Abstract
Aims: We sought to describe the differential effect of bleeding events in acute coronary syndromes (ACS) on short- and long-term mortality according to their type and severity.
Methods and results: The PLATO trial randomised 18,624 ACS patients to clopidogrel or ticagrelor. Post-randomisation bleeding events were captured according to bleeding type (spontaneous or procedure-related), with PLATO, TIMI, and GUSTO definitions. The association of bleeding events with subsequent short-term (<30 days) and long-term (>30 days) all-cause mortality was assessed using time-dependent Cox proportional hazard models. A model was fitted to compare major and minor bleeding for mortality prediction. Of 18,624 patients, 2,189 (11.8%) had at least one PLATO major bleed (mean follow-up 272.2±123.5 days). Major bleeding was associated with higher short-term mortality (adjusted hazard ratio [HR] 9.28; 95% confidence interval [CI]: 7.50-11.48) but not with long-term mortality (adjusted HR 1.28; 95% CI: 0.93-1.75). Spontaneous bleeding was associated with short-term (adjusted HR 14.59; 95% CI: 11.14-19.11) and long-term (adjusted HR 3.38; 95% CI: 2.26-5.05) mortality. Procedure-related bleeding was associated with short-term mortality (adjusted HR 5.29; 95% CI: 4.06-6.87): CABG-related and non-coronary-procedure-related bleeding were associated with a higher short-term mortality, whereas PCI or angiography-related bleeding was not associated with either short- or long-term mortality. Similar results were obtained using the GUSTO and TIMI bleeding definitions.
Conclusions: Major bleeding is associated with high subsequent mortality in ACS. However, this association is much stronger in the first 30 days and is strongest for spontaneous (vs. procedure-related) bleeding.
Introduction
Bleeding events in patients with an acute coronary syndrome (ACS) are relatively frequent and appear to be associated with a poor prognosis1,2. However, while this poor prognostic effect is well documented for short-term outcomes1-4, the long-term effect of bleeding events in ACS is much more controversial3,5. Moreover, the prognostic effect of bleeding may differ according to the severity3 and the type (spontaneous vs. procedure-related) of bleeding6. The present analysis, based on the Platelet Inhibition and Patient Outcomes (PLATO) trial cohort7, aims to describe the differential effect of bleeding events in ACS patients on short-term and long-term mortality according to their type –either spontaneous or induced by percutaneous coronary intervention (PCI), angiogram or coronary artery bypass graft (CABG) surgery– and severity.
Methods
The methods, patient population, and main results of the PLATO trial have been described previously7,8. Briefly, PLATO was a multicentre double-blind trial in which 18,624 patients with an ACS were randomised to clopidogrel or ticagrelor. Patients were eligible for inclusion if they had been hospitalised for an ACS with or without ST-segment elevation. Patients with non-ST-segment elevation ACS were moderate-to-high-risk individuals with at least one risk factor. For patients with ST-elevation myocardial infarction, the intention to perform primary PCI was required. Out-patient visits were scheduled at one, three, six, nine, and 12 months (except for those recruited later in the study who completed treatment after a minimum of six months), with a safety follow-up visit one month after the end of treatment. The trial demonstrated that ticagrelor, compared with clopidogrel, significantly reduced the primary endpoint (a composite of death from vascular causes, myocardial infarction, or stroke) without an increase in the rate of overall major bleeding, but with an increase in the rate of non-procedure-related bleeding.
Definitions
Major bleeding, according to the PLATO definition8, included major life-threatening bleeding or other major bleeding. Major life-threatening bleeding was defined as fatal bleeding, intracranial bleeding, intrapericardial bleeding with cardiac tamponade, hypovolaemic shock or severe hypotension due to bleeding and requiring pressors or surgery, a decline in the haemoglobin level of ≥5.0 g/dL, or the need for transfusion of ≥4 units of packed red blood cells. Other major bleeding events were defined as bleeding that led to clinically significant disability (e.g., intraocular bleeding with permanent vision loss) or bleeding either associated with a drop in haemoglobin concentration of ≥3.0 g/dL but <5.0 g/dL or requiring transfusion of two to three units of packed red blood cells. Minor bleeding was defined as any bleeding requiring medical intervention but not meeting the criteria for major bleeding. Minor bleeding, according to the PLATO definition, included bleeding requiring medical intervention to stop or treat bleeding, but not fulfilling the criteria for major bleeding8.
