Abstract
Aims: The aim of this study was to identify predictors of adverse events among patients with ST-elevation myocardial infarction (STEMI) undergoing contemporary primary percutaneous coronary intervention (PCI).
Methods and results: Individual data of 2,655 patients from two primary PCI trials (EXAMINATION, N=1,504; COMFORTABLE AMI, N=1,161) with identical endpoint definitions and event adjudication were pooled. Predictors of all-cause death or any reinfarction and definite stent thrombosis (ST) and target lesion revascularisation (TLR) outcomes at one year were identified by multivariable Cox regression analysis. Killip class III or IV was the strongest predictor of all-cause death or any reinfarction (OR 5.11, 95% CI: 2.48-10.52), definite ST (OR 7.74, 95% CI: 2.87-20.93), and TLR (OR 2.88, 95% CI: 1.17-7.06). Impaired left ventricular ejection fraction (OR 4.77, 95% CI: 2.10-10.82), final TIMI flow 0-2 (OR 1.93, 95% CI: 1.05-3.54), arterial hypertension (OR 1.69, 95% CI: 1.11-2.59), age (OR 1.68, 95% CI: 1.41-2.01), and peak CK (OR 1.25, 95% CI: 1.02-1.54) were independent predictors of all-cause death or any reinfarction. Allocation to treatment with DES was an independent predictor of a lower risk of definite ST (OR 0.35, 95% CI: 0.16-0.74) and any TLR (OR 0.34, 95% CI: 0.21-0.54).
Conclusions: Killip class remains the strongest predictor of all-cause death or any reinfarction among STEMI patients undergoing primary PCI. DES use independently predicts a lower risk of TLR and definite ST compared with BMS. The COMFORTABLE AMI trial is registered at: http://www.clinicaltrials.gov/ct2/show/NCT00962416. The EXAMINATION trial is registered at: http://www.clinicaltrials.gov/ct2/show/NCT00828087
Introduction
Mortality after acute ST-segment elevation myocardial infarction (STEMI) has considerably declined during the last two decades owing to improved access to and the widespread use of reperfusion therapy1. Primary percutaneous coronary intervention (PCI) has emerged as the reperfusion therapy of choice due to its lower risk of reinfarction and haemorrhagic stroke and improved vessel patency, translating into lower mortality as compared to fibrinolysis2. However, cardiac mortality is still observed in up to 4% of patients undergoing primary PCI at one-year follow-up3,4. Moreover, STEMI patients undergoing primary PCI remain at risk for reinfarction, stent thrombosis and repeat revascularisation, underlining the need to identify predictors of adverse clinical outcomes in this high-risk population.
The impact of clinical characteristics on prognosis among STEMI patients has been investigated in numerous studies during the fibrinolysis era, which consistently identified Killip class, age, heart rate, and anterior MI as independent predictors of mortality5,6. More recently, impaired left ventricular ejection fraction (LVEF) and angiographic variables –such as multivessel disease and small vessel diameter– have been reported to predict mortality among STEMI patients undergoing primary PCI7,8. However, these findings were based on studies without systematic use of P2Y12 receptor inhibitors, thrombus aspiration and stent implantation, which have significantly improved clinical outcomes9-13,14. In addition, the results of two large-scale randomised clinical trials have recently suggested that the use of new-generation drug-eluting stents (DES) among STEMI patients undergoing primary PCI is associated with a lower risk of repeat revascularisation, reinfarction and stent thrombosis (ST) compared with bare metal stents through to one year15,16. The purpose of the present study was therefore to identify predictors of all-cause death or any reinfarction and definite ST, target lesion revascularisation (TLR) among patients with STEMI undergoing primary PCI in a pooled analysis of two contemporary primary PCI trials (EXAMINATION and COMFORTABLE AMI)15,16.
