Abstract
Aims: In patients with ST-elevation myocardial infarction (STEMI), high thrombotic burden, subsequent distal embolisation and myocardial no-reflow remain a large obstacle that may negate the benefits of urgent coronary revascularisation. We aimed at assessing the predictors of: 1) thrombus grade in patients undergoing primary percutaneous coronary intervention (PPCI) and 2) infarct size, in order to optimise therapy to reduce thrombus burden.
Methods and results: One-hundred and fifty-three consecutive patients presenting with STEMI and undergoing PPCI were included. Thrombus was evaluated by angiography and scored according to the TIMI study group score. Next, patients were categorised into two groups that had either high thrombus grade (HTG; score 4-5) or low thrombus grade (LTG; score 1-3). We evaluated predictors of angiographic thrombus grade among a number of clinical, angiographic and laboratory data. We also assessed infarct size and scintigraphic left ventricular ejection fraction (LVEF) at three months in both patient groups. Ninety-four patients (58±11 years; 75% males) presented with HTG, whereas 59 patients (58±12 years; 78% males) presented with LTG. Pre-infarction angina (PIA) was more frequently encountered in the LTG group than in the HTG group (25% vs. 10%, p=0.009). Pre-procedural TIMI flow was significantly lower in the HTG group (p<0.001), and thrombosuction was more frequently applied in the HTG group (p<0.001). Absence of PIA (OR=0.29, 95% CI=0.11-0.75, p=0.01) and proximal culprit lesion (OR=2.10, 95% CI=1.02-4.36, p=0.04) were the only independent predictors of HTG. HTG proved an independent predictor of higher peak levels of creatine kinase (CK) (p<0.001) and troponin T (p<0.001), as well as lower LVEF (p=0.05) along with male gender and absence of prior statin therapy.
Conclusions: Absence of PIA and proximal culprit lesions are associated with higher thrombus grade. Higher thrombus grade is associated with larger infarct size and slightly worse LV function. This may have clinical implications in planning strategies, particularly regarding pharmacotherapy, that aim to decrease thrombus burden prior to stent implantation.
Introduction
Primary percutaneous coronary intervention (PPCI) for patients with ST-segment elevation myocardial infarction (STEMI) aims at early restoration of patency and adequate blood flow both in the epicardial and in the microvascular coronary circulation. However, despite adequate epicardial patency many patients fail to recover sustained myocardial perfusion due to microvascular obstruction1,2, which carries a prognostic indication of a poor outcome3,4.
A high thrombotic grade has been shown to predict distal embolisation, and subsequent microvascular obstruction, thus prompting the development of strategies aimed at decreasing thrombus grade before stent deployment such as thrombus aspiration5 and use of glycoprotein (GP) IIb/IIIa receptor antagonists6,7.
Pre-infarction angina (PIA) occurring shortly before the onset of acute myocardial infarction (AMI) has a cardioprotective effect due to the mechanism of ischaemic preconditioning8-10, i.e., the phenomenon by which brief episodes of ischaemia increase the tolerance of the heart to a subsequent major ischaemic insult. Moreover, PIA has been shown to preserve microvascular function after reperfusion11.
It has been shown that patients with AMI who have intermittent infarct-related pain or unstable angina in the seven days preceding the infarction have faster coronary artery reperfusion and smaller infarcts after thrombolytic therapy than patients without pre-infarction angina, suggesting two different types of thrombus growth and thus different responses to thrombolytic therapy12. During PPCI variable grades of thrombus formation are observed that can only be detected on the initial angiography. Ideally it would be possible to predict the thrombus grade clinically or through rapid laboratory investigations prior to PCI procedure. This may help to plan for earlier and enhanced pre-hospital management using adjunctive pharmacotherapy, particularly with the newly emerging rapid-acting reversible antiplatelet agents, which are currently under intense research and are expected to have higher efficacy and safety profiles than the existing treatments.
In this study we aimed to assess factors predicting angiographic thrombotic grade in a consecutive series of patients presenting with STEMI treated by PPCI. In addition, the subsequent infarct size and left ventricular function were assessed among patients with different thrombus grades.
