Abstract
Aims: To investigate incidence, predictors and prognosis of bleeding in ST elevation myocardial infarction (STEMI) patients who underwent primary percutaneous coronary intervention (PCI).
Methods and results: A large scale, prospective, observational study was performed between 1991 and 2004 in a single teaching hospital in The Netherlands including all consecutive STEMI patients who underwent primary PCI. The independent association between both major and minor bleeding and one-year mortality was evaluated using Cox proportional hazard models. A total of 5,030 patients were included, of whom 109 patients (2%) had cardiac surgery within 48 hours. Data on bleeding <48 hours were available in 4,717 patients (96%). Of these, 80 (1.6%) had major bleeding, whereas minor bleeding was observed in 266 patients (5.6%). Independent predictors of minor bleeding were advanced age, multivessel disease, Killip class ≥2 on admission, anterior MI location and TIMI 0 flow before PCI. Killip class ≥2 on admission was an independent predictor of major bleeding. Major bleeding (HR 3.5 [95% CI 2.3-5.4]) was associated with an increased risk of death at one year.
Conclusions: After primary PCI, the incidence of major bleeding is less than 2%. Although relatively infrequent, major bleeding complications are strongly and independently related to short- and midterm mortality.
Introduction
In patients with an acute coronary syndrome (ACS), including ST elevation myocardial infarction (STEMI), who undergo percutaneous coronary intervention (PCI), a number of antithrombotic and antiplatelet drugs are used.1-3 A major limitation of these drugs is the increased risk of bleeding, with a particularly increased risk in older patients, females and those with renal failure, diabetes or heart failure.4 In fact, bleeding may currently be the most common non-cardiac complication of therapy in patients with acute coronary syndromes, and anaemia can develop or worsen during hospitalisation, even in the absence of overt bleeding.5,6 An increased short-term and long-term mortality after bleeding complications has been demonstrated in a number of studies in patients with ACS.7-10 Not surprisingly, research to the effect of newer, and potentially safer, antithrombotic agents have focused on the prevention of bleeding.3,11,12 Although it is quite clear from randomised trials that primary PCI results in higher survival rates and less bleeding compared to fibrinolysis13-15, bleeding may still occur after primary PCI. There is, however, limited information about the incidence, predictors and prognostic importance of bleeding in patients who have primary PCI for STEMI. The aim of the present study was to evaluate the incidence and predictors of bleeding in patients undergoing primary PCI and whether bleeding is related to prognosis.
Methods
Population
From January 1991 to December 2004, individual patient data from all patients with admission diagnosis of STEMI admitted for primary PCI at the Isala klinieken (Zwolle, The Netherlands) were prospectively recorded. To avoid double inclusion of patients, only the first recorded admission for STEMI during the study period was used. Before the primary PCI procedure, all patients received 300-500 mg of aspirin intravenously, and 5000-10,000 IU intravenous heparin. In addition to aspirin and heparin, clopidogrel was given 300 mg orally since 1999. Primary PCI was performed with standard techniques if the coronary anatomy was suitable for angioplasty. Success of the PCI was determined by the classification system of the Thrombolysis in Myocardial Infarction (TIMI) trial16, in which a grade 3 blood flow indicates normal flow within the vessel, in combination with a myocardial blush grade 2 or 3.17 Additional treatment with glycoprotein IIb/IIIa inhibitors (12.5 µg/kg bolus tirofiban) or stents was left to the discretion of the treating cardiologist. In those who had treatment with glycoprotein IIb/IIIa inhibitors, only a half dose of unfractionated heparin was given. All patients were treated with an optimised drug therapy including angiotensin-converting enzyme inhibitors, β-blockers and lipid lowering drugs where appropriate.
Measurements (endpoints, definitions)
The frequency of bleeding during the first 48 hours was defined and classified according to the TIMI criteria.18 Major bleeding was defined as either intracranial bleeding or overt bleeding with a decrease in haemoglobin ≥5 g/dl (≥ 3.1 mmol/L) or a decrease in haematocrit ≥15% within 48 hours after admission. Minor bleeding was defined as observed bleeding with decrease in haemoglobin ≥3 g/dl (≥1.9 mmol/L), or >10% decrease in haematocrit. If a bleeding site was not identified: >4 g/dl decrease in the haemoglobin concentration or >12% decrease in haematocrit within 48 hours after admission or intracranial bleeding within 48 hours.
