
Abstract
Aims: This study aimed to evaluate the relationship between pre-infarction angina (PIA) and in vivo culprit lesion characteristics as assessed by intravascular optical coherence tomography (OCT) in patients with a first ST-segment elevation myocardial infarction (STEMI).
Methods and results: A total of 305 consecutive patients with a first STEMI who underwent OCT imaging of culprit lesions during primary percutaneous coronary intervention (PCI) were prospectively enrolled. OCT findings of the culprit plaque were compared between patients with (n=206) and without PIA (n=99). Patients with PIA showed lower rates of thin-cap fibroatheroma (TCFA) (62.6% vs. 80.8%, p=0.001) and plaque rupture (56.8% vs. 72.7%, p=0.007), smaller maximum ruptured cavity areas (1.10±1.04 mm2 vs. 1.53±1.20 mm2, p=0.002), and more severe residual luminal narrowing (p=0.015) with a higher incidence of white residual thrombus (68.4% vs. 50.0%, p=0.003) at the culprit lesions than patients without PIA. No significant differences in clinical outcomes were observed at the one-year follow-up.
Conclusions: In patients with a first STEMI, PIA was significantly associated with a lower incidence of TCFA and plaque rupture, a smaller ruptured cavity area, more white residual thrombi, and more severe lumen stenosis at the culprit lesions. Clinical Trials Identifier: NCT03107624
Abbreviations
AMI: acute myocardial infarction
IFC: intact fibrous cap
IRA: infarct-related artery
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
QCA: quantitative coronary angiography
STEMI: ST-segment elevation myocardial infarction
TCFA: thin-cap fibroatheroma
TIMI: Thrombolysis In Myocardial Infarction
Introduction
Pre-infarction angina (PIA) occurs frequently in patients with ST-segment elevation myocardial infarction (STEMI)1. Previous studies have shown that PIA is associated with smaller infarct sizes, preserved left ventricular function, reduced microvascular and myocardial damage and improved clinical prognoses2-5. Multiple mechanisms have been suggested to account for the myocardial protection of PIA in the setting of STEMI, including opening of pre-existing collateral vessels, an ischaemic myocardial conditioning effect, and different plaque morphologies at the culprit site limiting the thrombus burden6-8. Post-mortem and in vivo studies using intravascular ultrasound (IVUS) suggest that the composition of the culprit plaque may be different in patients presenting with PIA with less potent thrombogenicity from that in patients without PIA9,10. These studies were limited by case selection biases, small sample sizes and the inability of IVUS to assess intact fibrous cap (IFC) plaque and thrombus constituents. To date, significant uncertainty remains regarding the role of culprit plaque composition in the occurrence of PIA. Intracoronary optical coherence tomography (OCT) is a catheter-based imaging modality with a unique axial resolution (10 to 20 μm) that enables accurate in vivo detection and measurement of morphometric characteristics of the culprit lesion11, including ruptured versus IFC plaques, the cap thickness of fibroatheroma, and thrombus constituents.
Based on these premises, we prospectively investigated the relationship between PIA and the in vivo characteristics of culprit lesions in a large cohort of consecutive patients with a first STEMI using OCT at the time of primary percutaneous coronary intervention (PCI).
Methods
STUDY DESIGN AND POPULATION
This study was a prospective observational study (NCT03107624) conducted in 305 consecutive patients presenting with a first STEMI at The Second Affiliated Hospital of Harbin Medical University between June 2016 and January 2017.
All patients underwent primary PCI within 12 hours of symptom onset. Pre-intervention OCT evaluation of the culprit lesion in the infarct-related artery (IRA) was performed in all patients immediately after restoring an effective antegrade coronary flow. Inclusion/exclusion criteria are listed in Supplementary Appendix 1. The study flow chart is shown in Figure 1.
