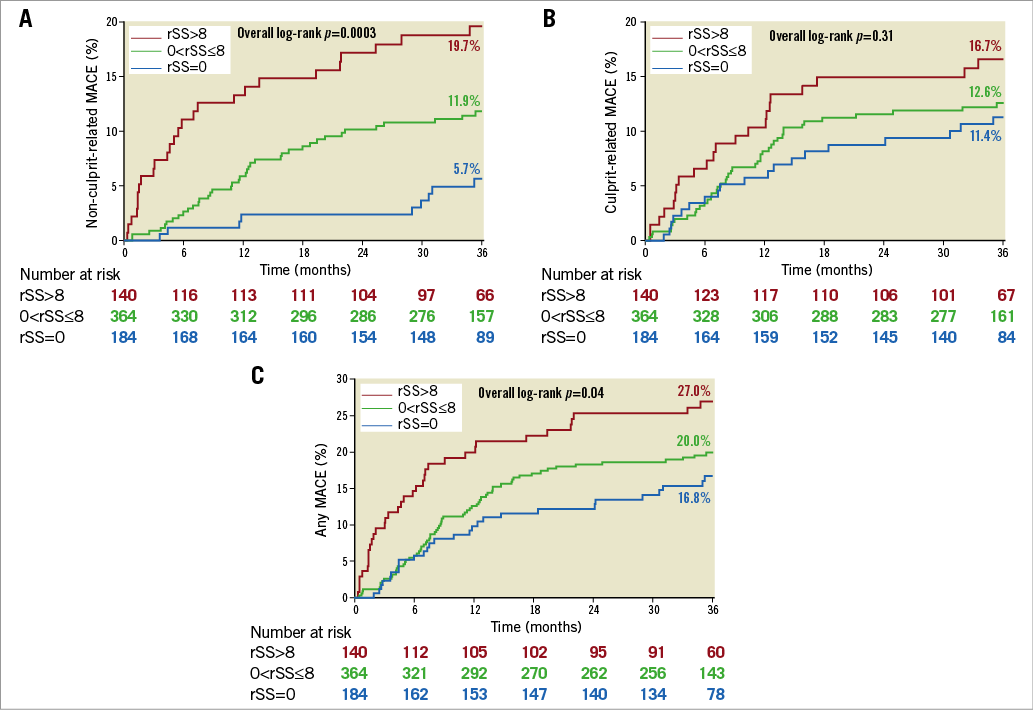
Figure 2. Kaplan-Meier curves showing major adverse cardiac events (MACE) up to three years. A) Non-culprit-related MACE. B) Culprit-related MACE. C) Any MACE. rSS: residual SYNTAX score
ROC ANALYSIS, NRI, AND IDI
When rSS was added to the model by clinical factors, prediction of non-culprit MACE was significantly improved using IDI, but not using NRI or ROC analyses (Table 7, Figure 3). When IVUS (patient with ≥1 lesion with VH-TCFA or plaque burden ≥70%) was added to the model based on clinical factors and rSS, non-culprit MACE prediction was significantly improved in all models.
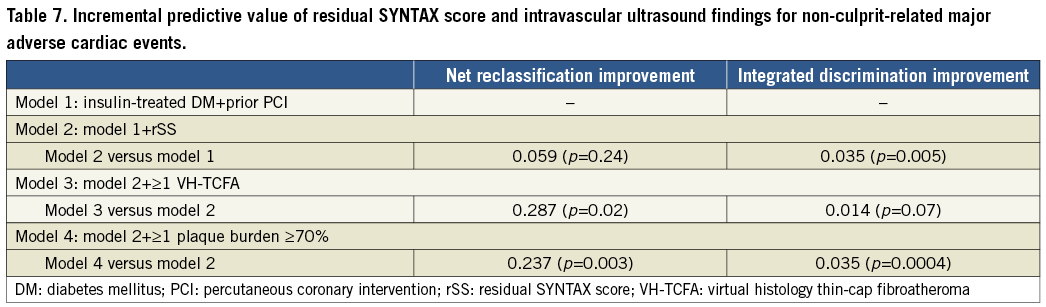
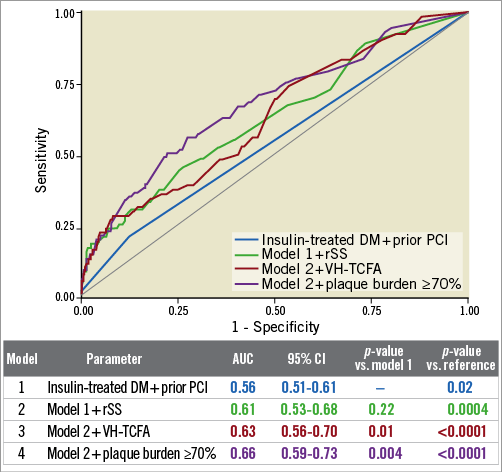
Figure 3. Receiver operating characteristic (ROC) curves for non-culprit-related major adverse cardiac events (MACE). On ROC analysis, the p-value for each model versus reference was significant. The p-values for model 3 versus model 1 and model 4 versus model 1 were also significant (p<0.0001 for both). Conversely, model 2 versus model 1 was not significant. AUC: area under the curve; CI: confidence interval; DM: diabetes mellitus; PCI: percutaneous coronary intervention; rSS: residual SYNTAX score; VH-TCFA: virtual histology thin-cap fibroatheroma
Discussion
The present study demonstrates a significant association among rSS, plaque morphology (based on greyscale IVUS and VH-IVUS), and non-culprit-related MACE, as well as an improved risk prediction of non-culprit-related MACE using rSS combined with greyscale IVUS and VH-IVUS findings (Figure 4).