Patients were diagnosed with STEMI if they had chest pain of >30 minutes duration and ECG changes with ST segment elevation >2 mm in at least two precordials and >1 mm in the limb leads. Recurrent myocardial infarction was diagnosed when there was a 50 percent decrease of creatine kinase-muscle-brain fraction (CK-MB) from a previous peak value, followed by a subsequent rise to a level exceeding the upper limit of normal.
Protocol specified blood sampling for CK and CK-MB levels was performed at baseline and at eight hours, 16 hours and 24 hours after PCI. Measurement of serum total CK and CK-MB levels was performed according to local hospital standards. A large enzymatic infarct size was defined as patients who had a CK release (peak CK) in the highest tertile of the study population. Peak CK and peak CK-MB are comparable independent predictors of LV function and one-year mortality in STEMI patients after primary PCI.19 Left ventricular ejection fraction was measured before discharge by radionuclide ventriculography or by echocardiography if the patient had atrial fibrillation. Radionuclide ventriculography was performed by using the multiple gated equilibrium method following the labelling of red blood cells of the patient with 99mTc-pertechnetate. A General Electric 300 gamma camera (GE Healthcare Ltd, Little Chalfont, Buckinghamshire, UK) with a low-energy all-purpose parallel-hole collimator was used. Global ejection fraction was calculated by a General Electric Star View computer using the fully automatic PAGE program. In patients with atrial fibrillation, standard 2-dimensional and Doppler imaging was performed and stored in cineloop format by well-trained echocardiographists and reviewed by experienced cardiologists who estimated the ejection fraction by visual assessment.
Data collection and follow-up
We collected the following variables from the patient files: age, gender, history of hypertension, diabetes, hyperlipidaemia, smoking, previous myocardial infarction, Killip class on admission, angiographic variables, laboratory measurements, pre-discharge LV function and discharge medication. Follow-up information was obtained from the patient’s general physician or by direct telephone interview with the patient. Study approval was obtained from the medical ethic committee of our hospital.
Statistical analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) version 12.0.1. Continuous data were expressed as mean±standard deviation and categorical data as percentage, unless otherwise denoted. Differences between continuous data were performed by students t test and the chi-square or Fisher’s exact test was used as appropriate for dichotomous data. Multivariable logistic regression analysis was performed to test the independent association between baseline characteristics and bleeding. Separate analyses were performed according to potential influence of GP IIb/IIIa inhibitors, since data on its use were only collected from 2000 to 2004.
Significant variables analysed are reported with their respective odd ratios and 95% confidence intervals. Cox proportional-hazards regression models were used to estimate hazard ratios of bleeding with regard to survival at one year. Survival was represented by Kaplan-Meier curves. For all analyses, statistical significance was assumed when the two-tailed probability value was < 0.05.
Results
Baseline characteristics
During the study period, 5,030 patients were included, of whom 109 patients (2%) had cardiac surgery within 48 hours. Mean age was 61.2±11.9 years (range 19-94 years) and 24% of those were female. Data on bleeding were available of 4,717 patients (96%), and these patients compromised the study population (Figure 1).
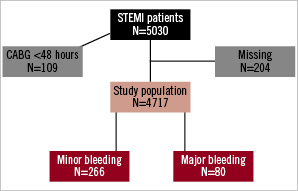
Figure 1. Flow chart.
Data on use of GP IIb/IIIa inhibitors were collected from 2000, and were available in 2,032 patients (87% of 2,330 patients admitted after 2000). A total of 80 patients (1.6%) had major bleeding, 75 patients with significant Hb decrease, and five patients with intracranial bleeding. Minor bleeding was observed in 266 patients (5.6%).
Predictors of bleeding
Differences of the baseline characteristics between patients with and without major bleeding are summarised in Table 1 and the differences between patients with and without minor bleeding are summarised in Table 2.
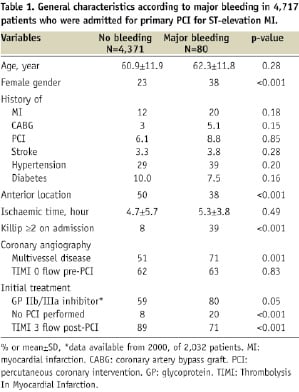
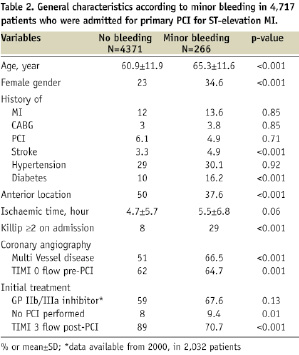
There was a significant increase in major bleeding compared to no bleeding when GP IIb/IIIa inhibitors during primary PCI were used. Patients with bleeding (both major and minor) had a significantly higher Killip class ≥2 on admission compared with patients with no bleeding (39% and 29.3% vs. 10%, P=0.001). Duration of ischaemic time was significantly longer for patients with major bleeding compared with those without bleeding (5.5 hours vs. 4.8 hours, P=0.04).
