Abstract
Background: Early spontaneous reperfusion (ESR) is not an uncommon phenomenon in clinical settings.
Aims: The aim of this study was to detect potential mechanisms of ESR in patients with STEMI.
Methods: This prospective study enrolled a total of 241 consecutive patients with STEMI undergoing optical coherence tomography (OCT) from July 2016 to August 2019. Forty-five patients (18.7%) met angiographic ESR criteria (TIMI 3 flow on the initial angiogram). Among those without ESR (TIMI 0 flow on initial angiogram), 45 patients were assigned to the control group according to propensity score matching with the ESR group.
Results: Although the baseline characteristics of the groups were comparable, non-ruptured plaque (62.2% vs 35.6%) predominated and plaque rupture (37.8% vs 64.4%) was less common in the ESR group (p=0.011). Red thrombus (44.4% vs 77.8%) was also less common in the ESR group (p=0.001). Lastly, compared to the control group, the ESR group underwent fewer emergent stent placements (68.9% vs 91.1%, p=0.008).
Conclusions: Relief of coronary occlusion induced by a non-ruptured plaque may contribute to ESR in patients with STEMI.
Introduction
Acute ST-segment elevation myocardial infarction (STEMI) is a life-threatening situation. Reperfusion, using techniques such as primary percutaneous coronary intervention (PCI), is recommended by the current guidelines to restore coronary blood flow promptly and save the maximum amount of jeopardised cardiomyocytes1,2. Interestingly, up to 30% of patients with STEMI have an initial Thrombolysis In Myocardial Infarction (TIMI) 3 flow during emergent angiography, without undergoing any invasive intervention or fibrinolytic therapy. This is known as early spontaneous reperfusion (ESR) and has been examined in previous studies3,4,5,6. ESR is associated with smaller infarct size, less stent placement as well as favourable short-term outcomes7. Although endogenous lysis of thrombi or relief of coronary spasms can cause ESR4, its pathologic and pathophysiologic mechanisms remain undefined. Furthermore, PCI timing (immediate or delayed) in patients with ESR is debatable; no specific recommendations are included in the current guidelines.
Optical coherence tomography (OCT) is an intracoronary imaging modality with a tenfold higher resolution (10-15 μm) compared to intravascular ultrasound (IVUS)8. OCT is recommended in the current guidelines for optimising stent implantation in selected patients9. OCT has also been used to identify potential mechanisms (such as plaque rupture, plaque erosion and calcified nodule) of STEMI10,11,12. OCT is more accurate than angiography or IVUS for detecting subtle morphological details of the culprit lesion and is both feasible and reproducible.
We sought to identify the potential mechanisms of ESR using OCT. We hypothesised that ESR may possess unique morphological characteristics.
Methods
STUDY DESIGN AND PATIENTS
This prospective study enrolled consecutive patients with STEMI who underwent OCT in a single centre from July 2016 to August 2019. Patients whose coronary blood flow of the culprit vessel achieved TIMI 3 flow on the initial angiogram, followed by OCT assessment, were assigned to the ESR group. The control group consisted of patients who had TIMI 0 flow, and who underwent OCT after achieving TIMI 3 flow by thrombus aspiration. The ESR and control groups were propensity score matched for age, sex, hypertension, diabetes mellitus, the location of the infarct-related artery, time from dual antiplatelet therapy (DAPT) to procedure and duration from the onset of symptoms to performance of coronary angiography. All patients underwent immediate OCT assessments. The exclusion criteria are reported in Supplementary Appendix 1.
This study was approved by the Xuanwu Hospital’s institutional review board, and informed consent was obtained from all patients.
CATHETERISATION PROCEDURES
All catheterisation procedures were carried out by experienced operators according to the hospital’s standard protocol. Coronary angiography was performed via a transradial or transfemoral approach with the use of a 6 Fr sheath and the default right radial access. Manual thrombus aspiration was performed in non-ESR patients using the Export Advance™ aspiration catheter (Medtronic, Minneapolis, MN, USA). After OCT assessment, the decision whether or not to stent the culprit lesion was based on the operator’s discretion, according to visual estimation of diameter stenosis ≥70%. ESR was defined as a culprit coronary vessel blood flow of TIMI 3 on the initial angiogram13. The definition of vasospasm is reported in Supplementary Appendix 1.
