Abstracts
Aims: New markers to help stratify coronary atherosclerosis are needed. Although attempts have been made to differentiate active lesions from those that are stable, none of these has ever been formalised into a discriminatory score. The aim of this study was to analyse the differences between culprit ACS lesions and culprit stable angina lesions with intravascular ultrasound-derived virtual histology and to construct and validate a plaque score.
Methods and results: Prior to percutaneous coronary intervention (PCI), we performed volumetric, intravascular ultrasound-derived virtual histology (IVUS-VH) analysis in acute coronary syndrome (ACS) culprit lesions (AC - n=70) and stable angina culprit lesions (SC - n=35). A direct statistical comparison of IVUS-VH data and multiple logistic regression analysis was undertaken. Four main factors were found to be associated (p<0.05) with an AC lesion phenotype: necrotic core/dense calcium (NC/DC) ratio; minimum lumen area <4 mm2 (MLA <4); remodelling index @MLA >1.05 and VH-TCFA presence. Calculation of each logistic regression coefficient and the equation produces an active plaque discrimination score with an AUC of 0.96 on receiver operating characteristics (ROC) analysis. Validation of the score in 50 independent plaques from the Thoraxcenter in Rotterdam revealed an AUC of 0.71, confirming continued diagnostic ability.
Conclusions: We have found four features on IVUS and VH that can predict and discriminate ACS culprit lesion phenotypes from those that are clinically stable. Subsequently, we have constructed and validated the Liverpool Active Plaque Score based upon these features. It is hoped this score may help diagnose active coronary plaques, in the future, to help prevent major adverse cardiac events.
Introduction
Post-mortem pathological studies have shown that a “vulnerable” plaque is the dominant pathophysiological mechanism responsible for acute coronary syndromes (ACS)1-3. One way to try to image these plaques in vivo is by using histological “surrogates” created by intravascular ultrasound-derived virtual histology (IVUS-VH)4. IVUS-VH has been validated in human pathological studies and atherectomy specimens5-7. The recent publication of the VIVA (Virtual Histology in Vulnerable Atherosclerosis) study8 has created a new interest in this potential role for IVUS-VH. This is because the study findings replicated the final results of the landmark PROSPECT (Providing Regional Observations to Study Predictors of Events in the Coronary Tree) study9. Both studies concluded that important non-culprit lesion-specific characteristics are: plaque burden >70%; VH thin-cap fibroatheroma (VH-TCFA – a virtual surrogate for unstable plaque) and a minimum lumen area (MLA) <4 mm2.
IVUS-VH is based upon the analysis of ultrasound backscatter as different plaque components produce a particular spectrum5-7. The power, amplitude and frequency of the signal undergo deconvolution through a trained classification tree. This process transforms signals into four colour-coded pixels: fibrous (green); fibro-fatty tissue (light green); necrotic core (red) and dense calcium (white). This has been found to correlate with histopathology and atherectomy specimens (predictive accuracy=87.1%, 87.1%, 88.3%, and 96.5%, for fibrous, fibro-fatty, necrotic core, and dense calcium, respectively)5-7.
Numerous other studies have analysed ACS and stable culprit plaques with IVUS-VH10-16; however, none has attempted to construct a discriminatory score based upon the specific anatomical and compositional changes seen in “active” culprit ACS plaques.
We therefore designed a prospective, observational study that would examine active culprit (AC) plaques from patients with an ACS presentation and stable culprit (SC) plaques from patients with clinically stable angina. The research aims were:
To define the anatomical and histological differences between ACS and stable disease.
To use these differences in a multiple logistic regression model which can assign a numerical value to each associated variable.
To test the ability of this model to discriminate active culprit plaque types in another independent cohort.