Methods
PATIENT POPULATION
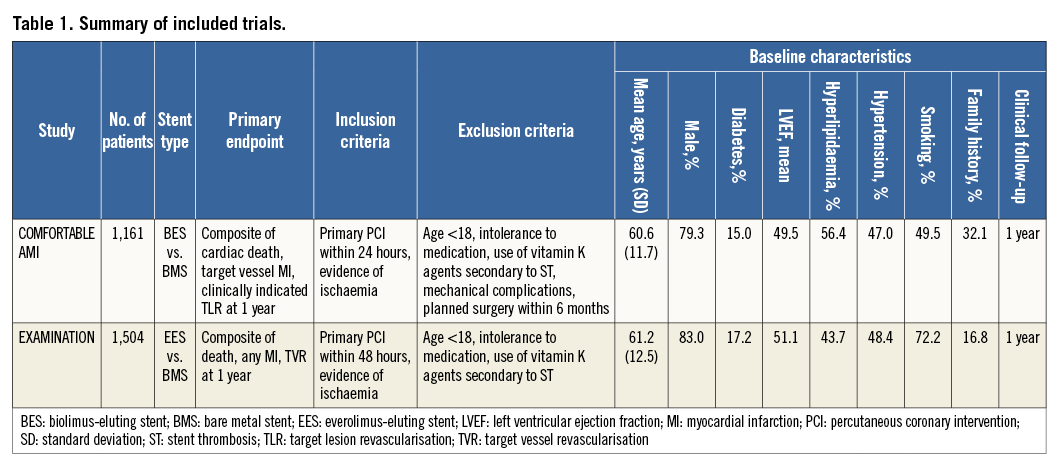
Individual patient data from COMFORTABLE AMI and EXAMINATION were pooled for the purpose of the present study. The details and primary results of the COMFORTABLE AMI and EXAMINATION trials have been described previously15,16. In summary, 1,161 STEMI patients undergoing primary PCI at 11 sites in Europe and Israel between September 2009 and January 2011 were randomly assigned to treatment with biolimus-eluting stents with biodegradable polymer coating (BioMatrix™; Biosensors Europe SA, Morges, Switzerland), or bare metal stents (Gazelle™; Biosensors Europe SA) in the COMFORTABLE AMI trial. In the EXAMINATION trial, 1,504 STEMI patients undergoing primary PCI at 12 sites in Europe between December 2008 and May 2010 were randomly assigned to treatment with everolimus-eluting stents with durable polymer coating (XIENCE V; Abbott Vascular, Santa Clara, CA, USA), or bare metal stents (MULTI-LINK VISION™; Abbott Vascular). Overall, 10 out of 2,665 patients withdrew consent after randomisation (six patients in EXAMINATION and four patients in COMFORTABLE AMI). For the purpose of this analysis, 2,655 patients allocated to new-generation DES (N=1,326) or bare metal stents (N=1,329) were pooled. A summary of the principal trial characteristics is reported in Table 1.
ENDPOINT DEFINITIONS AND EVENT ADJUDICATION
The same clinical endpoint definitions were used in COMFORTABLE AMI and EXAMINATION15,16. All-cause mortality included cardiac death, vascular death, and non-cardiovascular death. All deaths were considered cardiac unless an unequivocal non-cardiac cause could be established. Reinfarction was defined according to the WHO extended historical definition17. Target lesion revascularisation (TLR) was defined as any repeat revascularisation due to a stenosis within the index stent or within a 5 mm border proximal and distal to the index stent. Stent thrombosis (ST) was defined according to the Academic Research Consortium criteria18. In both the COMFORTABLE AMI and EXAMINATION trials, adverse events were independently adjudicated by a clinical events committee, chaired by the same investigator (PV), unaware of treatment allocation. The pre-specified primary endpoint of the present analysis was the composite of all-cause death and any reinfarction. Secondary endpoints were definite ST and target lesion revascularisation. Angiograms were centrally assessed by the independent core laboratory at Bern University Hospital, Bern, Switzerland, and Cardialysis, Rotterdam, The Netherlands by personnel blinded to stent type and outcome. Killip class I includes patients without clinical signs of heart failure. Killip class II includes patients with rales or crackles of <50% in both lung fields as well as the presence of an audible S3 heart sound, and elevated jugular venous pressure. Killip class III includes patients with rales or crackles of >50% in both lung fields as well as the presence of an audible S3 heart sound, and elevated jugular venous pressure. Killip class IV includes patients in cardiogenic shock or hypotension, and evidence of peripheral vasoconstriction.