OCT IMAGING ANALYSIS
Intracoronary images of the culprit lesion were acquired using frequency domain OCT (FD-OCT) through a non-occlusive technique with the C7XR™ system (Abbott Vascular, Santa Clara, CA, USA). The OCT images were identified by two experienced physicians using offline software (Abbott Vascular). The details of plaque characteristics and thrombus analysis are shown in Supplementary Appendix 1.
MEDICAL TREATMENT (Supplementary Appendix 1)
STATISTICAL ANALYSIS
Continuous variables are shown as means±SDs for normally distributed data or medians (25th-75th percentiles) for non-normally distributed data. Between-group differences were tested using an independent samples t-test or the Mann-Whitney U test. Categorical data are presented as counts (proportions) and compared using the χ2 test or Fisher’s exact test (if the expected cell value was <5). The propensity score analysis used a logistic regression model. We included age, sex, hypertension, diabetes mellitus, the location of the infarct-related artery, time from DAPT to the procedure, and the duration from symptom onset to coronary angiography. The variables with significant between-group differences – as indicated by p<0.05 during univariate analysis – were propensity score matched using a 1:1 ratio and based on an estimated calliper width of 0.1. The balance was deemed satisfactory when the standard mean differences were less than 10% (Supplementary Table 1). Statistical analysis was performed with SPSS, Version 22.0 (IBM Corp., Armonk, NY, USA).
ENDPOINTS
The primary endpoint was culprit lesion morphology in patients with ESR, as identified by OCT.
Results
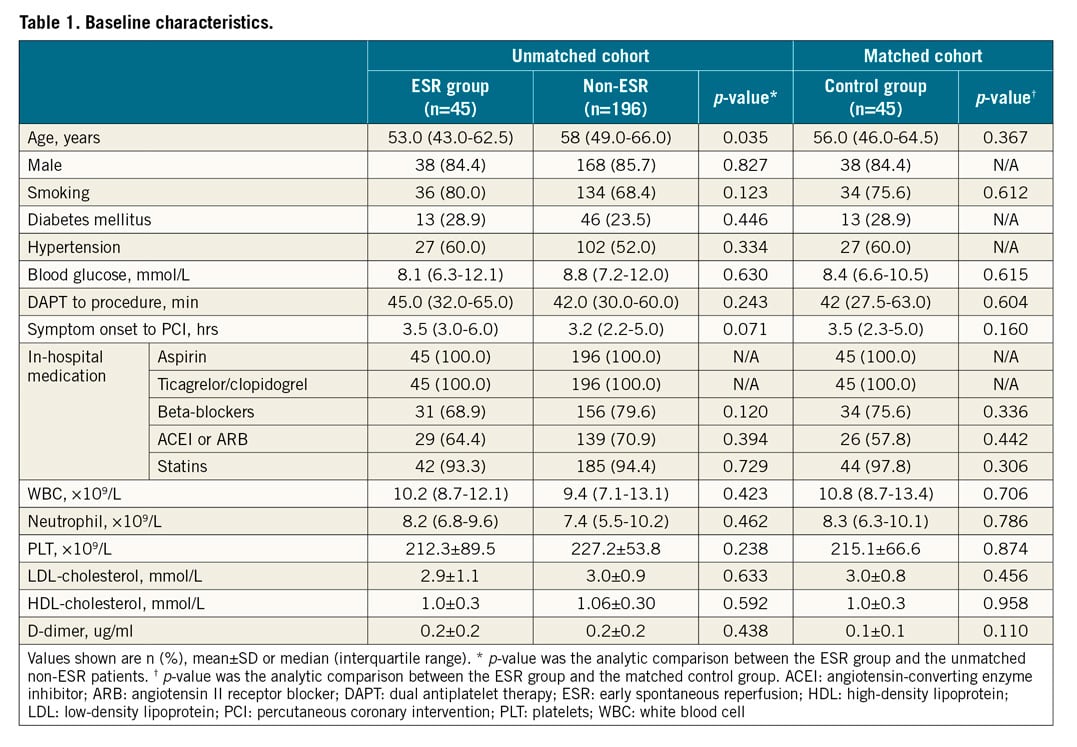
BASELINE CHARACTERISTICS
The study flow chart is provided in Figure 1. Among the 241 patients with STEMI and analysable OCT images, 45 (18.7%) met the angiographic ESR criteria. Patients in the ESR group were younger (53.0 [43.0-62.5] vs 58 [49.0-66.0] years, p=0.035). After propensity score matching, there were no significant between-group differences in baseline characteristics between the ESR (n=45) and control groups (n=45). The baseline characteristics of all patients are included in Table 1, Supplementary Table 2 and Supplementary Table 3.