Methods
This was a single-centre, prospective, observational cohort study that received ethical approval from the Cheshire National Health Service (NHS) ethics committee in the UK. Patients with a troponin positive ACS diagnosed by a general cardiologist were transferred urgently to our tertiary centre for PCI. These cases were screened and the patients gave written informed consent to be involved in the study. The main exclusion criteria were: ST elevation MI; unsuitable anatomy for PCI; left main stem disease; chronic kidney disease; previous PCI in the target vessel and heavily calcified or visibly thrombotic vessels. Active culprit (AC) plaques were defined as those meeting electrocardiographic (ECG) criteria for the ACS culprit vessel and where intervention was performed. Stable culprit (SC) plaques were those requiring PCI for progressive symptoms and failure of medical therapy in an elective setting. All vessels were studied prior to any percutaneous revascularisation. Imaging of the vessel in each group was undertaken using a phased array catheter (Eagle Eye® catheter, 2.9 Fr/20 MHz; Volcano Corp., Rancho Cordova, CA, USA). Proximal and distal marker frames were chosen in keeping with a consensus document on how to analyse both IVUS and VH17. Following administration of heparin 70 U/kg and a minimum of 1 mg of isosorbide dinitrate (ISDN) intra-arterially, the IVUS transducer was advanced beyond the presumed culprit lesion into a clean reference segment. The next distal side branch was then used as a marker for localisation during IVUS image assessment. The proximal border for image assessment was established in a similar fashion using another side branch or the mouth of the guide catheter. A continuous motorised pullback at 0.5 mm/s was performed (Volcano R100 pullback device; Volcano Corp.) until the probe had passed the proximal marker. The plaque composition and other analyses were performed by off-line IVUS-VH analysis (S5i Tower and PC VIAS 3.0.394 software; Volcano Corp.) after manual adjustment of border contours by a single individual blinded to the patient details and mode of presentation. Single operator analysis of ACS plaques produces less measurement error as shown in our validation work, which has been published previously18.
STATISTICS AND POWER CALCULATION
To guide the initial sample size for this study, we used the hypothesis that the mean necrotic core volume will be variable between the two groups. Previous studies in ACS patients and our own variability study suggested that the mean would be around 35 mm3 with a standard deviation (SD) for necrotic core ±28 mm3. A sample size of 100 lesions was calculated. From this we expected 80% power to detect a 7 mm3 difference in plaque components with an alpha of 0.05. This permitted the relative differences in the mean to be tested for statistical significance using a Student’s t-test. A p-value of <0.05 was considered significant. Continuous variables are expressed as means±standard error of the mean (SEM). Statistically significant variables in culprit plaques were entered into a multivariate logistic regression analysis with the forward stepwise technique19, to identify independent associations between plaque variables, with AC lesion phenotype being the dependent variable. Those variables that were strongest in the output from the regression each have a logistic regression coefficient. This coefficient is then used to multiply each numerical value for that variable (i.e., 0.675×NC/DC ratio=0.675×3.5=2.363). The summation of the output for each associated variable then creates the overall “Liverpool Active Plaque Score” (LAPS). To assess model fit and performance, the Hosmer-Lemeshow statistic (HLS), Akaike Information Criterion (AIC) and receiver operating characteristics (ROC) data were examined. The area under the curve (AUC), sensitivity, specificity and 95% confidence intervals (CI) were then estimated.
A larger area under the ROC curve reflects better performance of a discriminatory test. An AUC of greater than 0.5 suggests that the discriminatory power of the variable in question is superior to that of natural chance.
VALIDATION
Following construction of the score, the diagnostic equation was taken to the Thoraxcenter, Rotterdam, The Netherlands, and applied to an independent cohort of 50 plaques (17 “active culprit” and 33 “stable bystander” lesions) taken from a previous three-vessel IVUS-VH study in patients presenting with acute myocardial infarction10. Each plaque score was initially calculated with the operator blinded to the lesion status, until the final ROC curve was calculated.
SAMPLE SIZE CONSIDERATIONS FOR LOGISTIC REGRESSION
Sample size calculation for logistic regression is complex, but based on the work of Peduzzi et al20 the following guideline for a minimum number of cases can be suggested, with p as the smallest of the proportions of negative or positive cases for the total population and k the number of covariates (the number of independent variables). The minimum number of cases to include is: N=10 k/p. For our total population, p=0.44 (68 stable [35 construction and 33 validation]/155 total lesions [105 construction and 50 validation]) and k=4 variables. Therefore, from this equation: N=4×10/0.44; N=91 plaques. We have analysed a total of 155 plaques in our overall model and therefore the study should have sufficient statistical power.
