Abstract
Aims: This study was performed to assess the differences in culprit plaque composition between patients with and without pre-infarction angina (PA) by using spectral analysis of intravascular ultrasound (IVUS) radiofrequency (RF) data.
Methods and results: Of 57 patients consecutively admitted to our institution with acute myocardial infarction, pre-intervention IVUS RF data of culprit plaques were obtained and analysed in 35 patients after percutaneous aspiration thrombectomy. Among the 35 patients, 21 patients had PA. Culprit plaques of patients without PA consisted of a higher percentage of the necrotic core component than those with PA (minimum lumen area [MLA]) site, 21.2±8.9% versus 9.9±9.8%, p=0.0015; entire culprit lesion, 18.9±6.3% versus 12.0±9.6%, p=0.023). In contrast, culprit plaques of patients with PA contained a higher percentage of the fibrofatty component than those without PA (MLA site, 21.0±12.0% versus 11.5±7.6%, p=0.013; entire culprit lesion, 16.8±7.9% versus 12.1±5.5%, p=0.062). There was no significant difference in quantitative parameters between the patients with and without PA.
Conclusions: Culprit plaques of patients with PA were different from those without PA. Plaque composition may play an important role in the occurrence of PA.
Introduction
Pre-infarction angina (PA) has been shown to result in a smaller infarct size and better clinical outcomes1-5. However, the mechanisms of the occurrence of PA have not yet been fully elucidated. The occurrence of PA has been suggested to be associated with culprit plaque geometry or thrombus burden in the diseased vessel, which may be related to culprit plaque composition. However, the difference of culprit plaque morphology in vivo between patients with and without PA has not yet been fully evaluated.
Recently, spectral analysis of intravascular ultrasound (IVUS) radiofrequency (RF) data has been demonstrated to provide detailed quantitative and qualitative information on in vivo coronary plaque composition6-9. A study by Nasu et al revealed that in vivo tissue characterisation by Virtual Histology (VH) correlated favourably with the results of in vitro histopathologic examinations of tissue samples obtained by directional coronary atherectomy10.
In this study, we assessed the difference of culprit plaque composition in vivo between patients with and without PA by using VH.
Methods
Patients and lesions
Between June 2005 and April 2006, 57 consecutive patients with acute myocardial infarction (MI) were admitted to our institution. For these patients, coronary angiography was performed to identify culprit lesions, and subsequently, percutaneous coronary intervention (PCI) was performed. After percutaneous aspiration thrombectomy with TVAC™ (Nipro Corporation, Osaka, Japan) for removal of as many thrombi as possible to minimise the influence of thrombi on IVUS analysis, IVUS examinations were performed to assess the morphology of the infarct-related plaques11. However, IVUS examination was not attempted in 15 patients due to unstable vital signs. Four patients were excluded because of the failure to cross the lesion completely with the IVUS transducer. The remaining 38 patients were enrolled in this study. Informed, written consent was obtained from all the patients.
Diagnosis and definitions
The diagnosis of MI was based on the following findings: the presence of chest pain for >30 minutes, ST elevation or depression in two or more consecutive electrocardiogram leads, absence of improvement in subjective symptoms and electrocardiogram change after nitrates, and >2-fold increases of normal limits in plasma creatine kinase (CK) activity12.
Hyperlipidaemia was defined as a total cholesterol level ≥ 220 mg/dl, a high-density lipoprotein cholesterol level < 40mg/dl, a triglyceride level ≥ 150 mg/dl, a low-density lipoprotein level ≥ 140 mg/dl, and/or lipid-lowering medication use. Hypertension was defined as systolic blood pressure ≥ 140mmHg, diastolic blood pressure ≥ 90 mmHg, and/or use of an antihypertensive drug. Diabetes mellitus was defined by a previous physician’s diagnosis and treatment with diet, oral hyperglycaemic agents, or insulin.
Information on the presence of pre-infarction angina
PA was defined as prodromal symptoms such as: typical angina pectoris, atypical chest pain, shortness of breath and gastrointestinal symptoms occurring not more than one week before acute MI onset. The presence of PA was diagnosed by a physician from detailed clinical history taken before PCI, and was reconfirmed by another physician blinded to the results of IVUS examinations after PCI within one week.
