Acute myocardial infarction (AMI), with its subclassification into non-ST-elevation (NSTEMI) and ST-elevation myocardial infarction (STEMI), remains the most frequent cause of death on a global scale. Early reperfusion of the occluded coronary artery is an established groundbreaking treatment reducing mortality and morbidity in patients suffering AMI1. Extensive knowledge has been gained from seminal autopsy studies to determine the causes of coronary occlusion, which include plaque rupture, erosion, and calcified nodule2; nevertheless, therapeutic options remain limited to early reperfusion utilising coronary stents. Door-to-balloon time is the most critical factor for salvaging myocardium at risk in the setting of coronary thrombosis; however, a non-negligible fraction of patients presents with early spontaneous reperfusion (ESR), a phenomenon confirmed in major trials evaluating patients presenting with AMI3. Against this background, the most effective treatment for patients with ESR has not been established to date. Intravascular imaging using optical coherence tomography (OCT) has revolutionised the understanding of AMI and, for the first time, may permit differential treatment strategies based on underlying plaque pathology4. Whether certain plaque morphologies are associated with presentation as ESR has remained unknown until the publication by Guo et al5.
In the current issue of EuroIntervention, Guo and colleagues describe the findings in 241 patients presenting with STEMI and undergoing OCT imaging, where 45 of 241 (18.7%) patients had ESR defined as Thrombolysis In Myocardial Infarction (TIMI) 3 flow on the initial angiogram. Patients with ESR were overall younger as compared to STEMI patients without spontaneous reperfusion (control group) (53 [43-62.5] vs 58 [49-66] years, p=0.035). To overcome important imbalances in patient characteristics, the ESR group was compared to 45 propensity score-matched patients with STEMI and TIMI 0 flow. The authors report that non-ruptured plaques were significantly more frequent in the ESR versus the control group (62.2% vs 35.6%), while plaque ruptures were significantly more frequent in the control group (37.8% vs 64.4%). Furthermore, the presence of red thrombus was significantly more frequent in the control group (77.8% vs 44.4%). Therefore, it is not surprising that control patients needed stent implantation more emergently as compared to patients with ESR (91.1% vs 68.9%). In addition, OCT data showed larger minimal lumen area in patients presenting with ESR compared to the control group (2.5±1.7 vs 1.8±1.0 mm2).
The main findings of the current study align with key observations from prior research evaluating differences in plaque morphology among patients presenting with AMI. Non-ruptured plaques (i.e., plaque erosions) tend to show less severe vessel narrowing, less plaque burden, and smaller thrombus volume as compared to plaque rupture6,7, which supports the findings that patients with plaque erosion more likely present with non-STE-ACS8. Pathologic studies showed that white thrombus is friable and forms at the site of rupture as well as in erosion, while red thrombus is characteristically associated with slow blood flow, which is usually observed in venous thrombosis; however, propagated thrombus is also red and mostly observed in total occlusions9 (Figure 1). Erosion lesions have less luminal narrowing compared to plaque rupture, and consequently blood flow is often maintained in plaque erosion, which may provoke distal embolisation of non-occlusive thrombus. This has been documented in landmark pathology studies investigating intramyocardial thrombi in patients with plaque erosion and rupture (71% vs 42%)10. Thus, one possible mechanism of ESR is the dissolution and distal embolisation of thrombus in the culprit artery.