Methods
Study population
We studied 158 consecutive patients with a diagnosis of STEMI, who showed clear evidence of thrombus on the initial angiography, who underwent PPCI and who received abciximab prior to PPCI in the period from 2005-2009. Patients were selected from an ongoing registry (operational since 2004) in Leiden University Medical Center, which evaluates the effects of an all-phase integrated AMI care program (MISSION!) on short- and long-term outcomes13,14. Diagnosis of STEMI was made on the basis of typical electrocardiographic changes with clinical symptoms associated with elevation of cardiac biomarkers. All patients were treated according to the institutional AMI protocol (MISSION!). The MISSION! protocol is a rather stringent, rigorously standardised protocol. It comprises a well-organised pre-hospital, in-hospital and outpatient clinical framework for decision-making and treatment, so it is unlikely that procedural changes over time would have influenced the outcomes. Clinical data were prospectively entered into the departmental cardiology information system (EPD-Vision®; Leiden University Medical Center, Leiden, The Netherlands) and retrospectively analysed13. The tertiary centre provides a round-the-clock service of PPCI with highly experienced PCI physicians and dedicated nurses.
Medication
All patients received abciximab (Centocor B.V., Leiden, The Netherlands) as a bolus injection of 0.25 mg/kg bodyweight, followed by 0.125 µg/kg/min with a maximum of 10 µg/min as a continuous infusion for 12 hour abciximab administration started before PCI according to MISSION! protocol13. Furthermore, all patients received an equivalent of 300 mg of acetylsalicylic acid, 600 mg of clopidogrel as a loading dose before PCI and heparin given as a bolus of 5000 IU at the start of the PCI procedure. After the procedure, all patients received aspirin (75 mg/day) indefinitely and clopidogrel (75 mg/day) for one year. Other medications, including β-blockers, angiotensin-converting enzyme (ACE) inhibitors, nitrates and statins, were prescribed according to MISSION! protocol.
Invasive procedure and angiographic evaluation
All PPCI were performed through a 6 Fr femoral sheath. Patients underwent PPCI and stenting of the infarct-related artery according to standard techniques.
The choice of stent (bare metal stent or drug-eluting stent) was left at the operator’s discretion. Direct stenting was performed only in cases presenting clear views of the arterial lesion with adequate flow. Otherwise, the patient was subjected to balloon angioplasty and stenting was done subsequently. Thrombectomy was frequently, but not exclusively, performed when high thrombus burden was observed on the initial angiographic image of the target vessel. Thrombus score was graded as previously described by the TIMI study group6,15. Briefly, in TIMI thrombus grade 0, no cineangiographic characteristics of thrombus are present; in TIMI thrombus grade 1, possible thrombus is present with such angiographic characteristics as reduced contrast density, haziness, irregular lesion contour, or a smooth convex “meniscus” at the site of total occlusion, suggestive but not diagnostic of thrombus; in TIMI thrombus grade 2, there is definite thrombus, with the greatest dimensions ≤½ the vessel diameter; in TIMI thrombus grade 3, there is definite thrombus but with the greatest linear dimension >½ but <2 times the vessel diameter; in TIMI thrombus grade 4, there is definite thrombus, with the largest dimension ≥2 times the vessel diameter; and in TIMI thrombus grade 5, there is total occlusion. We further categorised the thrombus score into two overall grades: a high thrombus grade (grades 4 and 5), and a low thrombus grade (grades 1-3) (Figure 1). We decided to use this cutoff value in line with previous studies16-18 that showed the prognostic value of this cutoff. The inter-observer agreement was calculated with weighted Kappa statistics and showed good agreement (κ= 0.92, p<0.001). Coronary flow was graded according to Thrombolysis In Myocardial Infarction (TIMI) criteria19. TIMI flow grade was evaluated at baseline and after the PCI procedure.