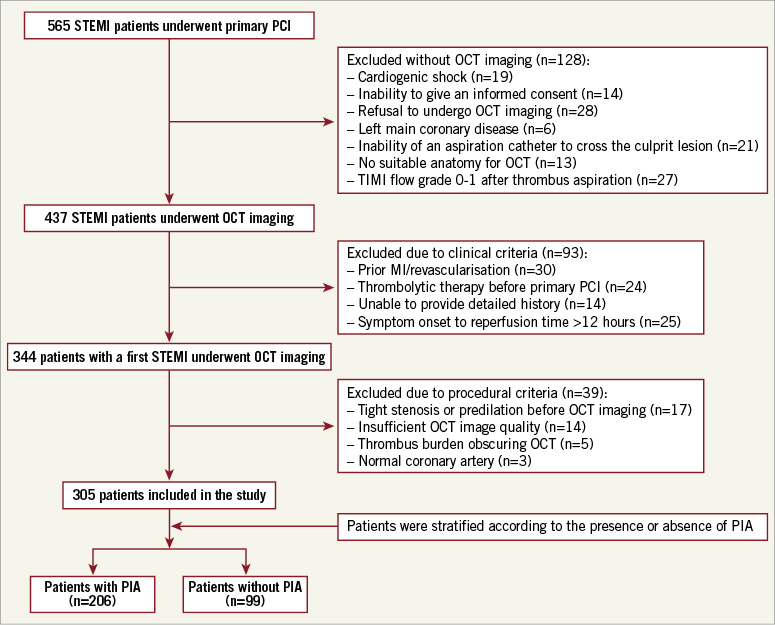
Figure 1. Study flow chart. Out of 344 patients who presented with a first STEMI, 305 patients were identified as eligible for the study and were enrolled based on the inclusion/exclusion criteria. MI: myocardial infarction; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; PIA: pre-infarction angina; STEMI: ST-segment elevation myocardial infarction; TIMI: Thrombolysis In Myocardial Infarction
The study was approved by the Ethics Review Committee of The Second Affiliated Hospital of Harbin Medical University, China, and all patients provided written informed consent before any procedure.
SYMPTOM DATA COLLECTION
Detailed information regarding patients’ clinical histories and symptoms, especially occurring in the month before the infarction, was collected in all cases before primary PCI. The information was assessed again within 48 hours after primary PCI and reported in a standardised questionnaire administered by researchers. Briefly, information regarding the presence or absence of angina, the patterns of angina and the time intervals between the last episode of chest pain and the onset of symptoms of acute myocardial infarction (AMI) was recorded.
DEFINITION OF PIA
PIA was defined as at least one episode of angina (typical chest pain, chest discomfort or radiating pain), either at rest or during exercise, lasting less than 30 minutes, and occurring prior to the onset of infarction-related pain. Conversely, no PIA was defined as a sudden-onset STEMI without angina before infarction. Following the classification of previous studies9,12, the type of PIA was further categorised as one of the following: 1) unstable PIA, defined as either new-onset (new development of angina during the month preceding infarction) or accelerated (angina that had been present for >1 month and had increased in severity during the month before infarction) angina; and 2) stable PIA, defined as angina that had been present for >1 month with no change in the angina pattern during the month before infarction. The timing of the closest episode of angina prior to myocardial infarction was divided into the following four categories: within 24 hours, between one and seven days, between seven days and one month, and more than one month before infarction.
ANGIOGRAPHIC ANALYSIS
The details of the angiographic analysis are reported in Supplementary Appendix 1.
OCT IMAGE ACQUISITION AND ANALYSIS
All OCT images were analysed using previously validated criteria11. Briefly, plaque rupture was identified by the presence of cavity formation in the culprit plaque with discontinuity of the fibrous cap13. An IFC culprit lesion was defined as the presence of a luminal thrombus overlying an intact cap fibroatheroma (definite erosion), or luminal surface irregularity at the culprit lesion in the absence of clear thrombus (possible erosion), or attenuation of an underlying plaque without superficial lipids or calcification immediately proximal or distal to the thrombus site13. Representative OCT images of culprit lesion features in patients with STEMI are presented in Figure 2.