After multivariable analyses, adjusting for all variables that were statistically significant in univariable analyses only Killip class ≥2 on admission (OR 2.3 95% CI 1.7-2.9) remained a significant independent predictor of major bleeding. Independent predictors of minor bleeding were age (OR 1.03 per year, 95% CI 1.01-1.04), multivessel disease (OR 1.5 95% 1.1-2.1), Killip class ≥2 on admission (OR 2.1 95% CI 1.8-2.4), anterior MI location (OR 1.3 95% CI 1.1-1.6) and TIMI 0 flow before PCI (OR 1.5 95% CI 1.1-2.1). Separate multivariable analyses revealed that use of GP IIb/IIIa was neither an independent predictor of major nor minor bleeding.
Bleeding and clinical outcome
Clinical outcome of the patients is summarised in Tables 3 and 4. Patients with major bleeding had a significantly larger enzymatic infarct size (P<0.001) and a lower LV ejection fraction (P=0.04) (Table 3).
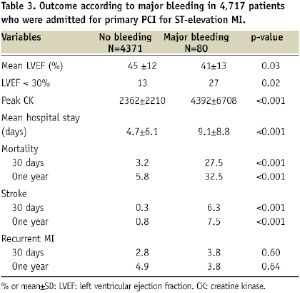
Also the prevalence of a severely depressed LV ejection fraction (< 30%) was much higher in those with major bleeding compared to patients without major bleeding (27% vs. 13%, P=0.02). The mean hospital stay was significantly longer in patients with major bleeding (P<0.001). Unlike re-infarction, both stroke (P<0.001) and death (P<0.001) after 30 days as well as one year were significantly higher in those with major bleeding compared to those without major bleeding. Similar to major bleeding, those with minor bleeding had a significantly larger enzymatic infarct size (P<0.001) and a lower LV ejection fraction (P=0.04) (Table 4).
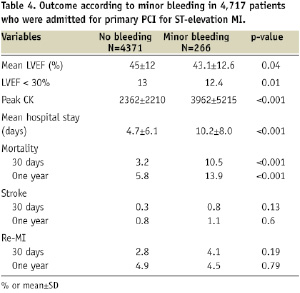
A total of 191 patients (4%) died within 30 days and 318 (6.7%) died within one year after admission. Both major bleeding, HR 7.0 (95% CI 4.7-10.5) and minor bleeding, HR 2.5 (95% CI 1.8-3.5) were associated with increased one-year mortality. Survival curves of patients with and without major or minor bleeding are depicted in Figures 2 and 3.
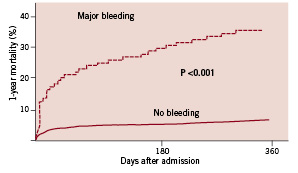
Figure 2. Kaplan Meier. One-year mortality in patients with (N=80) and without (N=4371) major bleeding.
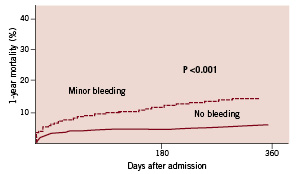
Figure 3. Kaplan Meier. One-year mortality in patients with (N=266) and without (N=4371) minor bleeding.
After adjusting for differences in age and gender, both major bleeding, HR 6.0 (95% CI 4.0-8.9) and minor bleeding, HR 1.8 (95% CI 1.3-2.6) were associated with increased one-year mortality. In the final survival multivariable model, adjustments were made for age, gender and for variables that were statistically significant different between those with and without bleeding in the multivariable analyses. Again, however, only major bleeding, HR 3.5 (95% CI 2.3-5.4) was associated with increased one-year mortality.
Discussion
In this large registry, the incidence of major bleeding within 48 hours after primary PCI was low, 1.6%. The incidence of minor bleeding was 5.6%. Major bleeding was an independent predictor of mortality.