Severe bleeding, according to the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) criteria, was defined as either an intracerebral haemorrhage or bleeding resulting in substantial haemodynamic compromise requiring treatment9. In practice, a bleeding event was defined as GUSTO severe if at least one of the following items was captured in the case report form: fatal, intracranial, intrapericardial bleed with cardiac tamponade, hypovolaemic shock or severe hypotension due to bleeding and requiring pressors or surgery, haemodynamic compromise, or surgical intervention required to stop or treat bleeding.
Thrombolysis In Myocardial Infarction (TIMI) major bleeding was defined as any intracranial bleeding or clinically overt signs of haemorrhage associated with a drop in haemoglobin concentration of ≥5 g/dL or fatal bleeding. In the main PLATO analysis7, bleeding was defined as a safety event and only counted when patients were on the study drug or within seven days of stopping study drug treatment. In the present analysis, bleeding occurring at any time after randomisation, regardless of treatment with study drug, was taken into account.
Bleeding events were further categorised into spontaneous or procedure-related. Procedure-related bleeds were defined as either angiogram- or PCI-related, CABG-related, or non-coronary-procedure-related (the latter category included all bleeding events associated with any type of procedure or surgery except those performed on the coronary arteries). Occurrence of PCI- or angiography-related bleeding was recorded at the discretion of the treating physician, without a specified time interval following the procedure.
Statistical analysis
Baseline characteristics of patients are described overall and according to whether individuals experienced at least one PLATO major bleed during the study period. Categorical variables are described as counts and percentages and continuous variables are described as medians with interquartile ranges (IQRs). A p-value, calculated by fitting a univariable Cox proportional hazards model for time to bleed or censoring, is provided for each variable.
The association between bleeding and subsequent all-cause mortality was assessed using time-dependent Cox proportional hazards models following patients from randomisation. Previous publications have suggested a non-proportional hazard following bleeding in ACS patients. Thus a decision was made a priori based on literature and investigator opinion to study separately the short-term and long-term associations of bleeding with mortality, where the short-term period would extend from the date of bleed to +30 days and long-term would be the remainder of available follow-up. Thus, bleeding is treated as a time-varying indicator during the study period, where all patients start with the indicator “off” at randomisation. Among patients with a bleed, the variable switched “on” a short-term indicator at the time of bleed and switched to a long-term indicator at +30 days. In patients who had more than one bleed during follow-up, all available bleeding events were analysed, returning to the short-term indicator following each sequential bleed.
The relationship between bleeding and all-cause mortality was also evaluated according to bleeding type (spontaneous or procedure-related). A separate comparison was made to evaluate the relationship between all-cause mortality and procedure-related bleeding, divided into four types: spontaneous, angiogram- or PCI-related, CABG-related, and non-coronary-procedure-related. The relationship between major bleeding and mortality, according to randomised treatment, was assessed using interactions. Finally, a model was fitted to compare major and minor bleeding for all-cause mortality within the short-term and long-term periods following bleeding. Each model used a time-varying formulation for bleeds, similar to the method described above.
Adjusted models were previously developed for all-cause mortality, based on backward selection repeated in 1,000 bootstrap samples where a significance level of 0.05 was required to stay in the model. Variables chosen in at least 75% of bootstrap samples were included in the final model. Candidate variables included baseline demographics, medical history, medications, laboratory values and disease characteristics. Continuous variables were assessed for linearity on the log-hazard scale for mortality and, when appropriate, linear splines were used. Two-way interactions were also considered. Covariates chosen for the adjustment model included age, region, history of myocardial infarction, non-haemorrhagic stroke, peripheral artery disease, diabetes mellitus, congestive heart failure, baseline heart rate, systolic blood pressure, Killip class, time from symptom onset to randomisation, white blood cell count, haemoglobin concentration, electrocardiographic changes at entry, ST-segment depression, elevated troponin, and final ACS diagnosis.