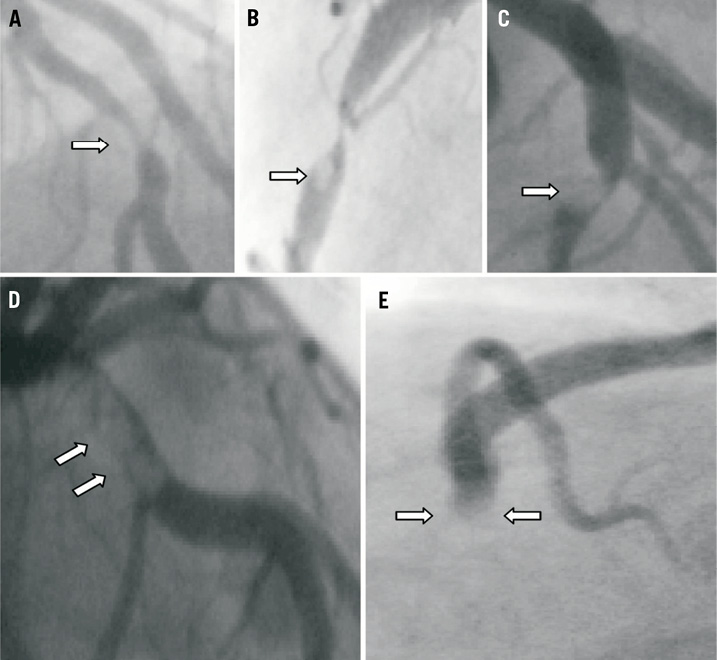
Figure 1. Thrombus (white arrows) graded according to TIMI working group classification: A) thrombus grade 1; B) thrombus grade 2; C) thrombus grade 3; D) thrombus grade 4; and E) thrombus grade 5; we further scored the thrombus into two overall grades: a high thrombus grade (grades 4 and 5), and a low thrombus grade (grades 1-3).
Procedural success was defined as residual stenosis <20% and TIMI flow grade 3. The coronary angiograms were reviewed off-line by two independent interventional cardiologists who were blinded to the clinical data.
Laboratory investigations
Cardiac troponin T concentration in plasma was measured on a third generation Elecsys 2010 analyser (Roche Diagnostics, Almere, The Netherlands). Creatine kinase (CK) activity in plasma was measured on a Roche Hitachi Modular P800 analyser (Roche Diagnostics). According to the MISSION! protocol13 blood samples were collected at admission and every 6 hr in the first 48 hr after PPCI. Subsequently these levels were determined every day up to discharge, unless clinical events prompted repeat measurements. Peak levels of CK and troponin T in plasma were calculated as a measure of infarct size in each patient by an investigator blinded to the assigned treatment.
LVEF assessment by gated-SPECT
According to the MISSION! protocol13 all included patients were enrolled for a myocardial perfusion study at 90 days post-PPCI. An ECG-gated single-photon emission computed
tomography (SPECT) acquisition at rest using intravenous 99mTechnetium-Tetrofosmin (MYOVIEW™, Amersham, Buckinghamshire, UK) was used to measure the left ventricular ejection fraction (LVEF) 90 days after PPCI. LVEF was calculated using an automated and validated method (QGS software, version 2.0; Cedars-Sinai Medical Center, Los Angeles, CA, USA). Detailed methods are described elsewhere20. In patients in whom gated SPECT could not be performed due to technical difficulties, LVEF was estimated by echocardiographic biplane method. LVEF assessment was done by an investigator blinded to the assigned treatment.
Definition of pre-infarction angina
Pre-infarction angina (PIA) was defined as at least one episode of typical chest or left arm or jaw pain, either at rest or during exercise, less than seven days before STEMI. The presence of PIA was diagnosed by a physician, blinded to the results of the PCI, from a detailed clinical history taken before PCI.
Tested variables
We evaluated predictors of thrombus grade among different clinical, angiographic and laboratory data. Clinical data included age, gender, traditional risk factors, PIA, and symptom to balloon time. Prior pharmacotherapy at admission was also recorded including aspirin, clopidogrel, statins, β-blockers (BB), angiotensin converting enzymes inhibitors or angiotensin receptor blockers (ACEI/ARB). Among laboratory data plasma troponin T levels at admission were recorded. Angiographic data included the culprit artery, location of the culprit lesion, and the number of diseased vessels.