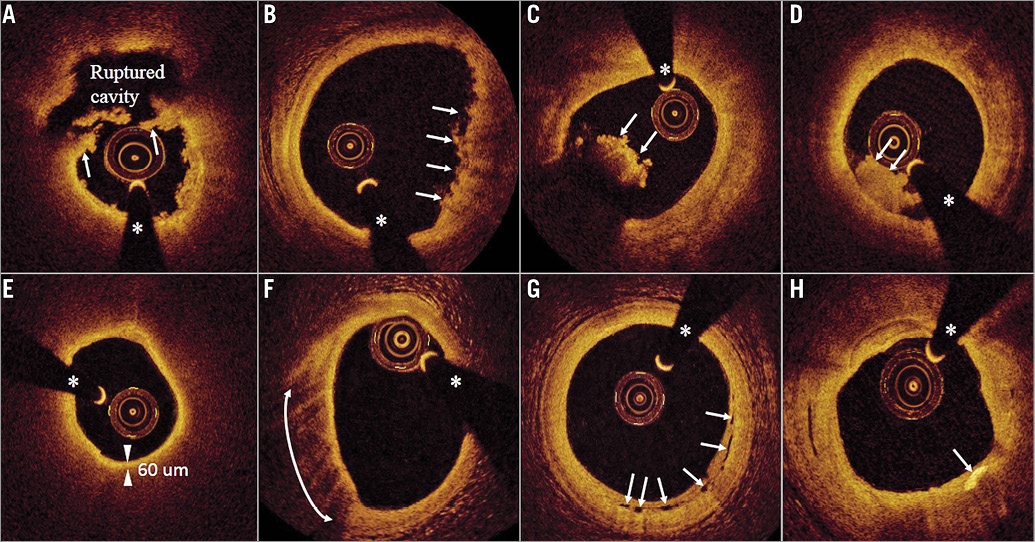
Figure 2. Representative OCT images of culprit lesion features in patients with STEMI. A) Ruptured plaque with remnants of the fibrous cap (arrows), a ruptured cavity and small residual white thrombus masses. B) Intact fibrous cap (IFC) fibrous-lipidic plaque with small protruding thrombi (arrows). C) Red thrombus (arrows) protruding into the lumen with high signal backscattering and attenuation. D) White thrombus (arrows) protruding into the lumen with homogeneous signal backscattering and low attenuation. E) Thin-cap fibroatheroma (TCFA) with circumferential lipid constituents and low signal backscattering. A minimum cap thickness of 60 µm (two arrowheads) was measured. F) A lipid-rich plaque with a superficial signal-rich band and high signal attenuation identifying macrophages (arrows from six to nine o’clock). G) Microchannels (arrows) appear as multiple voids with low signal backscattering. H) A cholesterol crystal (arrow) appears as a linear, high-intensity signal within the plaque. The asterisk indicates the guidewire shadow. OCT: optical coherence tomography; STEMI: ST-segment elevation myocardial infarction
The outlines of the lumen and flow areas were drawn for measurements on a cross-sectional image using the multiple-point trace function. The lumen area and flow area were measured for each interval within the culprit lesion, and the thrombus area (TA) was defined as the lumen area minus the flow area for the entire measurable segment14 (Supplementary Figure 1A, Supplementary Figure 1B). The minimum lumen area (MLA) was defined as the smallest lumen area measured along the length of the culprit lesion. In ruptured plaques, quantitative measurements, including lumen area and ruptured cavity area, were performed using manual tracing at each frame interval (0.2 mm) throughout the ruptured plaque. The maximal ruptured cavity site was identified based on these tracings, and the ruptured cavity area at the site of the largest intra-plaque cavity was reported15 (Supplementary Figure 1C, Supplementary Figure 1D). The details of OCT imaging acquisition and analysis methods are reported in Supplementary Appendix 1.
CLINICAL OUTCOMES
Major adverse cardiovascular events (MACE) (i.e., the composite of cardiac death or recurrent myocardial infarction related to the target vessel, clinically driven target lesion revascularisation, and unstable angina requiring hospitalisation), and individual components of MACE were assessed at one year. The definitions of the individual components of MACE are summarised in Supplementary Appendix 1.