Analyses were repeated using the TIMI and GUSTO bleeding definitions for models relating bleeding to risk of mortality. All tests were two-sided and a p-value <0.05 was considered to be statistically significant. All analyses used SAS version 9.2 or higher (SAS Institute Inc., Cary, NC, USA), or R version 3.0 or higher.
Results
The median time between randomisation and death or censoring was 303.4 (IQR 190.9-368.0) days. Mean time was 272.2±123.5 days. Among the 18,624 patients in the PLATO trial, 2,189 (11.8%) experienced at least one major bleeding event, according to the PLATO criteria. Most of these (2,014 patients) had only one major bleeding event during the study period; 148 patients had two major bleeding events, 20 patients had three events, and seven patients had four or more events. The median time between randomisation and the first major bleed was 12.8 (IQR 3.9-55.9) days.
Baseline characteristics
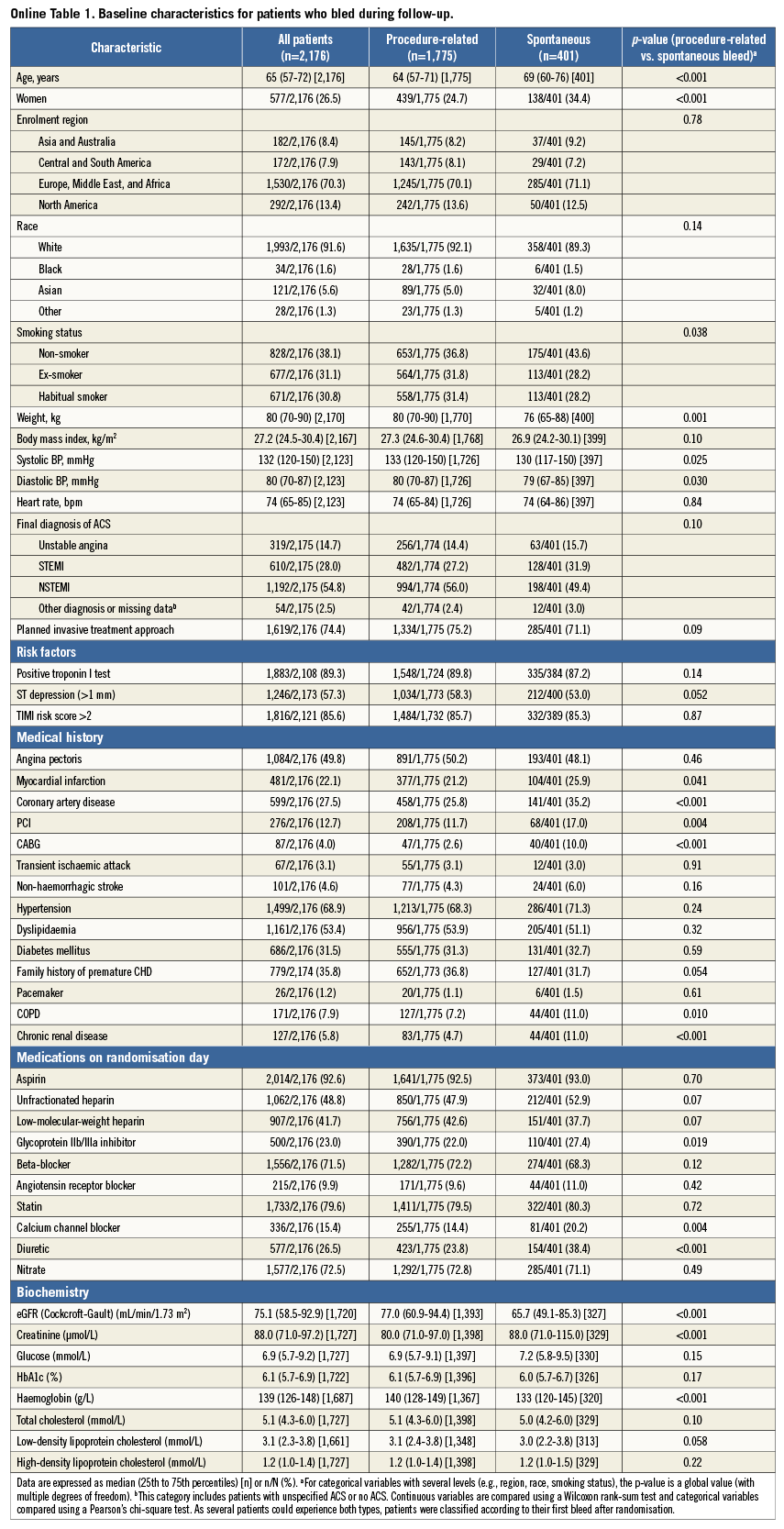
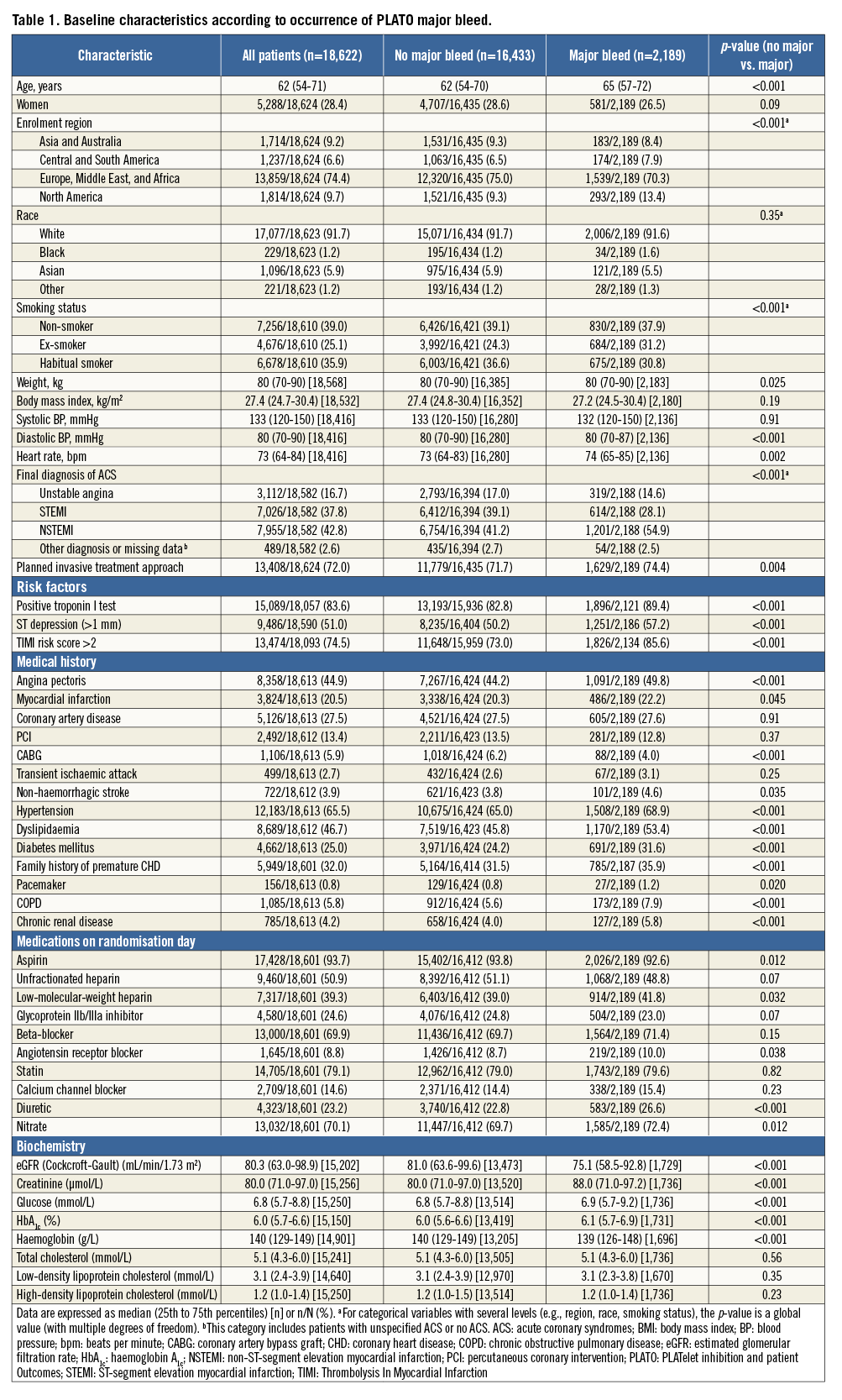
Baseline characteristics of patients, overall and with and without major bleeding, according to the PLATO definition, are shown in Table 1. Patients with major bleeding were at higher ischaemic risk, with a greater median age, higher median TIMI risk score, and higher prevalence of diabetes. They were also more likely to be male and to be enrolled at sites in North America.