Statistical analysis
Categorical variables were compared using the X 2 test or Fisher’s exact test. Continuous, normally distributed data were tested by Student’s t-test or, in the case of a non-Gaussian distribution, by a nonparametric test for independent samples (Mann-Whitney U test).The inter-observer agreements were calculated using weighted Kappa statistics. Variables that at univariate analysis had a p-value ≤0.15 were included in a multiple logistic regression model with the two categories of thrombus grade as the outcome. Infarct size as assessed by peak CK and peak troponin T (after logarithmic transformation), as well as three-months LVEF were analysed in a multivariate linear regression model among different potentially relevant variables. Correlation between the outcomes was tested using Spearman’s correlation. Data were expressed as mean±SD, or as median+interquartile range for continuous variables according to the data distribution; categorical variables were expressed as percentages. All analyses were performed using SPSS version 17.0 statistical software (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
One hundred and fifty-eight consecutive patients were selected for this evaluation. From this group, five patients were excluded due to incomplete data sets, thus the study population comprised 153 patients. Ninety-four patients had high thrombus grade (HTG), and 59 had low thrombus grade (LTG). Baseline clinical and angiographic characteristics of the studied population are presented in Table 1 and Table 2, respectively. The rate of PIA was significantly higher in the LTG group than in the HTG group (25% vs. 10%, p=0.009) (Figure 2). Among the angiographic characteristics, there was less initial TIMI flow grade (p<0.001) and a higher rate of aspiration thrombectomy in the HTG group (p<0.001), than in the LTG group. HTG tended to be more frequently encountered in proximal culprit lesions (54% vs. 39%, p=0.06).
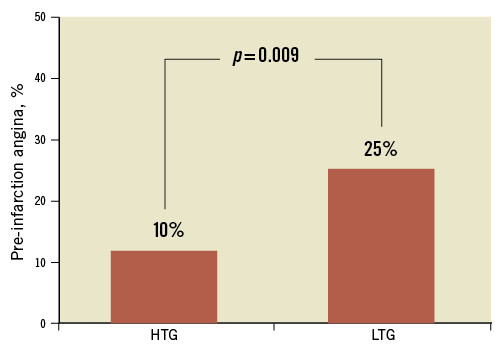
Figure 2. Rate of pre-infarction angina among the two categories of thrombus grade. HTG: high thrombus grade; LTG: low thrombus grade
Predictors of high thrombus grade (HTG)
Univariate and multivariate logistic regression analyses with thrombus grade as outcome revealed that only absence of PIA (OR=0.29, 95% CI=0.11-0.75, p=0.01) and presence of a proximal culprit lesion (OR=2.10, 95% CI=1.02-4.36, p=0.04) were independent predictors of HTG (Table 3).
Infarct size
Peak levels of CK and troponin T in plasma were significantly higher in the HTG group than in the LTG group (p<0.001 for both) (Table 4). Among the potentially relevant variables including age, sex, symptom to balloon time, hypertension, current smoking, hypercholesterolaemia, diabetes mellitus, PIA, prior drug therapy, number of diseased vessels, culprit artery, proximal culprit lesion, thrombus grade and use of thrombosuction, HTG predicted high peak levels of CK (B=–1.0, 95% CI=–1.4 -–0.6, p<0.001) and troponin T (B= –1.1, 95% CI=–1.6- –0.6, p<0.001).
Scintigraphic LVEF at three months
LVEF was not significantly different between both groups of thrombus grade (Table 4). However, when corrected for the aforementioned factors in a multivariate linear regression model, it was found that HTG predicted a slightly worse LVEF (B=4.9, 95% CI= –0.06-10, p=0.05), along with male gender and absence of prior statin therapy. The outcomes were moderately correlated (r=–0.45, p<0.0001 for LVEF and peak CK; and r=–0.5, p<0.0001 for LVEF and peak TnT).
Discussion
Key findings of the present study were: 1) the absence of PIA and a proximal location of the culprit lesion independently predicted higher angiographic thrombus grade, 2) higher thrombus grade was associated with significantly higher infarct size as assessed by peak CK and troponin T levels in plasma, as well as lower LVEF at three months along with male gender and absence of prior statin therapy.
The issue of identifying predictors of thrombus grade has gained wide interest from the time PPCI was established as the gold standard reperfusion strategy for STEMI. The presence of large thrombus burden has been found to be an independent predictor of major adverse cardiac events (MACE) and infarct-related artery stent thrombosis in patients treated with drug-eluting stents for STEMI21,22.
Cardioprotective effect of PIA
PIA is associated with improved prognosis after AMI9,12,23,24. Several mechanisms may explain this protective effect of PIA on myocardial reperfusion, such as myocardial ischaemic preconditioning9,24,25, enhanced collateral circulation towards the ischaemic myocardium26, and increased sensitivity to thrombolysis12.