STATISTICAL ANALYSIS
Categorical data are expressed as counts and percentages and were compared using χ2 tests or Fisher’s exact test. Continuous variables are presented as the mean±standard deviation and were compared using unpaired Student’s t-tests. A p-value <0.05 was considered statistically significant. Interobserver and intraobserver variability was assessed using kappa statistics for the major morphometric OCT variables and intraclass correlation coefficients for continuous measurements. All statistical analyses were performed using SPSS, Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
CLINICAL CHARACTERISTICS
STEMI onset with exertion was significantly less common in patients with PIA than in those without PIA (Table 1). Infarct size, as determined by the peak creatine kinase-myocardial band (CK-MB) levels and peak troponin I levels, was smaller in patients with PIA than in those without PIA (Table 1).
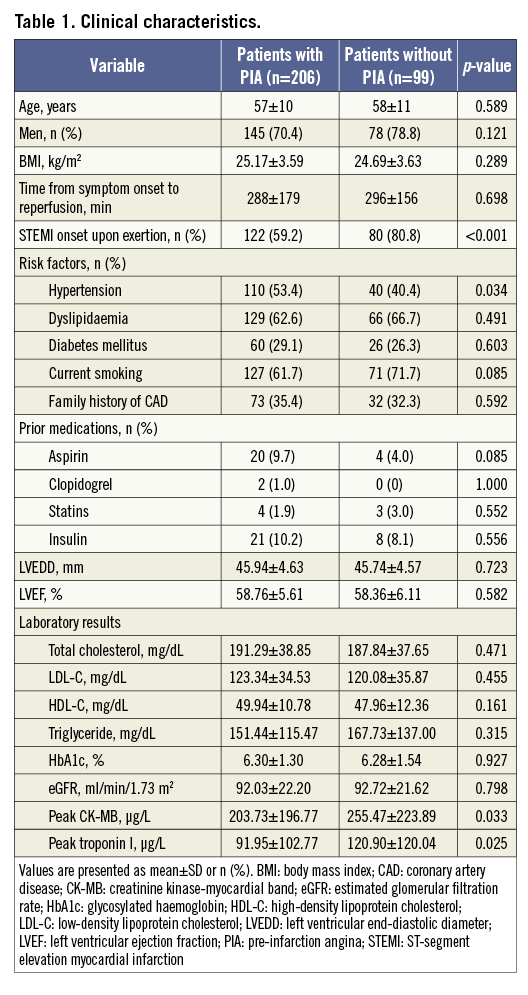
PIA CHARACTERISTICS
The PIA and clinical characteristics, and the angiographic and OCT findings according to the status of PIA are reported in Supplementary Appendix 2. The incidence and type of PIA are reported in Supplementary Figure 2. The time intervals between the last episode of angina and the STEMI onset in patients with PIA are shown in Supplementary Figure 3. Clinical characteristics, angiography, and OCT findings in patients without PIA and with unstable or stable PIA are provided in Supplementary Table 1-Supplementary Table 3.
ANGIOGRAPHIC FINDINGS
Collateral vessels were more frequently observed in patients with PIA than in those without PIA, although the presence of good collaterals was not significantly different between the two groups (Table 2).