STATISTICAL METHODS
For the purpose of the present study, patient-level data from the EXAMINATION and COMFORTABLE AMI trials were pooled and analysed. Continuous variables are expressed as means±standard deviation and compared by means of ANOVA. Categorical data are expressed as frequencies and percentages and are compared using the χ2 and Fisher’s exact tests. Mixed effects logistic regressions with random effect of the study (EXAMINATION or COMFORTABLE AMI) were used for the analyses of all-cause death or any reinfarction, TLR or definite ST. Odds ratios (OR) with 95% confidence intervals (95% CI) are reported. Univariable and multivariable mixed effects logistic regressions tested the association of single and multiple risk factors, respectively, with outcomes of interest (all-cause death or any reinfarction, TLR and definite ST). Multivariable risk factors with a p-value below 0.05 were retained for each minimal model, with OR (95% CI) reported. Estimates for the multivariable and minimal models are after multiple imputation of missing data per study using chained equations. For illustrative purposes, a classification and regression tree (CART) for time-to-death or reinfarction was constructed from the minimal model (with splits set at p-value <0.05, with each group after the split including a minimum of 10 patients and/or 10 events) using non-missing data only. All p-values shown are from two-sided tests, and the level of statistical significance was set at 0.05. Analyses were performed using Stata 12 (StataCorp, College Station, TX, USA).
Results
One-year follow-up was available in 2,617 of 2,655 patients (98.6%). At one year, the composite of all-cause death and any reinfarction occurred in 144 patients (5.4%), definite ST in 35 patients (1.3%), and TLR in 96 patients (3.6%).