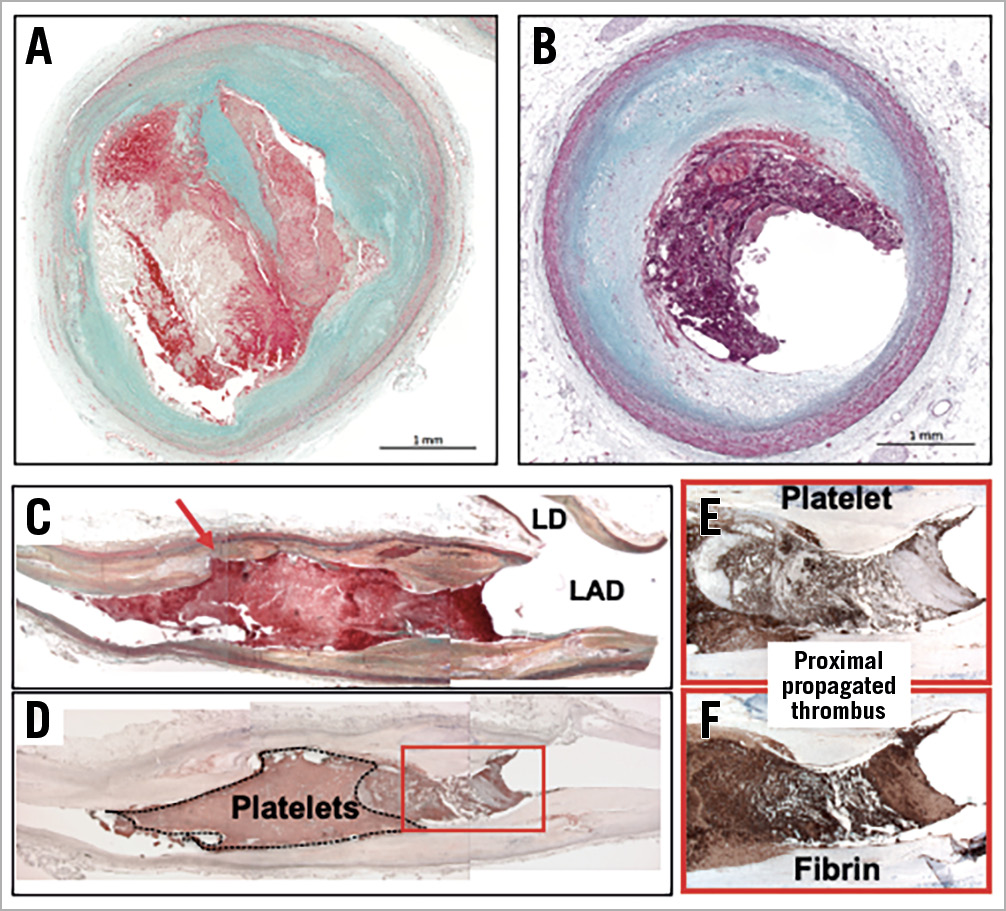
Figure 1. The pathology of plaque rupture and plaque erosion. A) Plaque rupture and (B) erosion histologic sections. Plaque ruptures tend to show more severe luminal stenosis (90% cross-sectional area stenosis), while plaque erosion has less severe narrowing (60% area stenosis). C) Longitudinal section of a left anterior descending artery (LAD) with plaque rupture. The red arrow shows the site of rupture with overlying white thrombus predominantly composed of platelets. Note the proximal thrombus is a propagated red thrombus and shows layered thrombus rich in fibrin and red cells (D-F). A) – C) Movat pentachrome stain, (D) CD61, (F) Fibrin II. Panels C and D are reproduced with permission from Alfonso F and Virmani R9.
Another possible mechanism of ESR is coronary artery vaso-spasm, which is associated with both plaque erosion and transient STEMI11,12. In contrast to lesions with plaque rupture, where necrotic core occupies large proportions of the intima and media, and causes destruction of structural vessel boundaries, both the internal elastic lamina (IEL) and external elastic lamina (EEL) with sandwiched medial wall remain intact and functional in plaque erosion, suggesting that vasospasm resulting from excessive contraction of medial smooth muscle cells is a possible underlying mechanism13. Although only one case (2.2%) showed vasospasm in the current study, the acetylcholine/ergonovine provocation test was not performed in this study and, consequently, the involvement of vasospasm cannot be confirmed. Evaluation of myocardial microvascular obstruction (e.g., myocardial blush grade14, cardiac magnetic resonance imaging, and coronary flow reserve) may be helpful to determine which aetiology, vasospasm or dissolving thrombus (with or without distal embolisation), contributed to ESR.