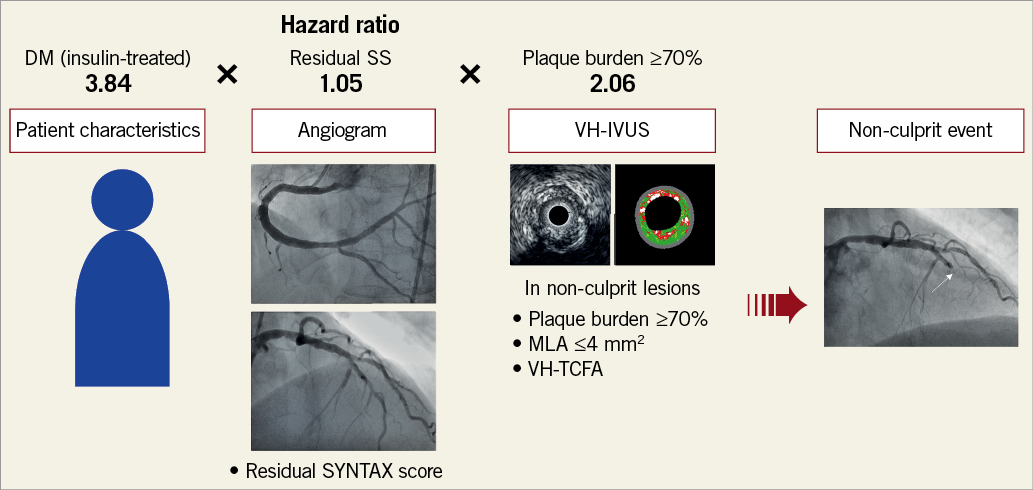
Figure 4. Prediction of future non-culprit lesion-related events. Patient characteristics (insulin-treated DM, hazard ratio [HR]=3.84), residual SYNTAX score (rSS, HR=1.05), and IVUS morphology (plaque burden ≥70%, HR=2.06) were independently associated with non-culprit lesion-related events (Table 6). Identification of each risk improved prediction of future events. DM: diabetes mellitus; MLA: minimum lumen area; VH-TCFA: virtual histology thin-cap fibroatheroma
Complete revascularisation is associated with more favourable outcomes versus incomplete revascularisation3,4,12,13. Using data from the PCI arm of the all-comers population of SYNTAX, Farooq et al12 reported that complete revascularisation was associated with significantly lower four-year mortality, revascularisation, stent thrombosis, and major adverse cardiac and cerebrovascular events. A meta-analysis also showed that complete revascularisation was associated with reduced cardiovascular events among PCI-treated patients, including long-term mortality, myocardial infarction, and repeat revascularisation13; however, those studies investigated the association between rSS and overall MACE including both culprit-related and non-culprit-related MACE. The present study is unique in focusing on non-culprit events to demonstrate that the rSS independently predicted non-culprit-related MACE and that complete revascularisation was associated with the lowest rate of non-culprit-related MACE. In addition to the rSS, insulin-treated diabetes mellitus and ≥1 lesion with plaque burden ≥70% were also independent predictors of non-culprit-related MACE. Insulin-treated diabetes mellitus was a predictor of non-culprit-related MACE in PROSPECT5, and having ≥1 lesion with a plaque burden ≥70% is a predictor of non-culprit-related MACE in ATHEROREMO-IVUS7 as well as in PROSPECT.