PREDICTORS OF ALL-CAUSE DEATH AND ANY REINFARCTION
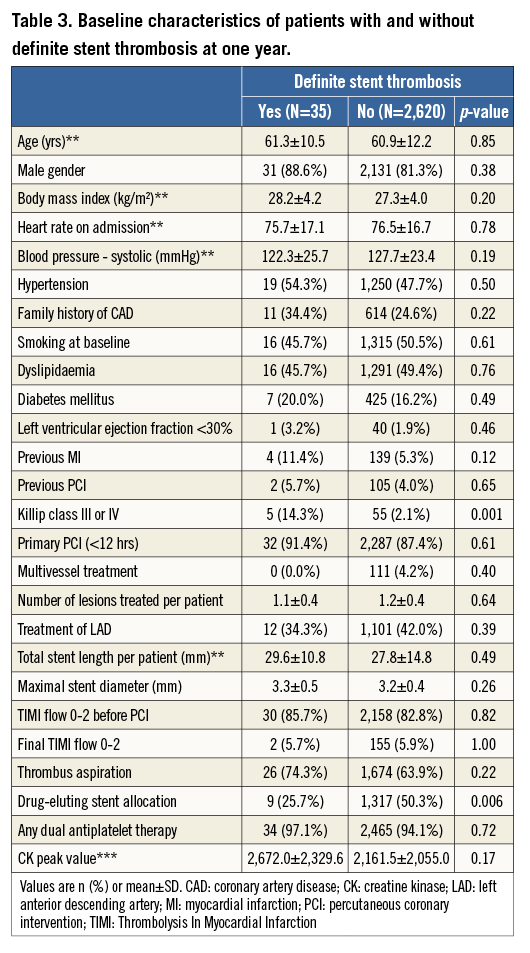
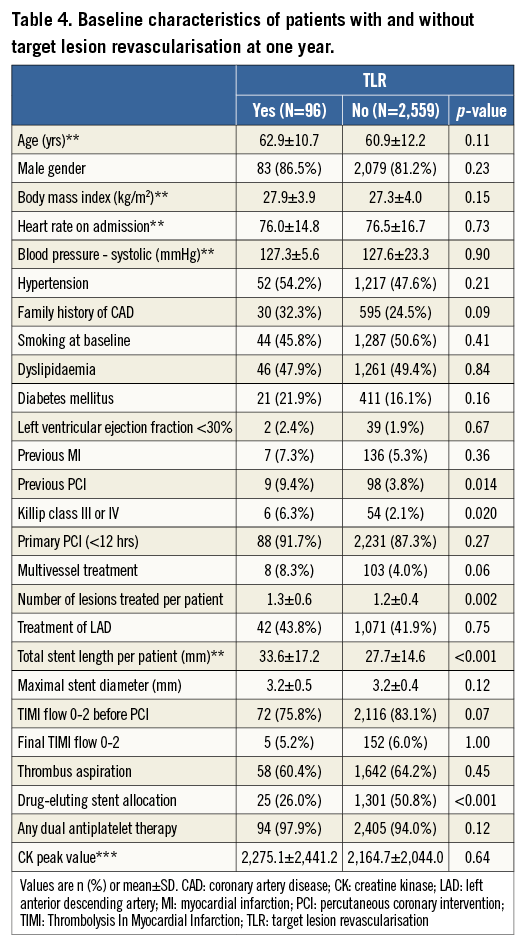
Baseline clinical, angiographic, and procedural characteristics according to the occurrence of all-cause death or any reinfarction at one year are summarised in Table 2. Patients who experienced all-cause death or any reinfarction after primary PCI were older, more likely to be female, and more frequently had increased heart rate, hypertension, non-smoking habits, diabetes mellitus, impaired left ventricular ejection fraction, previous MI, Killip class III or IV, longer stent length, final TIMI flow 0-2, and higher CK peak value. Independent predictors of all-cause death or any reinfarction after multivariable analysis are shown in Figure 1 and comprise in decreasing order: Killip class III or IV (OR 5.11, 95% CI: 2.48-10.52), left ventricular ejection fraction <0.30 (OR 4.77, 95% CI: 2.10-10.82), final TIMI flow 0-2 (OR 1.93, 95% CI: 1.05-3.54), hypertension (OR 1.69, 95% CI: 1.11-2.59), age (OR 1.68, 95% CI: 1.41-2.01), and CK peak (OR 1.25, 95% CI: 1.02-1.54).
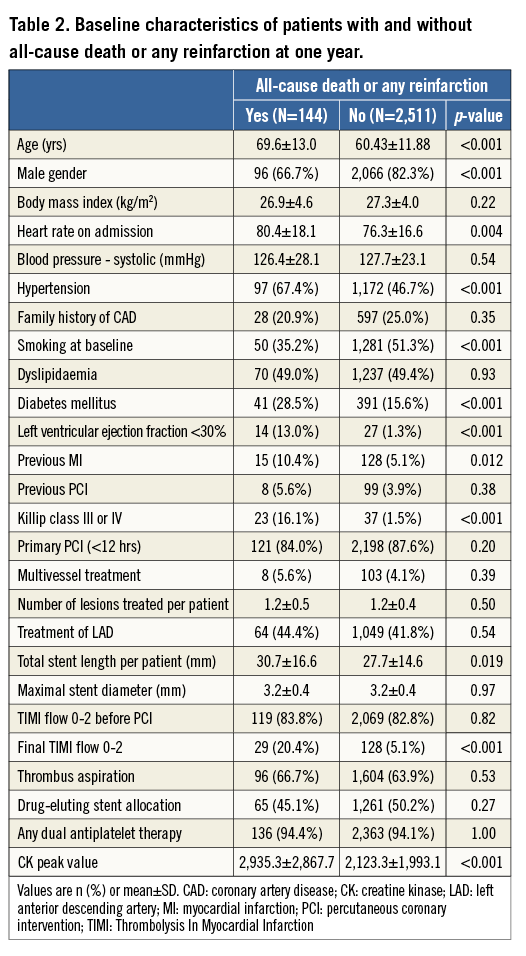
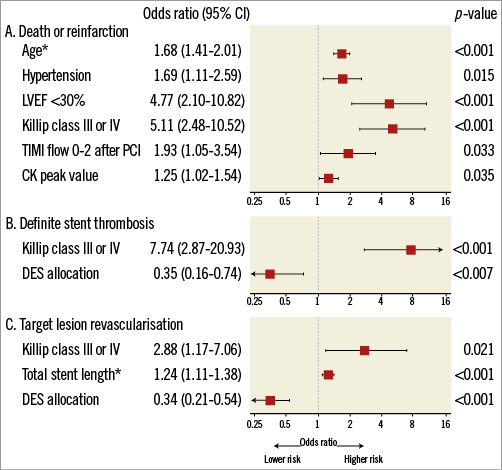
Figure 1. Independent predictors of adverse events. Multivariate analysis of predictors of (A) all-cause death or any reinfarction, (B) definite stent thrombosis, and (C) target lesion revascularisation. CK: creatine kinase; DES: drug-eluting stent; LVEF: left ventricular ejection fraction; OR: odds ratio; TIMI: Thrombolysis In Myocardial Infarction. *OR per 10 units increase for age (year) and total stent length (mm). **OR per log-transformed CK peak value.