Although the authors must be congratulated for performing such timely research applying intravascular imaging in the setting of AMI, several limitations must be discussed to help interpret the relevance of the current study with regard to implications in patients presenting with AMI. While OCT imaging is able to capture relevant plaque morphologies, its specificity to identify the underlying plaque pathology in the presence of coronary thrombus is limited. Therefore, the authors should have considered using glycoprotein IIb/IIIa inhibitors for better visualisation. Also, it has been well documented in a number of OCT studies that thrombus aspiration using export catheters may significantly modify plaque morphology, especially in the setting of AMI and coronary thrombosis15, which raises concerns as to the appropriate classification of these cases. As residual thrombus burden obscures underlying plaque pathology, interpretation of OCT imaging data may be misleading in the setting of AMI. Furthermore, patients with TIMI 1-2 flow were not included in the current analysis, which leaves room for introduction of selection bias. Patients presenting with TIMI 1-2 flow in the setting of STEMI may exhibit significant residual thrombus burden in the presence of ESR causing slow flow because of downstream thrombus embolisation. Consequently, a large number of patients with different underlying plaque patholo-gies may have been missed in the current trial. As a matter of fact, presentation of patients with either NSTEMI or STEMI may be determined by patient-specific factors such as individual ischaemia burden, pre-conditioning, collateral development, mismatch of demand versus supply, and preclinical anticoagulation, rather than only plaque pathology. While differential treatment options stratified by underlying plaque pathology may indeed be useful in improving patient outcome as exemplified in the EROSION trial16, re-classification of patients based on the presence or absence of ESR may hold prognostic value. However, modifying therapeutic options will require additional prospective randomised trials.
Conflict of interest statement
M. Joner reports personal fees from OrbusNeich, AstraZeneca, and ReCor, grants and personal fees from Biotronik, Boston Scientific, and Edwards Lifesciences, and grant support from Amgen outside the submitted work. M. Joner has received funding from the German Center for Cardiovascular Research: DZHK (FKZ 81Z0600502; FKZ 81X2600526) and from the Leducq Foundation (grant agreement number 18CVD02). R. Virmani has received grant/research/clinical trial support from NIH-HL141425, Leducq Foundation Grant, 4C Medical, 4Tech, Abbott Vascular, Ablative Solutions, Absorption Systems, Advanced NanoTherapies, Aerwave Medical, Alivas, Amgen, Asahi Medical, Aurios Medical, Avantec Vascular, BD, Biosensors, Biotronik, Biotyx Medical, Bolt Medical, Boston Scientific, Canon, Cardiac Implants, Cardiawave, CardioMech, Cardionomic, CeloNova, Cerus, EndoVascular, Chansu Vascular Technologies, Childrens National, Concept Medical, Cook Medical, Cooper Health, Cormaze, CRL, Croivalve, CSI, Dexcom, Edwards Lifesciences, Elucid Bioimaging, eLum Technologies, Emboline, Endotronix, Envision, Filterlex, Imperative Care, Innovalve, Innovative Cardiovascular Solutions, Intact Vascular, Interface Biologics, Intershunt Technologies, Invatin, Lahav, Limflow, L&J Bio, Lutonix, Lyra Therapeutics, Mayo Clinic, Maywell, MDS, MedAlliance, Medanex, Medtronic, Mercator, MicroPort, Microvention, Neovasc, Nephronyx, Nova Vascular, Nyra Medical, Occlutech, Olympus, Ohio Health, OrbusNeich, Ossio, Phenox, Pi-Cardia, Polares Medical, Polyvascular, Profusa, ProKidney, LLC, Protembis, Pulse Biosciences, Qool Therapeutics, Recombinetics, ReCor Medical, Regencor, Renata Medical, Restore Medical, Ripple Therapeutics, Rush University, Sanofi, Shockwave, SMT, SoundPipe, Spartan Micro, SpectraWAVE, Surmodics, Terumo Corporation, The Jacobs Institute, Transmural Systems, Transverse Medical, TruLeaf, UCSF, UPMC, Vascudyne, Vesper, Vetex Medical, Whiteswell, WL Gore, Xeltis. R. Virmani is a consultant for Abbott Vascular, Boston Scientific, CeloNova, OrbusNeich Medical, Terumo Corporation, W.L. Gore, Edwards Lifesciences, Cook Medical, CSI, ReCor Medical, Sino Medical Sciences Technology, Surmodics, and Bard BD, and is a scientific advisory board member for Medtronic and Xeltis. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