Assessment of thrombus burden
Thrombus burden was assessed by coronary angiography and scored in five grades as previously reported13. According to this classification, in thrombus grade 0 (G0), no cine-angiographic characteristics of thrombus are present; in thrombus grade 1 (G1), possible thrombus is present, with such angiography characteristics as reduced contrast density, haziness, irregular lesion contour, or a smooth convex meniscus at the site of total occlusion suggestive but not diagnostic of thrombus; in thrombus grade 2 (G2), there is definite thrombus, with greatest dimensions ≤ 1/2 the vessel diameters; in thrombus grade 3 (G3), there is definite thrombus, but with greatest linear dimension > 1/2, but < 2 vessel diameters; in thrombus grade 4 (G4), there is definite thrombus, with the largest dimension ≥ 2 vessel diameters; and in thrombus grade 5 (G5), there is total occlusion (unable to assess thrombus burden due to total vessel occlusion). In patients presenting with G5, thrombus was reclassified into one of the other categories after flow achievement.
PCI procedure
All of the patients received aspirin and intravenous heparin to achieve an activated clotting time of > 250 seconds. None of the patients received glycoprotein IIb/IIIa inhibitors because these drugs have not been approved in Japan.
After culprit lesions were identified, percutaneous aspiration thrombectomy was performed. Next, balloon dilatation or stent implantation was performed after IVUS examination. Decision-making concerning the need for stent implantation or balloon dilatation before and after stent implantation, stent size, stent type, and final pressure of balloon dilatation was left to the discretion of the individual PCI operators, who were blind to the results of IVUS RF data analysis. Stents were implanted in all of the patients except for one patient with PA.
IVUS RF data acquisition and analysis
IVUS RF data were acquired with a 20-MHz 2.9 Fr phased-array IVUS catheter (Eagle Eye Gold, Volcano Corp., Rancho Cordova, CA, USA) and a dedicated console (IVG3™, Volcano Corp., Rancho Cordova, CA, USA). An automated continuous pullback (0.5 mm per second) was performed with a dedicated pullback device (R-100, Volcano Corp., Rancho Cordova, CA, USA) after intracoronary administration of isosorbide dinitrate (2.5 mg). If a mobile mass with homogeneous echo density which was suggestive of thrombus was found on IVUS examination, percutaneous aspiration thrombectomy was performed until the mass was not detected on IVUS examination. IVUS run without the mass was assessed. The IVUS RF data were stored on a hard disk for off-line analysis.
Manual contour detection of both the lumen and the media-adventitia interface was performed for culprit lesions. Geometrical assessment and compositional analyses of culprit lesions were performed by using VH software (IVUS VH1.3j, Volcano Corp., Rancho Cordova, CA, USA). We assessed plaque compositions of minimum lumen area (MLA) sites and entire culprit lesions.
An entire culprit lesion was defined as the segment that was treated by PCI after the IVUS examination. The segment treated was identified by a post-intervention IVUS imaging run. With the use of reproducible landmarks (e.g. a calcium deposit or a side branch), the same segment was determined in the IVUS run before mechanical dilatation.
The MLA site was identified within the entire culprit lesion by IVUS quantitative analysis. If there were several MLA sites, the MLA site with the largest external elastic membrane (EEM) cross-sectional area (CSA) was chosen for evaluation14,15.
Plaque rupture sites were identified by grey-scale IVUS images. Plaque rupture was defined as ruptured capsule with an underlying cavity or plaque excavation by atheromatous extrusion with no visible capsule16. The prevalence and location of plaque rupture was assessed.
VH uses IVUS RF data to classify plaques into four components. Fibrous, fibrofatty, dense calcium, and necrotic core components can be identified within plaques, which was validated by preliminary in vitro and in vivo studies10,17. Four components were assigned colour codes. Fibrous, fibrofatty, dense calcium and necrotic core regions were labelled as green, greenish-yellow, white and red, respectively. From these data, colour-coded tissue maps can be constructed. Plaque composition was evaluated by both the absolute values and the percentage of plaque burden.
Statistical analysis
Continuous variables were expressed as mean±standard deviation. Discrete variables were presented as counts and percentages. Differences in means between the groups were analysed by two-tailed unpaired Student’s t test for normally distributed variables. As the data on the percentage of the dense calcium component at the MLA sites, the fibrofatty component area at the MLA sites and the fibrofatty component volume in the entire culprit lesions showed skewed distribution, those data were assessed by Mann-Whitney test. Frequencies were compared with Fisher’s exact probability test or chi-square test. A p value of less than 0.05 indicated statistical significance. Statistical analyses were performed using Stat View software version 5.0 (SAS Institute, Cary, NC, USA).