Results
Two hundred and thirty-eight patients were approached after urgent ACS transfer and case note review. Seventeen patients refused to participate and two hundred and twenty-one formally consented and were enrolled before angiography. One hundred and fifty-one had to be excluded after angiography due to exclusion criteria. The majority of exclusions were due to the presence of surgical disease, heavy calcification and/or heavy thrombus burden with reduced TIMI flow in the culprit vessel. Seventy culprit lesions in ACS patients were studied and analysed successfully with IVUS-VH. A further thirty-five culprit lesions from stable angina patients undergoing PCI were analysed separately, giving a total construction lesion cohort of one hundred and five.
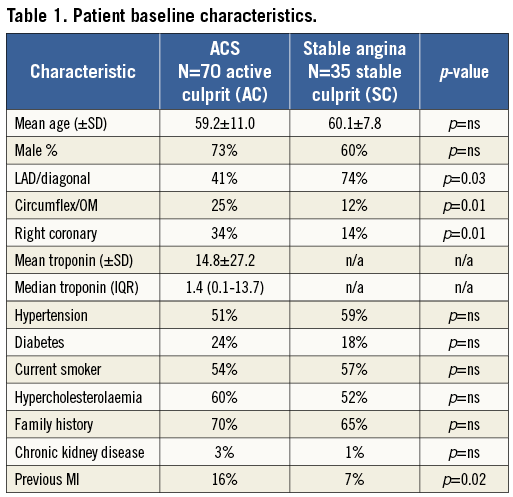
The patient baseline characteristics are displayed in Table 1. Of note, there was a statistically significant difference in the vessel interrogated between plaque types with more left anterior descending or diagonal (LAD/D1) arteries imaged in the stable cohort and a more even distribution in the ACS group: LAD/D1 (41%); circumflex (25%); right coronary (34%). There were no significant differences in conventional risk factors for coronary artery disease; however, those in the ACS cohort were more likely to have had a previous myocardial infarction (16% vs. 7%, p=0.02).
A comprehensive observational assessment of plaque anatomy and virtual histology is presented in Table 2 and Table 3. Total plaque length was statistically greater in the ACS culprit group, combined with a greater total plaque volume and burden, which heavily influenced the relative volume calculations for individual VH plaque components displayed in Table 3. Plaque volumes were therefore corrected for length and vessel size by displaying the measured percentage contribution of each plaque type. Subsequently, the only VH variable that showed a statistical difference between groups was dense calcium plaque, being increased within stable plaques. Creating a ratio between important plaque types also corrects for volume differences and we have shown that there is a statistical difference in the necrotic core volume/dense calcium volume (NC/DC) ratio between ACS culprit (AC) and stable culprit (SC) plaques. Figure 1 shows the angiographic appearance of a culprit ACS plaque (A) with VH frames shown at both the site of maximum necrotic core (B) and the minimum lumen area (MLA) (C). This highlights the eccentric nature and positively remodelled disease typically seen in ACS culprit plaques. In comparison, Figure 2 shows the angiographic image of a stable culprit coronary lesion in the proximal RCA (A). The minimum lumen area (B) shows a significant amount of circumferential calcification and relative negative remodelling. The maximum necrotic core frame confirms disease that is less eccentric and mixed in composition (C).
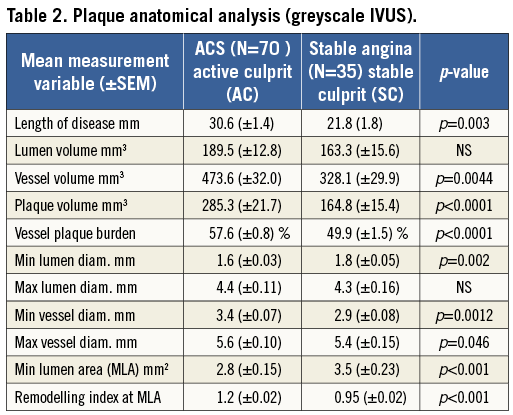
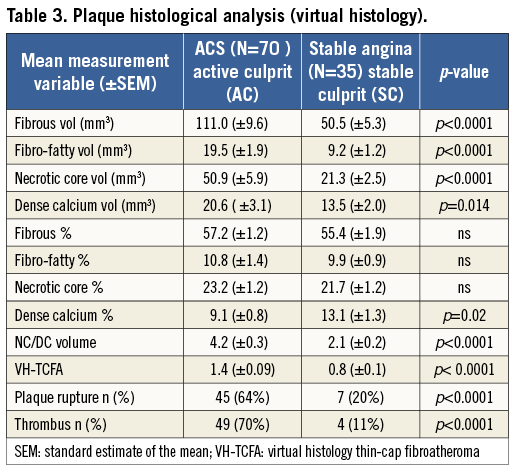
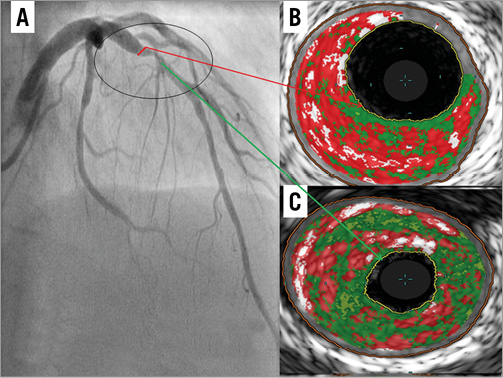
Figure 1. IVUS-VH appearance of LAD stenosis in a patient with a troponin positive NSTEMI and biphasic anterior T wave inversion on ECG (A). Site of MAX NC (B). Site of MLA (C).