Previous studies
Periprocedural bleeding is the most frequently reported non-cardiac complication after PCI for ACS. The incidence of major bleeding in patients with ACS as reported in randomised trials varied, depending on bleeding definition, clinical presentation and treatment. An incidence of major bleeding of 4.1% was observed in the TIMI II trial20, whereas an incidence as high as 15% was reported in the TIMI I trial.16 The GUSTO IV-ACS trial reported low rates of both major bleeding (1.2%) and minor bleeding (2.8%) in ACS patients treated with abciximab without early revascularisation.21 The GRACE study reported an incidence of major bleeding of 4.8% in STEMI patients, which was higher than in patients with NSTEMI and unstable angina.4
In the OASIS 5 trial12, investigating patients with ACS, major bleeding was 0.6% in the fondaparinux group versus 1.4% in the enoxaparin group. Possibly, incidence of major bleeding may decrease after use of fondaparinux. The ACUITY trial reported a higher rate of major bleeding in ACS patients treated with heparin plus glycoprotein IIb/IIIa inhibitors (5.7%) versus bivalirudin monotherapy (3.0%).10
Predictors of bleeding
In our study, Killip class ≥2 on admission was independently associated with an increased risk of major bleeding. Independent predictors of minor bleeding were advanced age, multivessel disease, Killip class ≥2 on admission, anterior MI location and TIMI 0 flow before PCI. An increased risk of minor bleeding among patients of an older age was also observed in previous studies.4,22-24
Patients with Killip class ≥2 were more at risk for developing bleeding post PCI, probably due to a more invasive therapy in these haemodynamically unstable patients. Both multivessel disease (more extensive coronary artery disease) and TIMI 0 flow pre-PCI may have been associated with more difficult PCI procedures, with longer procedure times.
In contrast to prior studies, our study showed that the use of GP IIb/IIIa inhibitors is not an independent predictor of major bleeding or minor bleeding. This may be explained by our use of only half-dose heparin when GP IIb/IIIa inhibitors were administered.
Prognostic importance of bleeding
A prognostic risk score for major bleeding in patients undergoing PCI via the femoral approach has been developed.25 Although this combined clinical and procedural model may be useful to determine which patients are at greatest risk after PCI, STEMI patients however, were excluded, and for these patients it is not useful.
Our study found that bleeding is an important independent predictor of prognosis in STEMI patients who underwent primary PCI. Major bleeding was associated with increased one-year mortality. Recently, Ndrepepa et al26 also reported an independent relationship between bleeding early after PCI and mortality at one year in patients with unstable angina and NSTEMI.
Several potential mechanisms might underlie the association between bleeding and deterioration of prognosis. Bleeding might lead to early cessation of dual antiplatelet therapy, which might result in ischaemia, haemodynamic decompensation, stent thrombosis, recurrent MI or death.27,28 Next, bleeding with hypovolemia and impaired oxygen carrying capacity might precipitate an hyperadrenergic state, hypotension and heart failure. Furthermore, bleeding leads to blood transfusions that might themselves lead to increased cardiovascular risk.7 Transfusion may induce microcirculatory disorder, nitric oxide, 2,3-DPG depletion and immunologic effects which may also affect long term outcome.29 Moreover, bleeding leads to a prolonged and complex hospital stay, and might require invasive monitoring. The impact of bleeding on outcome varies with the initial severity. The more severe the bleed, the greater the impact on outcome.30
Study strengths and limitations
During the 13 years of inclusion in this study, the techniques of PCI and drug treatment (after PCI) have changed. This may have affected outcome, but seems unlikely to have affected our principal conclusions. Our follow-up period was only one year, we had no data on causes of death or data of medication changes during follow-up, clopidogrel was not included as a variable in this analysis because of missing data during the first years. Also we recorded bleeding for just 48 hours after primary PCI. More importantly, this study period was before the era of routine measuring of activated clotting time (ACT), we had no data on body weight, anticoagulant agents employed in-hospital, situation requiring systemic anticoagulation, previous history of bleeding, rate of transfusion, location and cause of bleeding and timing and method of sheath removal (manual or with closure device). Finally, no data on use of IABP or duration of primary PCI procedure were collected.
Conclusion
After primary PCI for STEMI, the incidence of major bleeding is less than 2%. Although relatively infrequent, major bleeding complications are strongly and independently related to short- and midterm mortality. Trials on concomitant medication during primary PCI should pay special attention to the risk of bleeding complications.