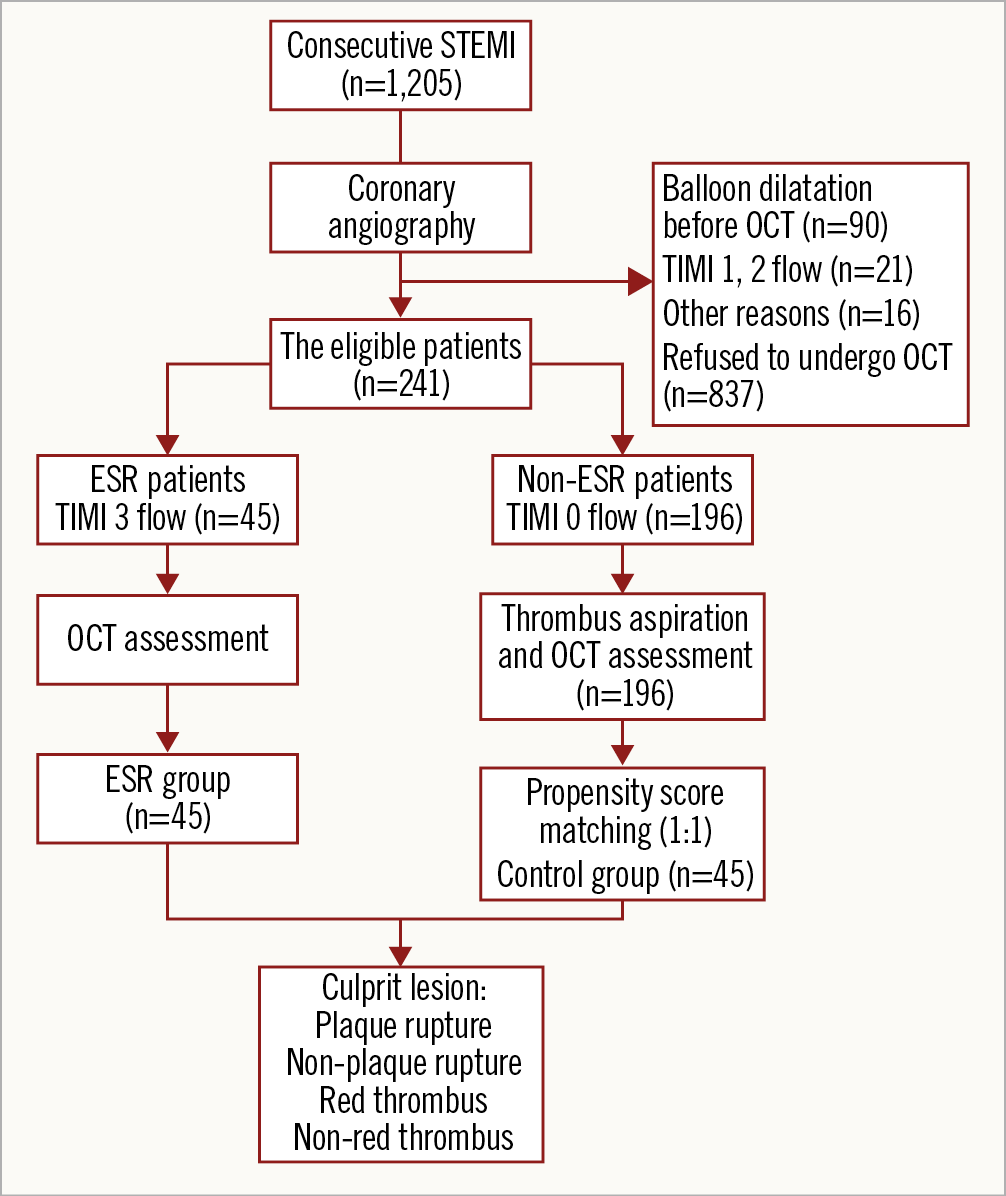
Figure 1. Study flow chart. ESR: early spontaneous reperfusion; OCT: optical coherence tomography
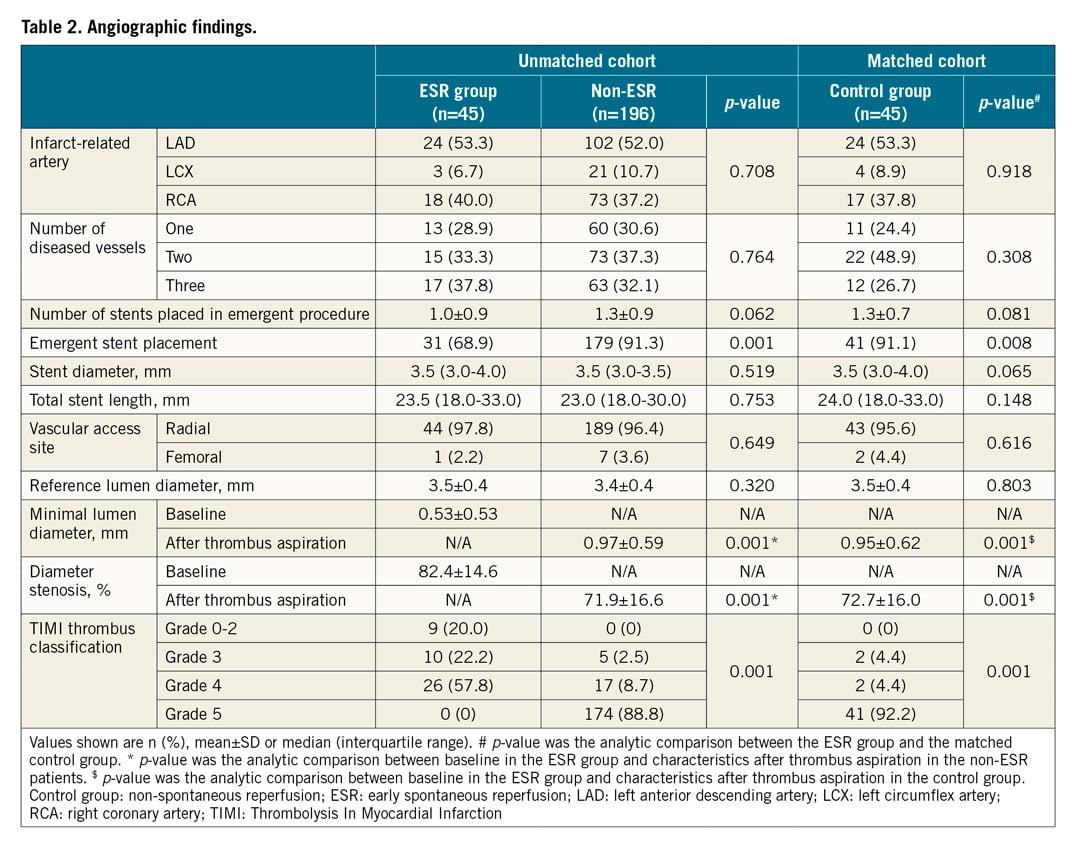
ANGIOGRAPHIC FINDINGS
The coronary angiography data for all patients are shown in Table 2. Emergent stent placement was significantly less frequent in the ESR group compared to controls (68.9% vs 91.1%, p=0.008). The minimal lumen diameter was significantly larger in the ESR group than in controls (0.53±0.53 vs 0.01±0.58, p<0.001). The TIMI thrombus classification was lower in the ESR group than that in controls (p<0.001).
OCT FINDINGS
The OCT findings in matched and unmatched populations are presented in Table 3 and Supplementary Table 4. ESR group patients had more non-plaque ruptures (62.2% vs 35.6%), whereas plaque ruptures (37.8% vs 64.4%) were less common in the ESR group. Moreover, non-red thrombi (55.6%) were more frequent in the ESR group, while red thrombi (77.8%) were frequent in the control group. The minimal lumen area was significantly larger in the ESR group than in the control group (2.5±1.7 vs 1.8±1.0 mm², p=0.025) (Figure 2, Figure 3).