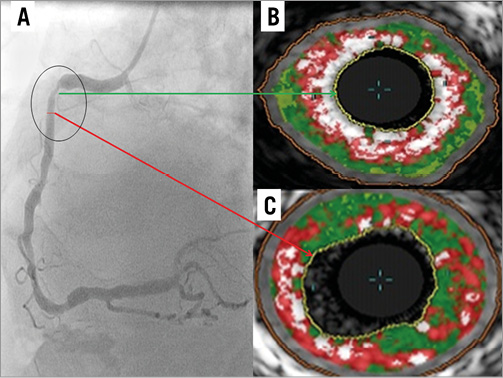
Figure 2. Angiography and IVUS-VH appearance of a stable angina culprit plaque in the RCA (A). MLA site (B). Site of MAX NC frame (C).
Figure 3 displays the ROC analysis for each plaque variable that was statistically significant in the construction logistic regression. Interestingly, this shows the relative importance of the remodelling index at the minimum lumen area (RI@MLA) and the necrotic core to dense calcium ratio (NC/DC) in comparison to minimum lumen area <4 mm2 (MLA <4) and VH-TCFA. Table 4 shows the output from the regression analysis and the relevant calculated coefficients that make up the score. Figure 4 displays the regression equation that calculates the Liverpool Active Plaque Score (LAPS) and the ROC curve showing the strong discriminatory power for an AC phenotype (in the construction cohort). Figure 5 is the ROC curve derived when the LAPS was applied to an independent cohort of plaques analysed at the Thoraxcenter, Rotterdam. As expected, the discriminatory ability of the score is reduced. However, the AUC value of 0.71 confirms that the score maintains a good predictive ability for AC plaque phenotypes. Figure 6 is a box and whisker plot showing the differences and full descriptive distribution of the LAPS between AC and SC presentations in 155 different plaques. Figure 7 is a visual illustration of the results from the application of the LAPS, where a score >6 is more typical of an active phenotype and a score <6 is more likely to represent a stable presentation. This is shown to emphasise how the score could be applied to stratify coronary artery plaques.
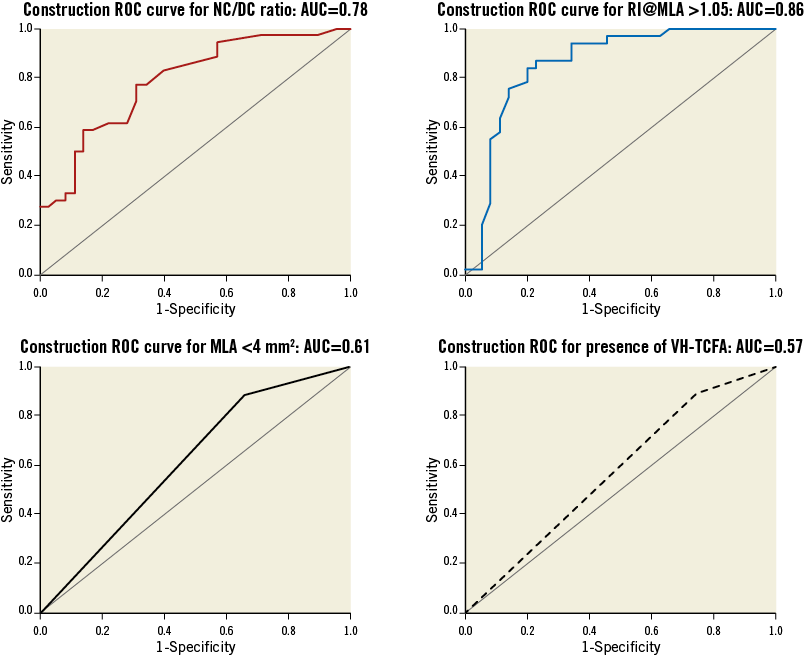
Figure 3. ROC analysis of significant plaque variables found by logistic regression.