CART ANALYSIS
Figure 2 shows the results of a CART analysis, depicting the most important variables which influenced the likelihood of all-cause death or any reinfarction in a hierarchical order through to one year. Killip class was the strongest predictor followed by age, left ventricular ejection fraction, and final TIMI flow 0-2. A patient with Killip class I or II, age ≤80, left ventricular ejection fraction ≥30%, and final TIMI 3 flow has a one-year mortality as low as 2.6%. Conversely, a patient with Killip class III or IV, age >80, left ventricular ejection fraction <30%, and final TIMI 0-2 flow has a considerably increased risk of all-cause death or any reinfarction ranging from 6.5% to 36.5% at one year.
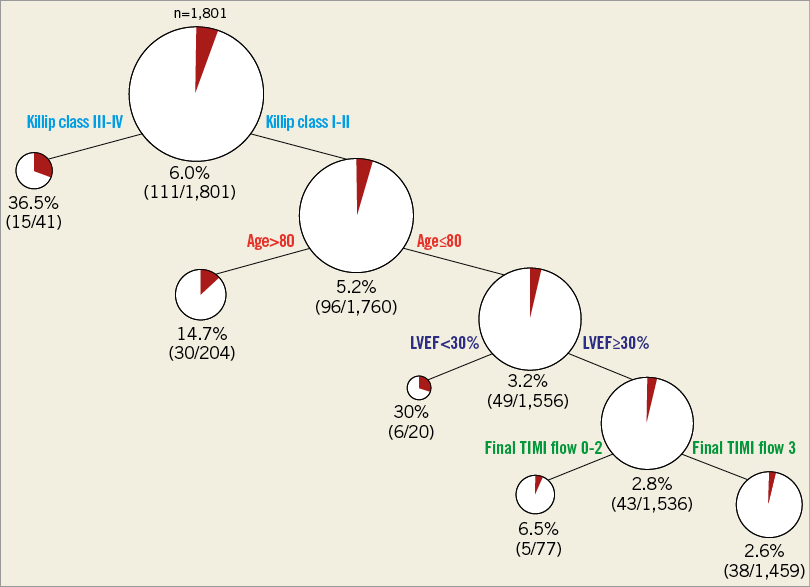
Figure 2. CART model of predictors of all-cause death or reinfarction. CART analysis showing in a hierarchical order the most important variables influencing the likelihood of all-cause death or reinfarction at one year. LVEF: left ventricular ejection fraction; TIMI: Thrombolysis In Myocardial Infarction
PREDICTORS OF STENT THROMBOSIS
Baseline clinical, angiographic, and procedural characteristics according to the occurrence of definite ST at one year are summarised in Table 3. Patients suffering definite ST more frequently presented with Killip class III or IV and were less frequently treated with DES. All definite ST events among patients with Killip class III or IV occurred within six days. After multivariable analysis, Killip class III or IV (OR 7.74, 95% CI: 2.87-20.93) and use of DES (OR 0.35, 95% CI: 0.16-0.74) emerged as independent predictors of definite ST (Figure 1).