Results
Among the 38 lesions, the images of three lesions could not be analysed because of poor image quality. Therefore, IVUS RF analyses were performed in the remaining 35 culprit lesions.
Patient and lesion characteristics
Patient and lesion characteristics are shown in Table 1.
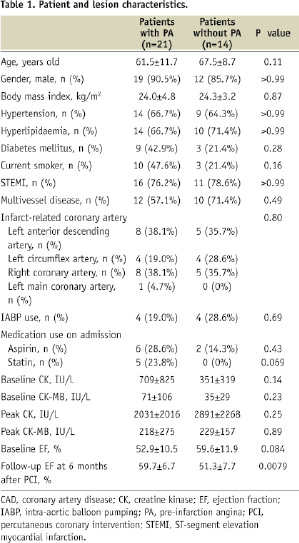
The infarct-related vessel was the left anterior descending artery in 13 lesions, the left circumflex artery in eight lesions, the right coronary artery in 13 lesions, and the left main coronary artery in one lesion. Out of the total of 35 patients, 21 patients had PA.
Patients with PA were younger than those without PA, although the difference was not statistically significant (61.5±11.7 years old versus 67.5±8.7 years old, p=0.11). In addition, the prevalence of diabetes mellitus tended to be higher in patients with PA than those without PA (42.9% versus 21.4%, p=0.28). There was no significant difference in the prevalence of ST-segment elevation MI and multivessel disease (ST-segment elevation MI, 76.2% versus 78.6%, p>0.99; multivessel disease, 57.1% versus 71.4%, p=0.49). All of the patients using statins had PA, although the difference in statin use between patients with and without PA was not statistically significant (23.8% versus 0%, p=0.069).
Procedural data demonstrated that TIMI 3 flow was acquired after percutaneous aspiration thrombectomy more often in patients with PA than in those without PA, although the difference was not statistically significant (95.2% versus 78.6%, p=0.28). There was no significant difference in the prevalence of patients with haemodynamic instability who needed intra-aortic balloon pumping between the two groups (19.0% versus 28.6%, p=0.69).
Laboratory data showed that there was no significant difference in baseline and peak CK or CK-MB level between the two groups. However, when we compared ejection fraction (EF) at the baseline and at the six months after PCI, baseline EF tended to be higher in patients without PA than in those with PA (59.6±11.9% versus 52.9±10.5%, p=0.084), whereas follow-up EF was significantly higher in the latter than in the former (59.7±6.7% versus 51.3±7.7%, p=0.0079).
Assessment on thrombus burden
Data on thrombus burden are shown in Table 2.
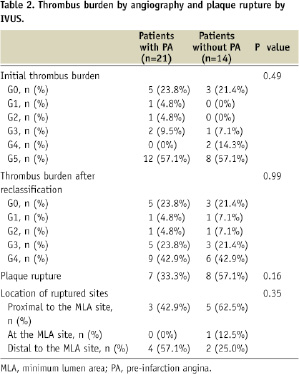
There was no statistically significant difference in the prevalence of thrombus burden categories in the initial assessment. No significant difference was found in the prevalence of thrombus burden after reclassification of the G5 group between the two groups, either.
Assessment on plaque rupture
Data on the prevalence and location of plaque rupture are shown in Table 2. Plaque rupture was more often observed in patients without PA than in those with PA, although the difference was not statistically significant (33.3% versus 57.1%, p=0.16). As for location of ruptured sites, there was no significant difference between the two groups. Plaque rupture was found at the MLA site in only one lesion.
Comparison of quantitative IVUS data between patients with and without PA
Quantitative IVUS data are shown in Table 3.
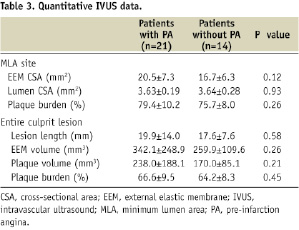
No significant geometrical difference was found between culprit plaques of patients with and without PA, including minimum lumen area (3.63±0.19 mm2 versus 3.64±0.28 mm2, p=0.93) and plaque burden (MLA site, 79.4±10.2% versus 75.7±8.0%, p=0.26; entire culprit lesion, 66.6±9.5% versus 64.2±8.3%, p=0.45).