Relationship between PLATO major bleeding and mortality
Among 2,189 patients with major bleeding, 236 (10.8%) subsequently died, whereas 669 of the 16,435 (4.1%) patients without a bleeding event died during follow-up (p<0.001). PLATO major bleeding was associated with a more than 12-fold increased risk in immediate short-term all-cause mortality (unadjusted hazard ratio [HR] 12.48; 95% confidence interval [CI]: 10.43-14.94; p<0.001). This increase persisted after adjustment (HR 9.28; 95% CI: 7.50-11.48; p<0.001). In comparison, major bleeding was associated with a modest increase in all-cause long-term mortality (unadjusted HR 1.57; 95% CI: 1.19-2.06; p=0.001), which was no longer significant after adjustment for baseline differences (adjusted HR 1.28, 95% CI: 0.93-1.75; p=0.128).
Relationship between PLATO major bleeding and mortality according to bleeding type
Among 2,189 patients with major bleeding events, there was a total of 485 spontaneous major bleeds and 1,796 procedure-related bleeds. The latter category included 1,503 CABG-related major bleeds, 236 PCI-related or angiography-related major bleeds (180 PCI-related and 56 angiography-related), and 86 non-coronary-procedure-related major bleeds (including any intervention or surgery). Of note, a patient could have had several types of bleeding; these categories are therefore overlapping.
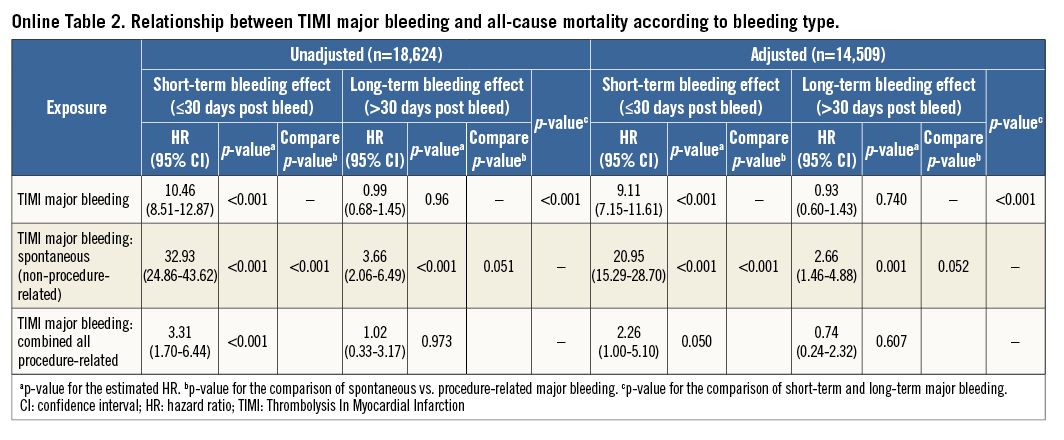
Baseline characteristics of patients, overall and with or without spontaneous and procedure-related major bleeding events are detailed in Online Table 1. The relationship between mortality and PLATO major bleeding according to the type of bleeding is detailed in Table 2, Figure 1, and Online Figure 1. Briefly, 122 of 485 (25.2%) patients with spontaneous bleeding vs. 139 of 1,796 (7.7%) with procedure-related bleeding died during follow-up. After adjustment, spontaneous bleeding was associated with both short-term and long-term large increases in all-cause mortality, whereas procedure-related bleeding was only associated with increased short-term mortality.