Recently, a new cardioprotective mechanism of PIA was proposed, which is the inhibition of microvascular obstruction phenomenon27-29. This was supported by a study conducted by Jesel et al30, who showed that absence of PIA was the only independent predictor of MRI-detected microvascular obstruction. This provides a new hypothetical mechanism for the clinical benefits of PIA, suggesting that PIA attenuates the development of the no-reflow phenomenon, not only through microvascular ischaemic preconditioning31, but also through limiting the microvascular obstruction induced by distal embolisation from large thrombus burden. This has to be further elucidated in larger studies reinforced by IVUS or OCT on one hand, and MRI or myocardial contrast echocardiography on the other for relating the extent of culprit plaque morphology and the extent of microvascular obstruction, respectively with PIA.
Angiographic thrombus burden
In 2010, Sianos et al18 published a score for stratifying thrombus burden in STEMI patients. It was actually a modification from the established TIMI study group score6, where they reclassified patients with thrombus grade 5 into the other categories. They did that either after crossing with a wire or predilating with a balloon. In our study we adopted the original TIMI classification based on the fact that wire crossing and balloon inflation may alter thrombus grade by inducing distal embolisation. The TIMI study group was precise in defining thrombus grade 5 not only on the basis of total occlusion, but also based on the shape of this total occlusion, which ended abruptly with a squared-off or an upstream convex termination, creating a stump or arterial cul-de-sac from which dye washout was delayed.
PIA and thrombus burden
Our study is the first to address the relation between PIA and thrombus grade in patients with STEMI. Previous studies have shown the benefits of PIA on surrogate markers of reperfusion29, as well as on clinical outcomes32.
Using IVUS, Higashikuni et al33 found that the culprit plaques of patients without PIA contained larger amounts of necrotic core component than patients with PIA, whereas the plaques of patients with PIA consisted of larger amounts of the fibro-fatty component than the plaques of patients without PIA. Moreover, they found more plaque rupture among patients without PIA than in those with PIA. This difference may explain the difference in thrombus burden and consequently the difference in clinical outcomes between both groups of patients. The necrotic core component was shown to be the most thrombogenic component in human atherosclerotic plaques34,35. Exposure of the necrotic core component (plaque rupture) leads to exposure of tissue factor, thereby increasing thrombogenicity and abrupt thrombus formation. Thus, necrotic core-rich plaques may produce large thrombus burden, which may often result in sudden onset of AMI without PIA. Previously, Kojima et al36 demonstrated that patients with PIA were more likely to have plaque erosion as a substrate rather than plaque rupture, with subsequent exposure of the proteoglycan-rich matrix without a large lipid core; this has less potent thrombogenicity than plaques with lipid-rich core and consequently leads to less thrombus burden. In a study by Capone et al37, the incidence of thrombus by angiographic analysis was higher in patients with a recent onset of rest angina than in those with slowly progressive PIA.
Proximal location of culprit lesion and thrombus burden
The present study revealed proximal location of the culprit artery as an independent predictor of HTG. To our knowledge, there was one previous study that has addressed this issue16, revealing no predictive role for the location of the culprit lesion on the thrombus grade. However, in their study they analysed the thrombus in the majority of the cases after the insertion of a 6 Fr perfusion catheter, in contrast to our study in which the thrombus was analysed and graded on the initial angiogram.
The PAMI study38 showed that patients with proximal culprits had worse angiographic features with higher rates of initial TIMI 0-1 flow, and consequently worse in-hospital clinical outcomes despite rapid and successful reperfusion in the vast majority, thus arguing for the inclusion of proximal culprit lesion in the angiographic prognostication of AMI patients.
Coronary thromboses leading to AMI are distributed in a non-uniform manner. They tend to cluster within the proximal third of the coronary arteries and the likelihood of clinically significant plaque rupture decreases by 13-30% for each 10 mm distally from the coronary artery ostia39,40.
Thrombus burden and infarct size
Our study showed that HTG was a predictor of larger infarct size and when corrected for other relevant risk factors, HTG was associated with a lower LVEF.
Although no previous study has related thrombus grade to infarct size or LVEF, previous studies have shown that distal embolisation and the subsequent no-reflow, which is partly related to higher thrombus burden, were associated with larger infarct size, LV remodelling, and depressed LV function41,42.
PIA: a predictor of infarct size?
Although the myocardial protective benefits of PIA have been established in previous studies10,11,43, we could not reproduce this finding. A possible explanation is that a study with a relatively small sample size (only 24 patients had PIA) lacks statistical power. In a previous study44, it was concluded that the protective effect of PIA in AMI is overwhelmed by the protective effects of complete revascularisation provided by PPCI.