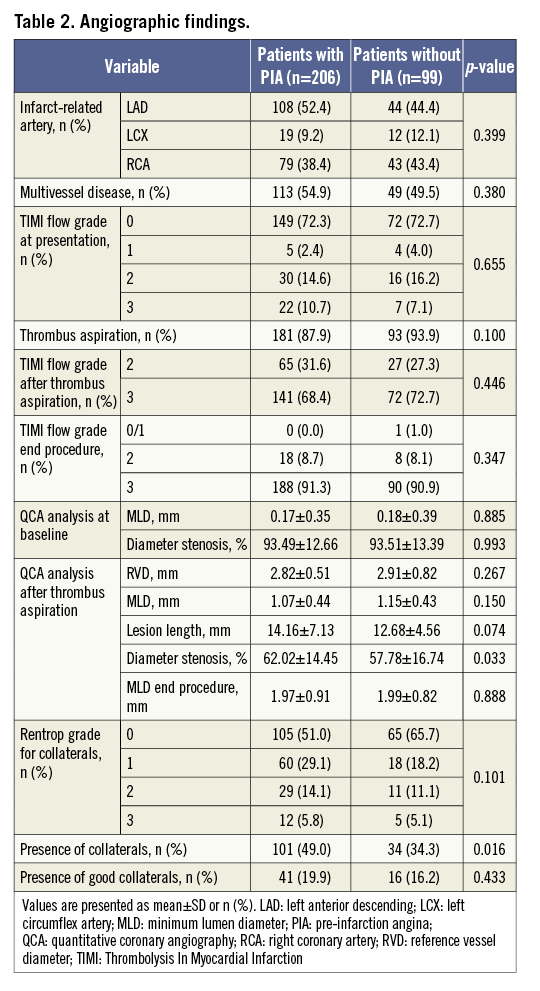
OCT FINDINGS
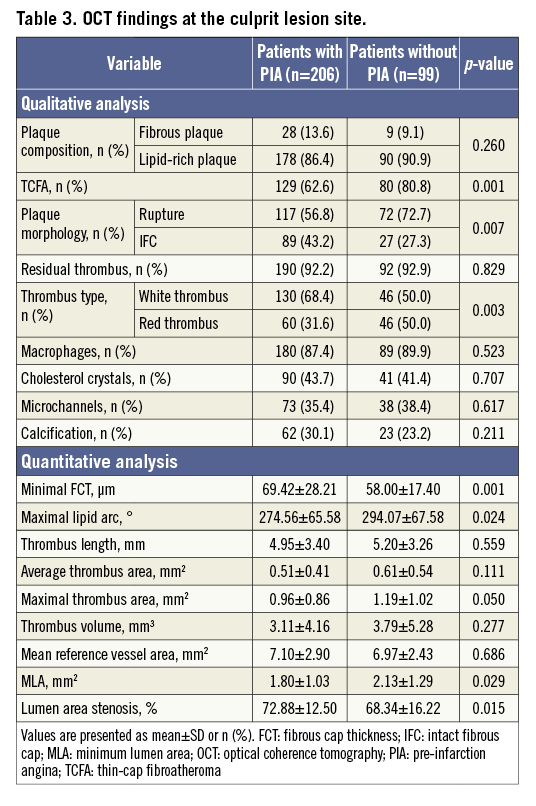
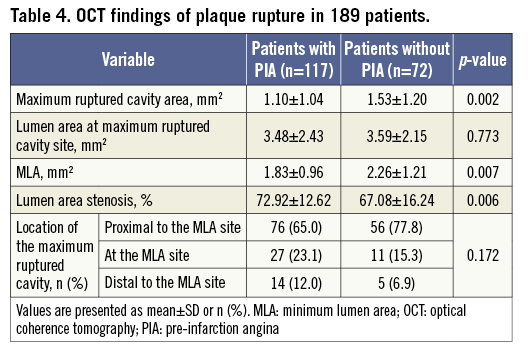
Patients with PIA exhibited a significantly lower incidence of thin-cap fibroatheroma (TCFA) and plaque rupture, a thicker minimal fibrous cap, a smaller lipid core, more white residual thrombi and more severe luminal narrowing at the culprit lesions than patients without PIA (Table 3). The maximum ruptured cavity area and the MLA were significantly smaller in patients with PIA than in patients without PIA (Table 4). The interobserver and intraobserver kappa statistics for the qualitative assessment of plaque rupture/IFC were 0.917 and 0.922, respectively. The intraclass correlations for the maximum ruptured cavity area were 0.988 and 0.990, respectively.
CLINICAL OUTCOMES
Clinical follow-up data were available for 301 of the 305 patients (98.7%). The median follow-up time was 13.8±3.8 months. No significant differences in clinical outcomes were observed at the one-year follow-up (Supplementary Table 4).