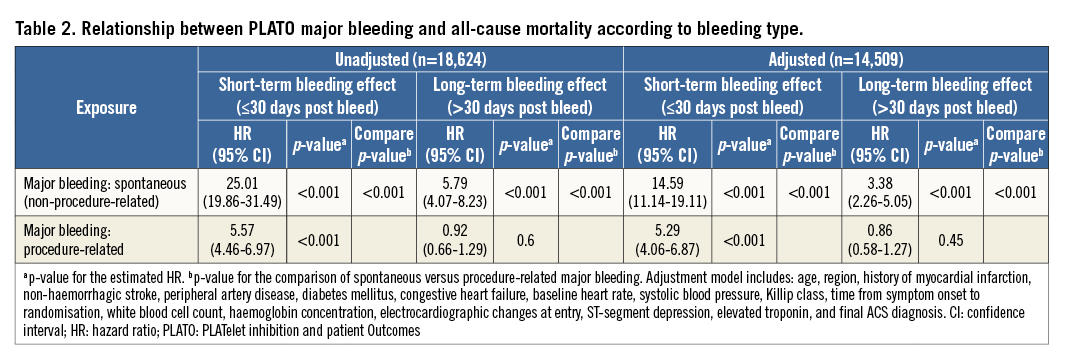
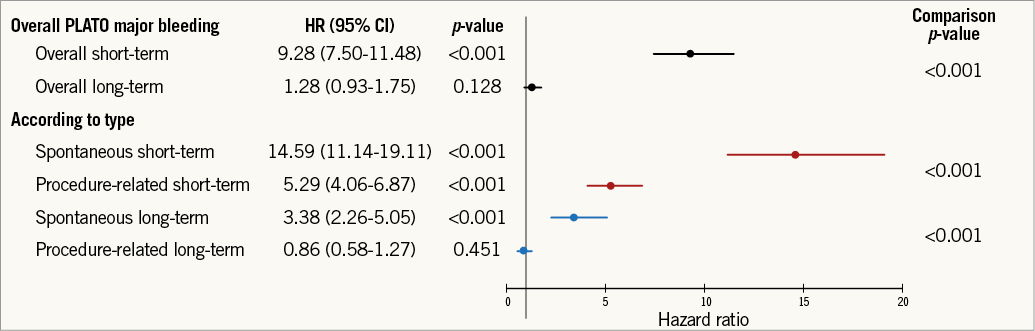
Figure 1. Relationship between PLATO major bleeding and mortality according to bleeding type. Adjusted HR, 95% CI, and relevant comparison p-values shown. PLATO: PLATelet inhibition and patient Outcomes. Adjustment model includes: age, region, history of myocardial infarction, non-haemorrhagic stroke, peripheral artery disease, diabetes mellitus, congestive heart failure, baseline heart rate, systolic blood pressure, Killip class, time from symptom onset to randomisation, white blood cell count, haemoglobin concentration, electrocardiographic changes at entry, ST-segment depression, elevated troponin, and final ACS diagnosis.
Procedure-related bleeding deaths after PLATO major bleeding were distributed as follows: 100 of 1,503 (6.7%) patients with CABG-related bleeding; 25 of 236 (10.6%) patients with catheter-related (PCI or angiography) bleeding; and 22 of 86 (25.6%) patients with non-coronary-procedure-related bleeding.