Comparison of culprit plaque composition between patients with and without PA
Compositional IVUS data are shown in Table 4.
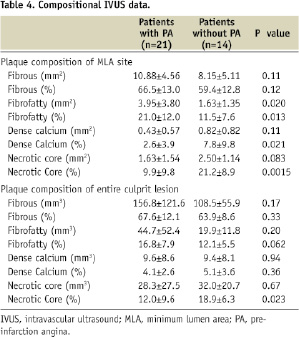
Analysis of plaque composition of MLA sites demonstrated that the necrotic core component area tended to be higher in patients without PA than in those with PA (2.50±1.14 mm2 versus 1.63±1.54 mm2, p=0.083), whereas the fibrofatty component area was higher in patients with PA than in those without PA (3.95±3.80 mm2 versus 1.63±1.35 mm2, p=0.020). The percentage of the necrotic core component was also higher in patients without PA than in patients with PA (21.2±8.9% versus 9.9±9.8%, p=0.0015), whereas the percentage of the fibrofatty component in patients with PA was higher than in patients without PA (21.0±12.0% versus 11.5±7.6%, p=0.013). Furthermore, a higher percentage of the dense calcium component at MLA sites was found in patients without PA than in patients with PA (7.8±9.8% versus 2.6±3.9%, p=0.021). There was no significant difference in the fibrous component. Representative examples of grey-scale IVUS images and colour-coded plaque compositional maps of MLA sites of patients with and without PA are shown in Figure 1.
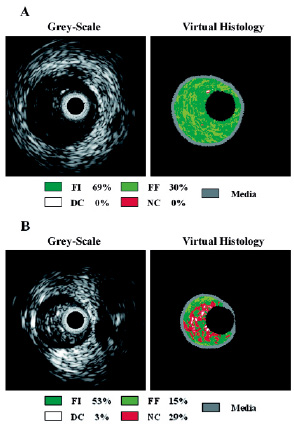
Figure 1. Representative examples of IVUS grey-scale image and colour-coded plaque compositional map of the MLA sites of patients (A) with PA and (B) without PA. FI, fibrous; FF, fibrofatty; DC, dense calcium; NC, necrotic core.
Analysis of plaque composition of entire culprit lesions showed that there was no significant difference in the volume of each plaque component. However, there was a significant difference in the percentage of the necrotic core component between the two groups. Culprit plaques of patients without PA contained larger percentage of the necrotic core component than those with PA (18.9±6.3% versus 12.0±9.6%, p=0.023). No significant difference was found in the percentage of the fibrofatty component in the entire culprit lesions; however, there appeared to be a trend towards significance (16.8±7.9% versus 12.1±5.5%, p=0.062). There was no significant difference in the other plaque components.
Discussion
Using spectral analysis of IVUS RF data, we demonstrated that culprit plaques of patients without PA had more of the necrotic core component than those with PA, whereas those with PA contained larger amounts of the fibrofatty component than those without PA. There was no significant difference in quantitative data between patients with and without PA.
The mechanism of the occurrence of PA has not yet been fully elucidated. Our findings suggest that plaque composition may play an important role in the occurrence of PA. The necrotic core component has been shown to be the most thrombogenic component in human atherosclerotic plaques18,19. The exposure of the necrotic core component (plaque rupture) leads to heavy deposition of the tissue factor, thereby increasing thrombogenicity and abrupt thrombus formation. Thus, the necrotic core-rich plaques may produce large thrombus burden, which may often result in sudden onset of MI without PA. In fact, in the present study, we found more plaque rupture in patients without PA than in those with PA. On the other hand, previous studies have shown that plaques consisting of a proteoglycan-rich matrix without a large lipid core also cause acute coronary thrombosis (plaque erosion)20,21. It has been suggested that this kind of plaques have less potent thrombogenicity than plaques with a large lipid core and consequently lead to a lesser thrombus burden. Therefore, small thrombus burden, produced by culprit plaques rich in the fibrofatty component, may often result in the occurrence of PA. In fact, the previous study by Kojima et al demonstrated that patients with PA were more likely to have plaque erosion as a substrate22.