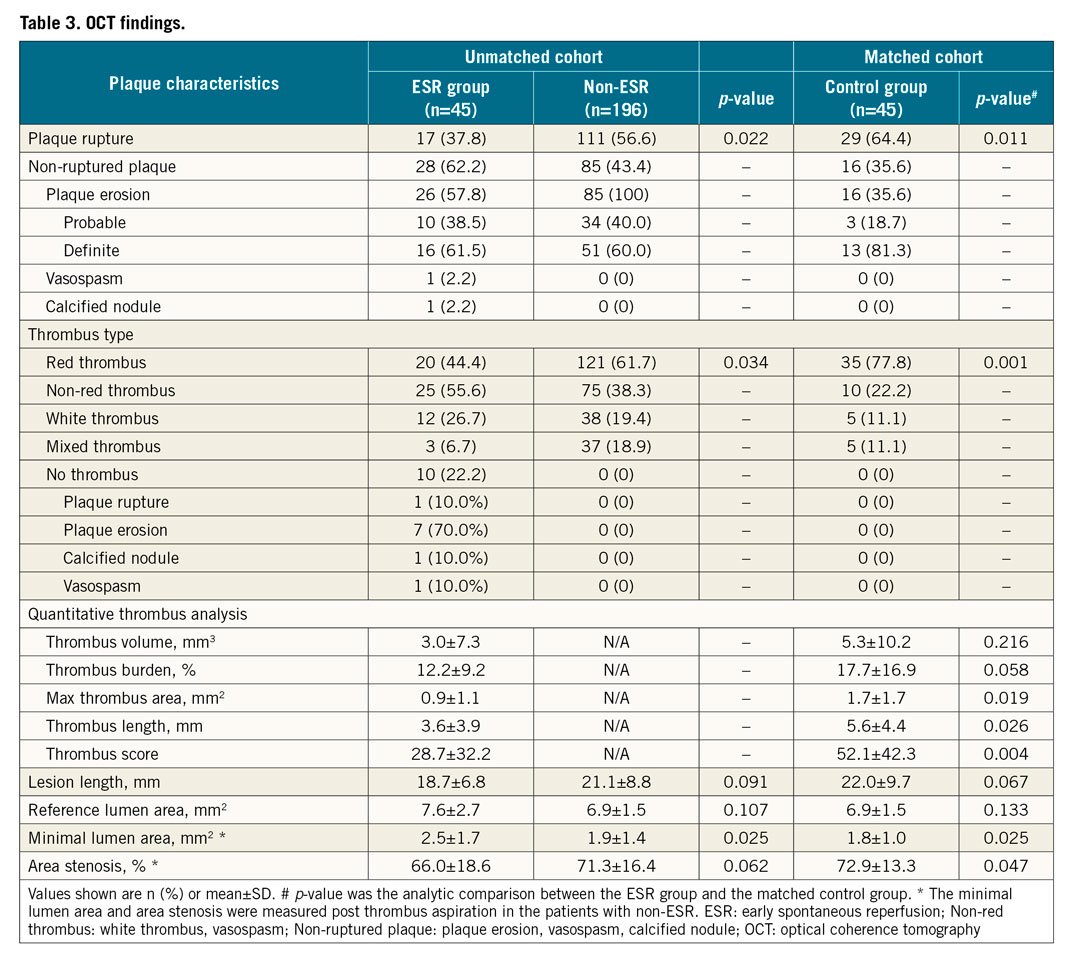
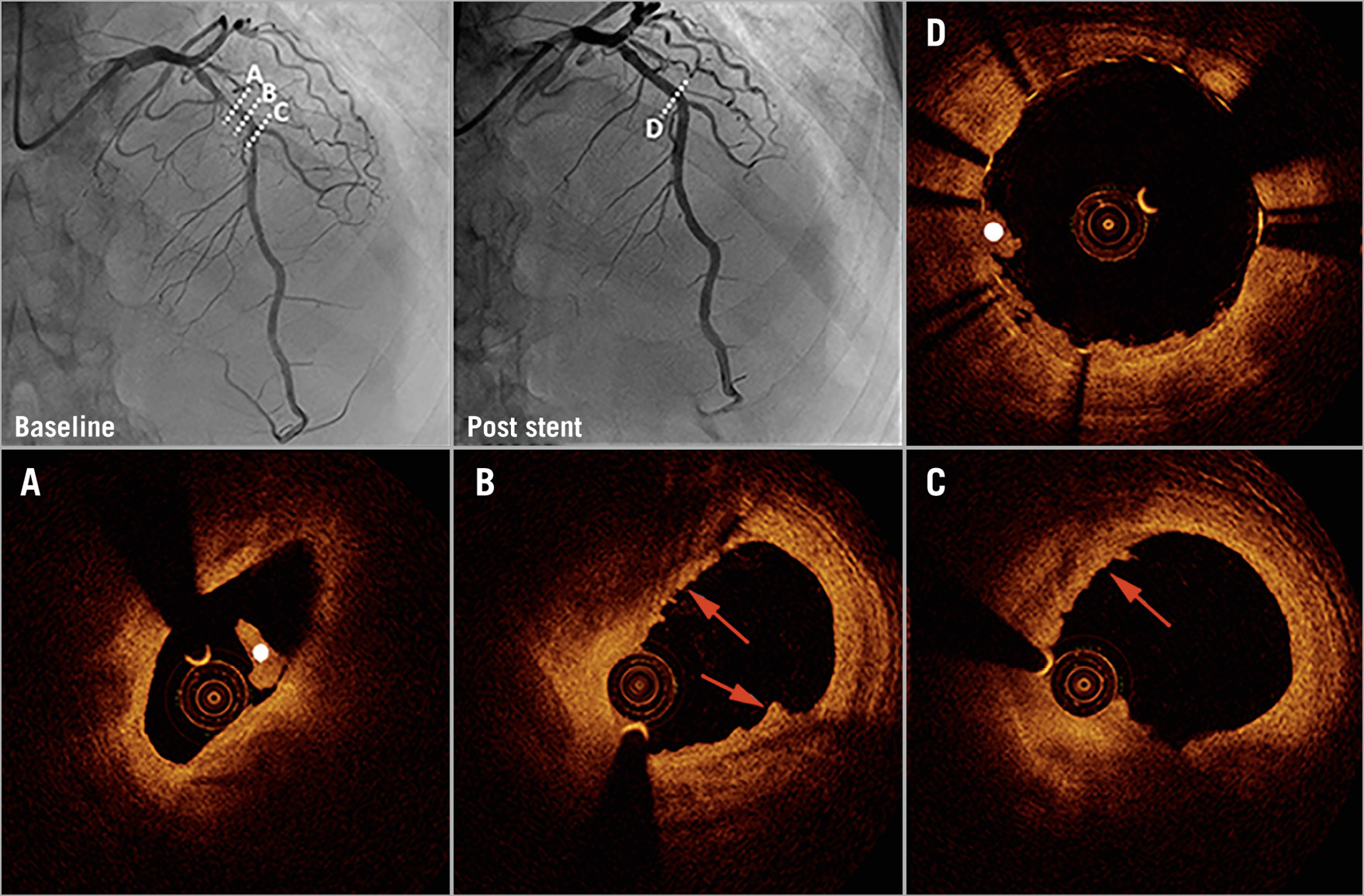
Figure 2. Coronary angiography and optical coherence tomography in early spontaneous reperfusion. Baseline angiography (upper left) shows a patient with TIMI 3 flow. Optical coherence tomography (OCT) shows plaque erosion (red arrows) and white thrombus (white dot) (A-C). Coronary angiography (upper middle) and OCT show imaging of the region after stent implantation (D).
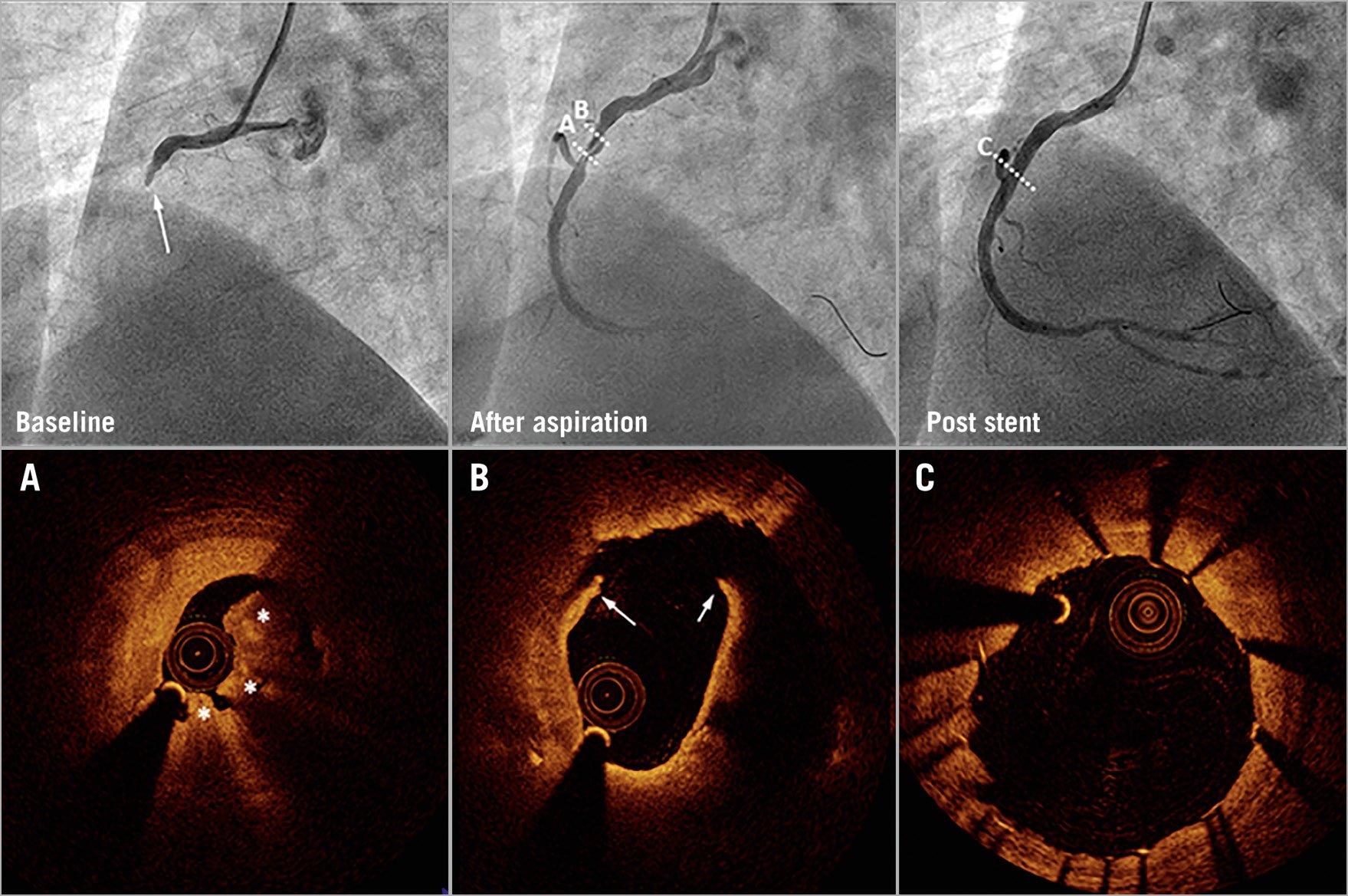
Figure 3. Coronary angiography and optical coherence tomography in a control patient. Baseline angiography (upper left) shows a patient with complete occlusion and TIMI 0 flow. After thrombus aspiration, the coronary arterial flow was restored to TIMI 3 flow. Optical coherence tomography (OCT) shows plaque rupture (white arrows) and red thrombus (white stars) (A & B). Angiography and OCT show imaging of the region after stent implantation (C).