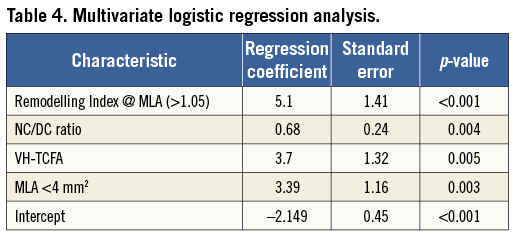
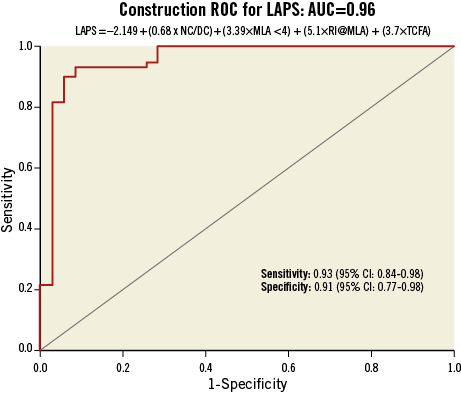
Figure 4. Construction ROC curve for LAPS.
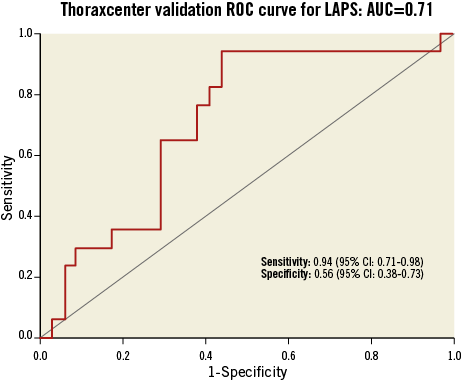
Figure 5. Validation ROC curve for LAPS in an independent cohort.
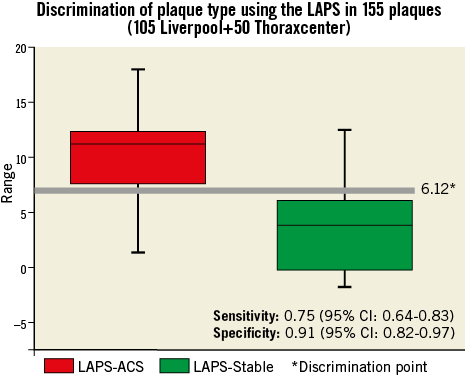
Figure 6. LAPS data for AC plaques (red) and SC plaques (green).
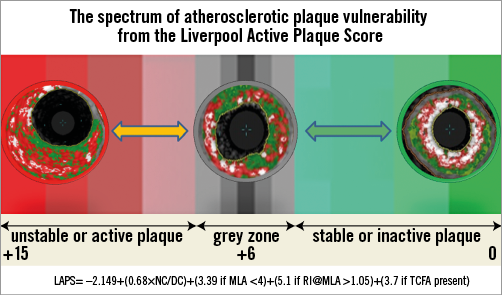
Figure 7. Visual illustration of LAPS with plaque examples.