Discussion
This is the first in vivo study to compare prospectively the culprit lesion phenotypes observed by OCT in patients with a first STEMI with or without PIA. The main findings are as follows: PIA occurred frequently in patients with a first STEMI and was significantly associated with a thicker fibrous cap, smaller lipid arc, fewer TCFAs, lower incidences of plaque rupture and red residual thrombi, smaller rupture cavities, and more severe luminal narrowing at the sites of culprit lesions.
PIA: INCIDENCE AND IMPACT ON INFARCT SIZE
In this study, the incidence of PIA in patients with a first STEMI was 68%. It has been demonstrated that many patients suffering from AMI may experience PIA in the hours, days, or weeks preceding the acute coronary event12. The reported incidence of PIA in the literature varies from 10% to 69%, reflecting an extremely broad span that may be related to differences in the definitions of PIA, the study populations recruited, or the methods used to collect patient information12,16. In our prospective study, we confirmed that unstable patterns of PIA are very common in patients presenting with a first STEMI, with 80% of cases of instability occurring within one week of myocardial infarction.
Numerous studies conducted in STEMI patients have shown associations of PIA with smaller infarct size, preserved left ventricular function, less microvascular damage and better clinical outcomes2-5. The beneficial effects of PIA have been proposed to be related to ischaemic preconditioning, the development of collateral vessels, improved reperfusion and preserved microvascular circulation4,6-8. The results of the present study support existing data indicating that PIA is associated with smaller infarct sizes and a higher incidence of collaterals in patients with a first STEMI. The lack of significant differences in the degree of collaterals and the presence of good collaterals between patients with and without PIA could be explained by the exclusion of patients with prior myocardial infarction or prior coronary interventions. In our study, a low rate of target vessel-related events with no difference in outcome was observed at the one-year follow-up. The high success rate of reperfusion achieved with primary PCI in both groups with normal ejection fraction and preserved left ventricular diameters may have concealed further differences in outcome.
PIA AND PLAQUE MORPHOLOGIES
Consistent with all previous investigations, we found that plaque rupture was the most common cause of coronary thrombosis in STEMI, regardless of the presence or absence of PIA17. However, in patients with PIA, we observed a lower incidence of TCFA and plaque rupture, a smaller lipid core with a smaller rupture cavity area and a significantly thicker fibrous cap than in patients without PIA, despite similar lipid-rich culprit plaque constituents. Further, patients with PIA less frequently experienced the symptom onset upon exertion compared with those without PIA, in line with the results of a prior study18. This observation may simply reflect a reduced daily physical activity in patients with PIA, especially those with rest angina. Rupture of a TCFA and subsequent thrombosis may occur spontaneously but, in some cases, a temporary increase in emotional or physical stress may act as a final trigger for the clinical event19. In our study, patients with sudden onset of STEMI without anticipating angina had a significantly thinner fibrous cap and larger lipid core with a higher incidence of TCFA than patients with PIA, suggesting that patients without PIA may have a lower threshold for abrupt coronary occlusion due to greater vulnerability of the underlying plaque than patients with anticipatory symptoms. The greater intrinsic plaque vulnerability (thinner cap with larger lipid core) and the more frequent myocardial infarction induced by exertion observed in patients without PIA could explain the higher rate of plaque rupture and the larger ruptured cavity area in these patients.
PIA AND THROMBUS COMPONENTS
In the current study, we did not observe any difference in thrombus volume between patients with and without PIA after manual thrombectomy. However, we found differences in thrombus composition. Patients with PIA had significantly more white (platelet-rich) thrombi, while those without PIA had more red (erythrocyte-rich) residual thrombi. Differences in culprit plaque characteristics might have contributed considerably to the difference in thrombus composition observed in patients with/without PIA. We noticed a greater intrinsic plaque vulnerability (thinner cap with larger lipid core) and increased incidence of plaque rupture in patients without PIA and a higher rate of IFC atheroma in patients with PIA. We showed in prior studies that ruptured plaques are mostly associated with red thrombi while eroded plaques without rupture are preferentially accompanied by white thrombi13,20. The difference in thrombus components detected by OCT between patients with and without PIA is consistent with these pathophysiological principles.