Discussion
In this prospective study, we defined ESR in 20% of patients with STEMI. Compared with controls, the ESR group demonstrated a lower rate of plaque rupture and a higher rate of plaque erosion. In the ESR group, non-red thrombi were more common, whereas red thrombus accounted for 77.8% of control group cases. Significantly fewer patients in the ESR group underwent emergent stent implantation compared to the control group.
ESR is not an uncommon phenomenon in the clinical setting. We previously reported that 18.4% of patients with STEMI had angiographic patency in the infarct-related artery within 12 hours of chest pain onset7. Four primary angioplasty in myocardial infarction (PAMI) primary PCI trials found that 16.0% of patients had ESR13. A recent large-scale study found that the prevalence of ESR was 17.0% among patients with ST-segment elevation acute coronary syndrome (ACS).
Although some factors – such as endogenous activity of thrombolysis, the size of thrombus, atherosclerotic conditions and vasomotor tone – were believed to be associated with ESR4,7,14, the mechanisms of plaque morphology remain uncertain. Generally, coronary occlusion due to a ruptured atherosclerotic plaque followed by immediate thrombosis was regarded as the main cause (70.0%) of STEMI12. Also, plaque erosion and calcified nodules contributed to thrombi formation in STEMI without plaque rupture. These patients exhibited either missed endothelium replaced by exposed intima or disruptive nodular calcifications that protruded into the lumen13. In the current study, non-ruptured plaque (62.2%) was predominant in the ESR group. To our knowledge, this is the first report which suggests that ESR is mainly a result of non-ruptured plaque-related transient coronary occlusion.