PREDICTORS OF TARGET LESION REVASCULARISATION
Baseline clinical, angiographic, and procedural characteristics according to the occurrence of TLR at one year are summarised in Table 4. Patients undergoing TLR more frequently had previous PCI and Killip class III or IV, had a higher number of lesions to be treated resulting in longer total stent length, and had less frequently received DES. Independent predictors of TLR after multivariable analysis are shown in Figure 1, and include Killip class III or IV (OR 2.88, 95% CI: 1.17-7.06), stent length (OR 1.24, 95% CI: 1.11-1.38), and DES allocation (OR 0.34, 95% CI: 0.21-0.54).
Discussion
The findings of this analysis reporting predictors of adverse events among STEMI patients undergoing contemporary primary PCI in two large-scale randomised trials can be summarised as follows:
– One-year rates of all-cause death and any reinfarction are relatively low in the contemporary primary PCI era.
– Killip class III or IV remains the strongest predictor of all-cause death or any reinfarction, definite ST, and TLR.
– The use of DES independently predicts a lower risk of TLR and definite ST as compared with BMS.
The introduction of reperfusion strategies with the use of fibrinolytic agents as well as aspirin have significantly reduced mortality in STEMI patients19,20. More recently, mechanical reperfusion by means of primary PCI with stenting, in conjunction with potent platelet inhibitors, has further reduced in-hospital mortality to as low as 3%. It is worth noting that the decline in early mortality was consistently observed in nationwide surveys in the United States and Europe21,22. However, there is a paucity of information on predictors of all-cause death and reinfarction among patients with STEMI undergoing contemporary reperfusion with primary PCI.
PREDICTORS OF ALL-CAUSE DEATH AND ANY REINFARCTION
The present analysis shows that independent predictors of all-cause death and any reinfarction have not changed significantly despite changes in reperfusion and adjunctive therapy. Killip class at presentation was identified as a predictor of mortality in the fibrinolysis era, as reported in the GUSTO-I and TIMI trials5,23. Similar findings have been observed in the BMS era (CADILLAC), and more recently in the HORIZONS-AMI trial comparing early-generation paclitaxel-eluting stents and BMS7,24,25. The fact that Killip class remains the strongest predictor of all-cause death and any reinfarction, irrespective of left ventricular function and extent of coronary artery disease at baseline, reflects the degree of haemodynamic compromise in these patients which can be recognised easily on clinical grounds.
In addition to Killip class, other predictors of death and reinfarction identified in our analysis included age, hypertension, left ventricular ejection fraction, final TIMI flow and CK peak value, which have traditionally been considered major risk factors of ischaemic adverse events after STEMI and have been consistently identified in previous reports as predictors of death and reinfarction among STEMI patients treated with fibrinolysis as well as primary PCI5,7,23,26.
PREDICTORS OF STENT THROMBOSIS AND TARGET LESION REVASCULARISATION
Beyond Killip class, other independent predictors of definite ST and TLR are all device-related. Given the fact that all definite ST among patients with Killip class III or IV occurred within six days of PCI, it appears important to ensure optimal procedural results avoiding residual dissections, inadequate stent expansions, and to install adequate antithrombotic treatment during and after the procedure.