Discussion
The in vivo assessment of high-risk atherosclerotic plaque characteristics may help improve our understanding and ability to help to identify vulnerable plaques reliably in the future. We have analysed the differences between the anatomical and virtual histological features in culprit coronary plaques from both unstable and stable presentations. Using logistic regression, we have both determined the important features found in plaques responsible for ACS events and assigned a numerical value to these, allowing the calculation of a summative score with discriminatory power. Although many of the features within our score have been previously discussed in the IVUS-VH literature10-16 and found to be more prevalent in culprit plaques, this is the first attempt at modelling a plaque score from IVUS and VH data. The aim of this was to generate a concept that could be taken forward in larger trials and applied to future lesions and studies, possibly with more advanced imaging techniques, such as coronary CT, optical coherence tomography (OCT) and near infra-red spectroscopy (NIRS). Identifying the high-risk features of plaques that have been shown to correlate with future MACE in previous studies8-9 is of paramount importance from both a preventative and an interventional point of view. Moreover, creating a reproducible score could allow both proper validation and invasive assessment of pharmacological agents aiming to modify vulnerable plaque features21. It may also be conceivable that in the future a “master” plaque risk score could be produced following further studies. The overall aim of this concept would be to guide cardiologists towards plaques where the risk of a future event occurring may be greater than the risk of treatment with a particular novel drug or device, such as high-dose statins, PC SK9 inhibition, darapladib or bioabsorbable vascular scaffolds (BVS)22. The variables that have formed our score and are associated with culprit ACS plaque presentations concur with similar findings from both VIVA and PROSPECT8-9 with MLA and VH-TCFA again found to be important. However, given that our study focused on culprit plaque morphologies, we added to this the remodelling index at the MLA (RI@MLA) and the necrotic core/dense calcium ratio (NC/DC). Both of these findings have previously been found to indicate an unstable lesion phenotype10,23-30 and have even been linked to sudden death31.
Our study examined the most pathologically important part of the vessel, responsible for the recent ACS event, before PCI. This allowed us to observe vital information about residual plaque characteristics in a contemporary setting. The PROSPECT trial examined the VH appearance of non-culprit, angiographically insignificant lesions that eventually caused readmission to hospital with unstable angina. The actual myocardial infarction (MI) rate in the follow-up cohort was low at 1%. This is a direct reflection of the study protocol, which required all significant angiographic lesions to undergo PCI before IVUS-VH analysis. Therefore, the results of PROSPECT do not inform us about the composition of culprit plaques implicated in a confirmed MI presentation. The VIVA study acquired three-vessel IVUS in both unstable and stable atherosclerosis before PCI, but again the rate of MI during follow-up was low, with endpoints being driven by revascularisation not MI. Although the total number of plaques analysed in this study is small compared to PROSPECT and VIVA, the number imaged in the context of a confirmed MACE event (n=70 [construction] + n=17 [validation]=87) is greater than in PROSPECT (n=55) and VIVA (n=19) combined.
The inclusion of the NC/DC ratio within the score merits discussion, as previously in the literature the impact of dense calcium measurement on necrotic core coding on VH has been investigated32. We found that in stable culprit (SC) plaques there are statistically higher values for dense calcium but not for necrotic core. It has previously been shown in some analyses33 that dense calcium in VH analysis begets and increases the identification of necrotic core behind the calcium signal. Although this is certainly observed in some situations, it does not seem to have statistically influenced our overall volume measurements. Moreover, calcium in a spotty distribution within relatively larger necrotic cores (i.e., a high NC/DC level) appears to be a more important phenotype, and this has previously been observed, pathologically, in sudden coronary death victims3.
The NC/DC ratio in our cohort proved to be a significant independent predictor of a culprit lesion phenotype with an individual AUC of 0.78.
The influence of positive remodelling at the site of the MLA was very strong in our cohort (individual AUC – 0.83). This may be explained by the eccentric plaque burden seen in culprit ACS plaques or by the fact that extensively remodelled plaques are biologically active and have an increased tendency to weaken at the luminal surface, when the fibrous cap thins or an unknown Glagovian expansive limit is reached10,23-30. The presence of more negatively remodelled plaques with greater dense calcium, in stable angina, may be explained by plaque contraction, as necrotic core and macrophages “burn out” and are “contained” by calcification.