The previous studies have demonstrated that PA has been shown to result in better clinical outcomes1-5. In the present study, follow-up EF at the six months after PCI was larger in patients with PA than in those without PA. The difference in culprit plaque composition may explain these findings. The culprit plaques of patients without PA, rich in the necrotic core component, may produce larger amounts of thrombi than those with PA, rich in the fibrofatty component. This difference may lead to more distal circulatory disturbances and more distal embolisation, and then, lead to a larger infarct size and poor clinical outcomes in patients without PA than in those with PA. Furthermore, in patients with PA, small amounts of thrombi may cause long-term ischaemia before MI, and this condition may induce protective reactions in endothelial cells and myocardium.
In the present study, there was no statistically significant difference in the prevalence of thrombus burden categories in the initial assessment. However, it is noteworthy that in the initial assessment, there was no patient of the G4 group in patients with PA, whereas 14.3% of the patients belonged to the G4 group in patients without PA. Furthermore, there was no patient of the G1 and the G2 group in patients without PA. As the number of patients in this study was relatively small, studies with the large number of patients may be needed to assess the difference in thrombus burden between the two groups. We found no significant difference in the prevalence of thrombus burden after reclassification of the G5 group between the two groups, either. This might be because we performed percutaneous aspiration thrombectomy before flow achievement.
However, we found that TIMI 3 flow was acquired after percutaneous aspiration thrombectomy more often in patients with PA than in those without PA. Thus, there was more difficulty in aspirating thrombi in patients without PA than in those with PA. This might result from the difference in thrombus burden or the nature of thrombi. In the present study, lower success rate of percutaneous aspiration thrombectomy might have led to larger myocardial damage in patients without PA than in those with PA.
There is another explanation for the difference in culprit plaque composition between patients with and without PA. It has been shown that sudden coronary occlusion is often preceded by a variable period of plaque instability and thrombus formation23. Patients with PA could have undergone early plaque disruption with extrusion and embolisation of the necrotic core component, thereby explaining why these sites contained smaller amount of the necrotic core component. Even if this might be the case, small thrombus burden, which did not cause MI, might lead to ischaemic preconditioning and might have a beneficial effect on clinical outcomes.
The present study showed that plaque rupture sites were not often located at the MLA site, which is consistent with the previous study16. As MLA sites may not often represent the nature of culprit plaques, it may be more appropriate to assess the entire culprit lesions than MLA sites when we analyse plaque composition.
Recently, statin therapy has been shown to reduce the lipid component of coronary plaques and then lead to coronary plaque stabilisation24,25. In the present study, all of the patients using statins had PA. Statin therapy might have prevented large deposition of the necrotic core component in culprit plaques, and consequently, might have reduced the event of plaque rupture.
Limitations
There were some limitations in this study. This study was performed at a single centre and had a relatively small sample size. Patient selection bias might occur because 15 patients were excluded due to the patients’ unstable condition. As contour detection of the media-adventitia interface was difficult in some cases with heavily calcified lesions, those lesions were excluded from the analysis as poor image quality. This might also lead to selection bias. In the present study, geometrical assessment was performed by VH software. As geometrical validation of VH software is not fully established, this might lead to the inaccuracy of geometrical data in this study. In the current study, patients with PA tended to be younger than those without PA. In addition, the prevalence of diabetes mellitus was higher in patients with PA than in those without PA, although the difference was not statistically significant. Those differences might affect our results. Further studies, with a larger number of patients, will be needed. In the process of plaque rupture or plaque erosion, parts of plaques might disappear, and the composition of the remaining plaques could be altered from the original plaque composition. As thrombi and blood are not validated on IVUS RF data, these components might be assigned to one of the four components. In fact, the previous studies have shown that thrombi are often classified into the fibrofatty component. Therefore, the difference in the fibrofatty component might be affected by thrombi. However, by performing percutaneous aspiration thrombectomy prior to IVUS data acquisition, this limitation could be minimised. In the procedure of percutaneous aspiration thrombectomy, the parts of plaques might also be aspirated and plaque composition might be altered.
Conclusions
Culprit plaques of patients without PA contained larger amount of the necrotic core component than those with PA, whereas those with PA consisted of larger amounts of the fibrofatty component than those without PA. This difference in plaque composition may lead to the difference in thrombus burden and then to the difference of clinical characteristics and outcomes between patients with and without PA. Thus, plaque modification therapy may be important in daily practice. Further studies, multicentred, with a larger number of patients, will be warranted to confirm our findings and to assess the relationship between plaque composition and clinical outcomes in patients with acute MI.