Importantly, DES allocation predicted a reduced risk of both ST and TLR. The higher effectiveness of DES compared with BMS, leading to a reduced risk of restenosis and repeat revascularisation procedures, has been evidenced since the introduction of DES in clinical practice among patients with stable angina as well as STEMI13. However, safety concerns were raised with respect to the use of early-generation sirolimus-eluting and paclitaxel-eluting stents in the setting of STEMI27. Two recent network meta-analyses have reported a lower risk of ST with new-generation DES compared with BMS28,29. Similar results were observed in the EXAMINATION trial30. These findings can be explained at least in part by a lower thrombogenicity observed in vitro with new-generation DES compared with BMS31. In line with these recent reports, the present large-scale patient-level analysis shows that DES allocation reduced the risk of definite ST by 70% in patients with STEMI at one year. Of note, BioMatrix and Gazelle have identical stent platforms, and the same applies to XIENCE V and MULTI-LINK VISION. Therefore, the differences between the DES used and their BMS comparators were limited to polymer coatings and antiproliferative agent release. Whether this benefit persists beyond the first year after PCI will need to be confirmed by longer-term follow-up of available trials as well as by future studies powered for safety endpoints.
As it relates to device effectiveness, stent length independently predicted TLR in our analysis. Several reports have associated stent length with an increased risk of repeat revascularisation after PCI in patients with stable angina and STEMI32,33. This may be related to the higher degree of vascular injury after implantation of longer and/or overlapping metallic stents34.
Study limitations
Some limitations of the present analysis deserve discussion. First, time from symptom onset to reperfusion was not available. However, both the COMFORTABLE AMI and EXAMINATION trials were conducted in European countries with developed STEMI networks. Therefore, one can assume a low degree of variability in time-to-reperfusion across the centres involved in the two trials. Second, baseline characteristics captured in the COMFORTABLE AMI and EXAMINATION trials were not identical. Therefore, we cannot exclude that unmeasured variables, such as renal failure and anaemia, might have emerged as independent predictors of adverse events if available for analysis. Third, high-volume European tertiary centres recruited patients in the COMFORTABLE AMI and EXAMINATION trials. For this reason, our findings may not be generalisable to different healthcare settings. Fourth, the currently available universal definition of MI was not applied in this study which would probably have resulted in somewhat higher event rates, particularly during the follow-up phase. However, one of the strengths of this pooled analysis is the uniform definition as well as the adjudication of the endpoint MI in both studies. Fifth, the aim of this analysis was to identify baseline predictors of adverse events. Evaluating the impact of any changes in adherence to medications over time was beyond the scope of this analysis. Finally, clinical follow-up was limited to one year. It cannot be excluded that predictors of longer-term outcomes might differ from the ones identified in this analysis. Nevertheless, it is notable that over 60% of adverse events in patients with STEMI occur during the first year of follow-up35. Therefore, predictors of one-year outcomes remain of importance to identify high-risk subsets among patients with STEMI undergoing percutaneous reperfusion in the contemporary era of primary PCI.
Conclusions
Killip class remains the strongest predictor of all-cause death or reinfarction among STEMI patients undergoing primary PCI. DES use independently predicts a lower risk of TLR and definite ST compared with BMS.
| Impact on daily practice Patients with ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI) remain at high risk of adverse events, including death, reinfarction, stent thrombosis and repeat revascularisation. This underlines the need to identify predictors of impaired clinical outcomes in order to recognise higher-risk patients in contemporary practice of primary PCI. According to our findings, Killip class remains the strongest predictor of death or reinfarction in STEMI patients undergoing primary PCI, which highlights that a simple clinical assessment remains of key importance in this setting. In addition, the use of DES independently predicts a lower risk of definite stent thrombosis and target lesion revascularisation compared with bare metal stents, suggesting that DES should be considered the standard of care in STEMI patients undergoing primary PCI. |
Conflict of interest statement
M. Taniwaki has received research support from Abbott and OrbusNeich. S. Windecker has received research grants to the institution from Biotronik and St. Jude. The other authors have no conflicts of interest to declare.