Further interesting insights from our data relate to the influence of the vulnerable plaque surrogate VH-TCFA in plaque diagnostics. This has previously been linked with future MACE events8,9. In our cohort, VH-TCFA presence within the interrogated plaque disease was greater in the culprit ACS plaques compared to stable angina. However, on a discriminatory level, VH-TCFA has an individual AUC of 0.57, suggesting it has very modest diagnostic ability. The limitations of the VH-TCFA concept have been described elsewhere7,17,22. IVUS-VH cannot define true thin caps, due to the resolution of a 20 MHz IVUS catheter being in the order of 150 µm. Also, VH-TCFA lesions are often found within stable angina lesions and this weakens their discriminatory power. Moreover, it has been shown that these lesions are dynamic and fleeting in nature, with around 75% “healing” over one year34, which further weakens their overall influence on lesion diagnostics. In addition, a recent study from the VIVA group has shown unacceptable variability in VH-TCFA recognition and the process of individually naming plaque subtypes (such as thick-cap fibroatheroma [ThCFA], etc.)35. Critically, this leads us to the concept generated by this investigation. It is clear that our work provides a basic “pilot” idea in this field, that could be taken forward in future investigations, hopefully with the addition of information gained from other imaging tools such as OCT (which can define true thin caps <65 µm) and NIRS (highly sensitive for lipid core).
More importantly, most of the plaque variables we have found in this investigation can now be measured by non-invasive cardiac computerised tomography. Therefore, there may be a role for this theory in the preventative screening of asymptomatic plaques found in individuals with familial hazard or abnormal conventional risk factors.
Limitations
This is a single-centre, observational study with the inherent limitations of this design. Although efforts were made to blind the analysing operator to the presentation type, it can occasionally be clear when a plaque is a culprit, due to the presence of residual thrombus and plaque rupture. This is a potential source of bias. The analysis of MLA <4 mm2, RI >1.05 and VH-TCFA with logistic regression required these datasets to be converted to binary responses, which may not be representative of what is reported in the real world. In this study we imaged the full volume data from culprit plaques a few days after an event had occurred within the plaque. Although this is as close as we can realistically come to phenotyping “event-associated” unstable plaques, we still cannot say with certainty what the anatomy and histology was at the point of rupture/erosion. Furthermore, analysis of high-risk ACS culprit plaques required interrogation of IVUS images containing rupture cavities and thrombus at varying stages of progression, and therefore we followed previously published guidance by including thrombus in the plaque analysis where possible17. This is notoriously difficult and previous studies have shown a difference between independent IVUS-VH measurements and measurements performed in a core lab facility35-37. However, we have published our magnitude of measurement variability in high-risk ACS plaques, following a previous in-depth study18. There are also several peer-reviewed publications involving the identification of thrombus on IVUS38-40. The validation cohort from the Thoraxcenter contained VH calculations from an earlier iteration of the VH concept (IVUS lab version 4.4), which could theoretically have affected plaque score calculations. Moreover, this cohort of lesions was from “active culprit vessel” and “stable bystander vessel” rather than “active culprit” and “stable culprit” lesions. This was justified by the fact that we still validated the discrimination of active culprit disease from other forms of atherosclerosis, using the derived Liverpool Active Plaque Score.
Conclusion
We have found four features on IVUS and VH that can predict and discriminate ACS culprit lesion phenotypes from those that are clinically stable. Subsequently, we have constructed and validated the Liverpool Active Plaque Score (LAPS) based upon these features. Although the main outcome of this study is concept-generating, it is hoped that in the future a similar score may allow the prospective identification and treatment of active coronary plaques to prevent major adverse cardiac events.
| Impact on daily practice The in vivo assessment of high-risk atherosclerotic plaque characteristics may help improve our understanding and ability to identify vulnerable plaques reliably in the future. We have analysed the differences between the anatomical and virtual histological features in culprit coronary plaques from both unstable and stable presentations. Using logistic regression, we have both determined the important features found in plaques responsible for ACS events and assigned a numerical value to these, allowing the calculation of a summative score with discriminatory power. It is hoped that in the future a similar score may allow cardiologists to prospectively identify and appropriately treat active coronary plaques in order to prevent major adverse cardiac events. |
Guest Editor
This paper was Guest Edited by Henning Kelbaek, MD from the Rigshospitalet, Copenhagen, Denmark.
Funding
Liverpool Heart and Chest Hospital, Johnson Interventional Cardiology Research Fellowship.
Conflict of interest statement
The authors have no conflicts of interest to declare. The research was funded entirely by an independent local fellowship. The Guest Editor has no conflicts of interest to declare.