PIA AND LESION SEVERITY
The current OCT study demonstrated that PIA is associated with more severe lumen narrowing than that observed in patients without PIA, which agrees with the results of a prior study21. Although the difference in lesion severity between the two groups was statistically significant, the difference was marginal from a clinical standpoint. In this study, patients without PIA showed less severe luminal narrowing but a larger lipid arc with a higher incidence of TCFA at the culprit site. This result suggests that attained compensatory enlargement could be responsible for preventing significant stenosis; however, lesions with a large plaque burden and positive remodelling may be more vulnerable to rupture leading to sudden thrombotic occlusion. Consistent with the results of a previous study22, we found that most STEMI events occur in the presence of significant lumen stenosis regardless of the presence or absence of PIA. Histopathological findings reported in some cases of AMI with PIA showed that the arterial lumen was partially narrowed by an old healed atheroma with organised thrombus23. Such a pathological process might be a possible explanation for the more severe luminal narrowing observed in patients with PIA compared with patients without PIA.
Limitations
This study has several limitations. First, this was an observational study conducted at a single centre. Although the data were prospectively collected and all patients were consecutively enrolled, several patients were excluded from the current analysis, possibly resulting in case selection bias. Therefore, this study cannot be generalised to all patients presenting with STEMI. Second, in patients with TIMI flow grade 0/1 or large angiographic filling defects, manual thrombectomy was performed to re-establish effective vessel patency, allowing safe and high-quality OCT imaging data collection at the culprit site. However, the potential effect of the thrombus aspiration catheter on superficial plaque integrity and atherothrombotic components assessed by OCT must be given serious consideration. Third, the clinical descriptions of the symptoms characterising PIA were subjective. Although the use of a standardised questionnaire administered by researchers at multiple time points near the acute event to confirm the data on PIA may have improved the reporting quality, uncertainties remain regarding the reliability of our group classifications. Fourth, histopathological examination of thrombus aspirates was not performed. Therefore, no conclusions can be drawn regarding the relationships of thrombus compositions to the presence and type of PIA. Fifth, because a large residual thrombus burden at the culprit site may obscure the underlying plaque morphology and impede accurate measurement of the lumen area, we excluded patients with massive residual thrombus precluding visualisation from the analysis.
Conclusions
The present study demonstrates that patients with a first STEMI and PIA have a significantly lower incidence of TCFA and plaque rupture, a smaller ruptured cavity area, more white residual thrombi, and more severe lumen stenosis at the culprit lesion compared with patients without PIA.
| Impact on daily practice Differences in the in vivo culprit lesion morphologies observed in STEMI patients with PIA may suggest different underlying pathophysiological mechanisms that may eventually enable tailored treatment strategies. Additional studies are warranted to elucidate better the impact of PIA on the development of STEMI. Increased public awareness of the clinical relevance of prodromal symptoms that precede STEMI is needed, especially in large countries where primary PCI systems are currently being developed. |
Funding
This study was funded by the National Key R&D Program of China (grant no. 2016YFC1301100), and the National Natural Science Foundation of China (grant no. 30871064/C140401 and no. 81330033).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Methods.
Supplementary Appendix 2. Results.
Supplementary Figure 1. Methods for the quantitative assessment of ruptured plaques and thrombi on OCT images.
Supplementary Figure 2. Incidence and type of PIA in patients with a first STEMI.
Supplementary Figure 3. Time interval between the last episode of angina and STEMI onset in patients with PIA.
Supplementary Table 1. Patient clinical characteristics according to the status of PIA.
Supplementary Table 2. Angiographic findings according to the status of PIA.
Supplementary Table 3. OCT findings according to the status of PIA.
Supplementary Table 4. Clinical outcomes at the 1-year follow-up.
To read the full content of this article, please download the PDF.