There are four potential explanations for our findings. In coronary thrombosis, the initial flow obstruction is usually caused by an early and fragile platelet thrombus. The fate of thrombus is largely determined by the balance among platelet activation, pro-coagulant factors, antithrombotic therapy and the fibrinolytic system. Non-ruptured plaques usually do not contain a large necrotic core exposed to circulating blood10. This results in lower local thrombogenicity15. A previous study indicated that white thrombus, identified by OCT, was predominant in plaque erosion and calcified nodules16. Our ESR patients exhibited results similar to ACS patients in a previous study. The occurrence of ESR has been associated with antithrombotic and vasodilator drug treatment before primary PCI in previous studies17. The dissolution of the white thrombus following re-opening of the infarct-related artery might be one of the main mechanisms underlying ESR. Moreover, in non-ruptured plaque-related STEMI, the thrombus volume and degree of lumen stenosis are smaller. Both of these factors might contribute to a higher incidence of ESR in this group4. Kramer et al demonstrated that plaque erosion (60.0%) had a higher incidence of <75.0% area stenosis when compared with plaque rupture (35.4%)18. Jia et al reported that the mean diameter stenosis in plaque erosion was 55.4% and about 70.0% of patients had diameter stenosis <70.0%10. In our study, the ESR patients had a higher minimal lumen diameter and minimal lumen area. Also, 2.2% of ESR cases were diagnosed with coronary spasm, yet this was not the case for the control group. ESR may be followed by prompt relief of coronary spasm. Lastly, lower thrombus burden was another likely mechanism of ESR. The study by Lee et al indicated that heavy thrombi were independent predictors of less frequent ESR in patients with STEMI4, in agreement with our findings.
Patients with ESR usually have resolved symptoms and normalised ST segments, similar to “transient STEMI”19,20. Patients with transient STEMI had more rapid efficient endogenous fibrinolysis and reduced platelet reactivity21. Clinically, these patients had less extensive coronary artery disease, better coronary flow on angiography, lower peak creatine kinase levels, and higher left ventricular ejection fractions22. Another plaque feature of ESR was analogous to “non-ST-segment elevation ACS”. In a previous study23, plaque erosion (61.5% vs 38.5%) and calcified nodules (100% vs 0%) were more common, but there were fewer instances of plaque rupture (29.1% vs 70.9%) in patients with non-ST-segment elevation ACS compared to STEMI.
Although our ESR group had a higher percentage of three-vessel disease, the difference was non-significant. Lee et al4 also reported that the number of diseased vessels had no significant relationship with the occurrence of ESR. Moreover, we found that the lumen area and diameter stenosis were associated with ESR prevalence. Therefore, the relation between the number of diseased vessels and ESR may be influenced by other factors. According to the clinical manifestation, morphological features, and prognosis, ESR seems to be an intermediate state between STEMI and non-ST-segment elevation ACS.
Jian Wang et al17 found that most cases of ESR were not actually spontaneous but rather the result of antithrombotic and vasodilator drug treatment before primary PCI. However, our data did not show a significant difference in pre-hospital antiplatelet drug treatment between the ESR group and the control group. Therefore, future studies should examine whether antithrombotic drugs are associated with ESR occurrence. Moreover, both the TRANSIENT trial19,20 and the EROSION study10 showed no differences in short-term outcomes between primary and delayed PCI in patients with either transient STEMI or non-ruptured plaque-induced acute myocardial infarction. OCT was performed in our study, but the invasive strategy was still left to the operator’s discretion, according to angiographic images. Therefore, our results suggest that more clinical studies should explore whether plaque morphology could guide the decision concerning interventional treatment in ESR patients.
Study limitations
Limitations of the study should be acknowledged. First, the data were collected at a single medical centre from a relatively small study cohort. Second, it was performed in a heterogeneous, non-selected population within a clinical setting. Propensity score matching may mitigate bias after adjusting for confounding factors, but unmeasured indicators leave room for residual bias. Third, thrombus aspiration was performed in the non-ESR patients, resulting in plaque modification and inaccurate assessment. Fourth, due to the balloon dilatation that would affect the plaque morphologic characteristics by OCT, we excluded patients with more severe stenosis, and a selection bias might have existed. Fifth, because OCT was not covered by health insurance, about 70 percent of the 1,205 STEMI patients were unable to complete OCT; this could also have influenced patient selection. Sixth, we excluded patients with TIMI 1 or 2 flow; this constitutes an additional source of potential selection bias.
Conclusions
Non-ruptured plaque is closely involved in the potential mechanism of ESR. Since ESR is associated with favourable clinical outcomes, future studies should explore whether plaque morphology assessment could guide the decision concerning interventional treatment in these patients.
|
Impact on daily practice Non-ruptured plaque is a potential mechanism of ESR. Future studies should explore whether plaque morphology could guide therapeutic strategy in ESR patients. |
Funding
This work was supported by grant No. 81470491 from the National Natural Science Foundation of China and No. 7192078 from Beijing Municipal Natural Science Foundation to Dr Jing Li, and grant No. 2018-2-7082 from the Capital’s Funds for Health Improvement and Research (CFH) to Dr Jincheng Guo.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