The present study also showed that greyscale IVUS and VH-IVUS plaque morphology was significantly correlated with rSS. Plaque burden ≥70% and minimum lumen area ≤4 mm2, the high-risk plaque characteristics in PROSPECT5, were independent correlates of rSS in the present study. Because rSS focuses on luminal stenosis and lesion complexity and does not assess plaque vulnerability, IVUS findings could add complementary information to the angiographic rSS. Combining rSS and IVUS may be a better predictor for future non-culprit-related events. Moreover, the present study also demonstrated the significant correlation between rSS and total dense calcium volume. This is reasonable because calcification is a component of the SS9.
Several studies have reported that rSS3,4,12,13 or IVUS findings such as plaque burden ≥70%, minimum lumen area ≤4 mm2, and VH-TCFA5-7 were independent predictors of cardiac events. Bourantas et al14 showed that demographic factors had poor discrimination in detecting patients with high-risk plaque characteristics related to non-culprit-related MACE and that discrimination was slightly improved when angiographic factors were considered. The present study showed that the presence of ≥1 lesion with plaque burden ≥70% or ≥1 VH-TCFA significantly improved risk prediction of non-culprit-related MACE beyond rSS and clinical factors such as insulin-treated DM and prior PCI. Thus, morphological assessment by IVUS may contribute to better prediction of high-risk patients compared with angiographic assessment alone.
Study limitations
First, as a post hoc analysis, the results of the present study should be considered exploratory and hypothesis-generating. Angiographic and IVUS characteristics of patients enrolled in this study had low-intermediate lesion complexity with relatively low baseline SS. Further studies with higher lesion complexity are warranted. Second, significant differences in non-culprit-related MACE rates were mainly due to rehospitalisation for unstable or progressive angina; the impact of rSS on survival should be confirmed in larger populations. Third, as the indication of PCI during the index procedure was left to the operators’ discretion in PROSPECT, there could be a selection bias for untreated non-culprit lesions. Interpretation of IVUS findings at the time of intervention was not performed by a core lab; thus, lesions with high-risk plaque assessed by IVUS should not have been treated during the index procedures.
Conclusions
This PROSPECT substudy demonstrated the significant associations between rSS, plaque morphology based on greyscale IVUS and VH-IVUS, and non-culprit-related MACE. IVUS findings, such as plaque burden ≥70%, minimum lumen area ≤4 mm2, and total dense calcium volume, were significantly correlated with rSS, and rSS and plaque burden ≥70% independently predicted non-culprit-related MACE.
| Impact on daily practice IVUS findings, such as plaque burden ≥70%, minimum lumen area ≤4 mm2, and total dense calcium volume, were significantly correlated with rSS, and higher rSS and plaque burden ≥70% are independent predictors of the risk of non-culprit-related MACE. |
Guest Editor
This paper was guest edited by A. Colombo, MD; Department of Interventional Cardiology, EMO GVM Centro Cuore Columbus, Milan, Italy.
Acknowledgements
The authors thank Dominic P. Francese, MPH, for assistance in preparing the manuscript.
Conflict of interest statement
A. Fujino has received grant support from Asahi Intecc. A. Maehara has received grant support from Boston Scientific, and Abbott Vascular for research fellows, is a consultant for Boston Scientific, and has received speaker fees from Abbott Vascular. P.W. Serruys is a consultant for Abbott Laboratories, AstraZeneca Pharmaceuticals, Biotronik, Cardialysis B.V., GLG Research, Medtronic, Sino Medical Sciences Technology Inc., Société Europa Digital & Publishing, Stentys France, Svelte Medical Systems Inc., Volcano Europe BVBA, and Q3 Medical Devices Limited. G.S. Mintz is a consultant for Boston Scientific and ACIST, and has received fellowship/grant support from Volcano, Boston Scientific, and Infraredx, and honoraria from Boston Scientific and ACIST. P. Généreux has received speaker’s fees from Abbott Vascular and Edwards Lifesciences, consulting fees from Cardiovascular Systems Inc, PiCardia, and Soundbite Medical Solutions, and institutional research grants from Boston Scientific and Tryton Medical. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