Future clinical implications
The present study argues for the consideration of PIA as a clinical predictor of thrombus burden in STEMI patients, thus setting the basis for implementing strategies aiming to decrease thrombus grade before stent implantation such as thrombus aspiration and the use of platelet glycoprotein IIb/IIIa antagonists in selected patients. Earlier administration of IIb/IIIa antagonists results in higher pre-interventional TIMI flow with subsequently improved perfusion post-PCI45-47, which in turn reflects less thrombus burden. Earlier administration of IIb/IIIa antagonists requires treatment in the pre-hospital setting, which for many dedicated primary PCI centres may pose substantial logistical obstacles. Therefore, the early administration of IIb/IIIa antagonists could be limited to patients in whom high thrombus burden is predicted.
This may set a strategy for pre-hospital triage of STEMI patients receiving early pre-hospital antithrombotic treatment. Since high-dose clopidogrel administration takes 3-4 hr to reach the top of inhibition of platelet aggregation48, Gp IIb-IIIa inhibitors are considered to more rapidly inhibit platelet aggregation, with subsequent benefits in mortality according to the risk profile. The use of risk scores, such as the TIMI risk score49, should be strongly encouraged to identify a high risk population with thrombotic complications, which largely outweighs the risk of bleeding complications, and in whom a selective strategy of pre-hospital/pre-PCI aggressive anti-thrombotic therapy is to be adopted.
Recently, a great deal of research has focused on development of new antiplatelet agents that could be administered orally or intravenously and, unlike the currently applied thienopyridines, could provide direct-acting reversible inhibition of the platelet P2Y12 receptor. New agents such as “cangrelor50-53” and “elinogrel54,55” are characterised by a rapid onset of action, more effective platelet inhibition, and favourable safety profile with rapid reversal of its antiplatelet effect post-infusion, thus allowing for surgery without a significant delay. Furthermore, “vorapaxar” (SCH 530348)56,57 and “atopaxar” (E5555)58,59 are orally administered agents that reversibly inhibit platelet protease activated receptor 1 (PAR 1), through which thrombin induces its effect on platelet aggregation, and thus, thrombus formation. This concept of rapid action and rapid reversibility of platelet inhibition could fuel further research about the triage of pre-hospital treatment among STEMI patients with suspected high thrombotic burden.
We believe that the next step towards further optimisation of care among STEMI patients is to improve pre-hospital triage, not only by diagnosing STEMI patients in the ambulance, but also starting early pre-hospital pharmacotherapy which necessitates the implementation of a risk/benefit scoring system. This scoring system should take into consideration clinical (such as PIA), as well as rapid bedside laboratory data (cardiac biomarkers), among other things, to identify patients who would benefit most from early pre-hospital treatment.
Limitations
This study may have been limited by its observational design and the relatively small sample size, which may hinder the prognostic power. However, implementation of a stringent protocol for the study population (MISSION! protocol13) reinforces our results. No long-term clinical follow-up was performed. Therefore, a correlation between thrombus burden and clinical outcomes is lacking.
Owing to the small number of included population, and the retrospective nature of the study, we could not perform reliable testing of each single thrombus subgroup. However, Sianos et al18 validated this categorisation in a large cohort of 900 STEMI patients, and found that large thrombus (≥twice the vessel diameter) independently predicted mortality and MACE.
Many factors can influence the angiographic assessment of thrombus burden (such as the TIMI flow, vessel size, culprit complex lesions with ulcers or intra-plaque dissections that can confuse the analyst). More reliable methods to assess thrombus burden should be considered, such as the amount of aspirated thrombus, or thrombus assessed by optical coherence tomography.
Conclusion
PIA is associated with a decreased angiographic thrombus grade, whereas proximal culprit lesions are associated with higher thrombus grade. Higher thrombus grade was in turn associated with larger infarct size as well as slightly worse LV function. This may have clinical implications in planning strategies, particularly regarding pharmacotherapy, that aim to decrease thrombus burden prior to stent implantation, particularly in high-risk patients without PIA.
Conflict of interest statement
M. Schalij received grants from Eli Lilly, Boston Scientific, Medtronic and Biotronik. All other authors have no conflict of interest to declare.