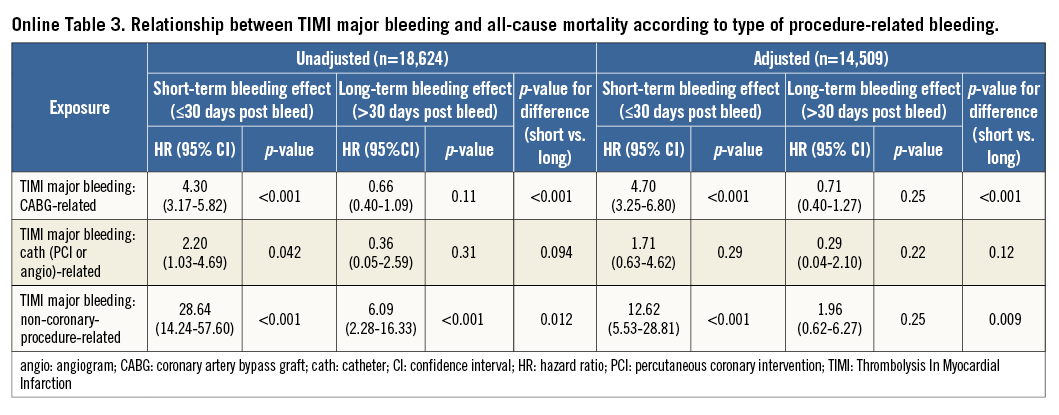
When detailing the relationship between type of procedure-related bleeding and all-cause mortality, it appeared that CABG-related and non-coronary-procedure-related bleeding were only associated with a higher short-term mortality, whereas PCI- or angiography-related bleeding was not associated with either short-term or long-term mortality (Table 3).
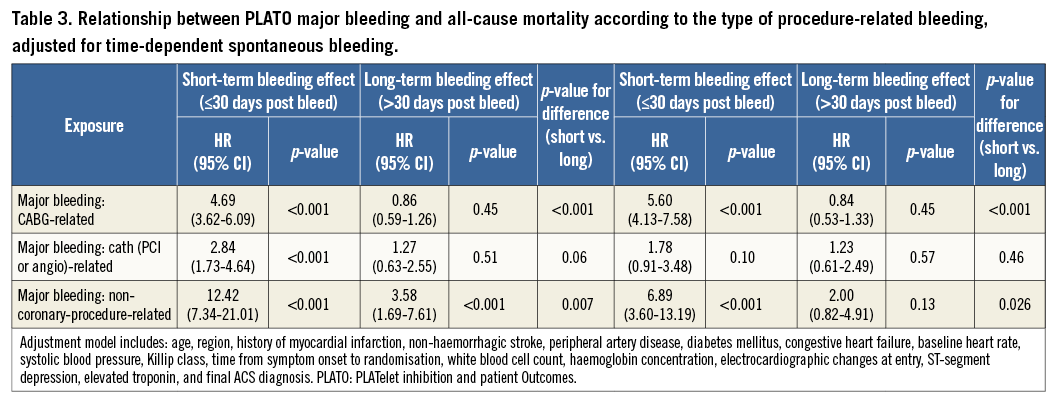
Relationship between bleeding and mortality according to bleeding definition
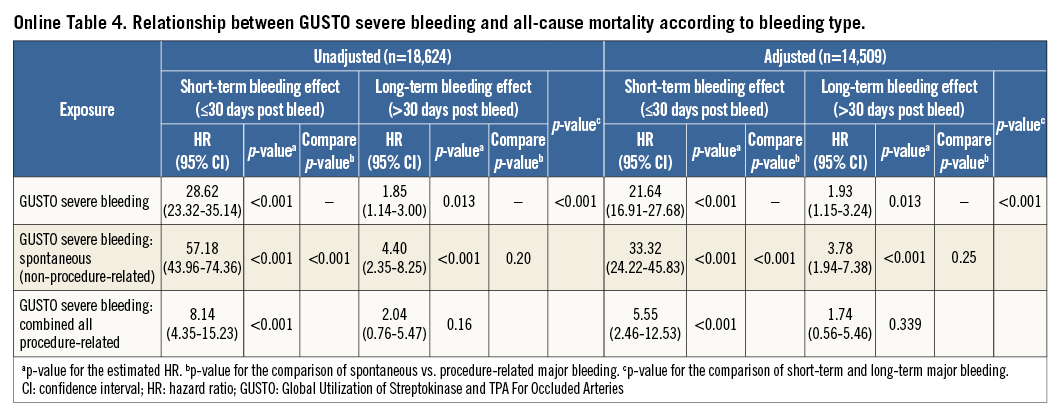
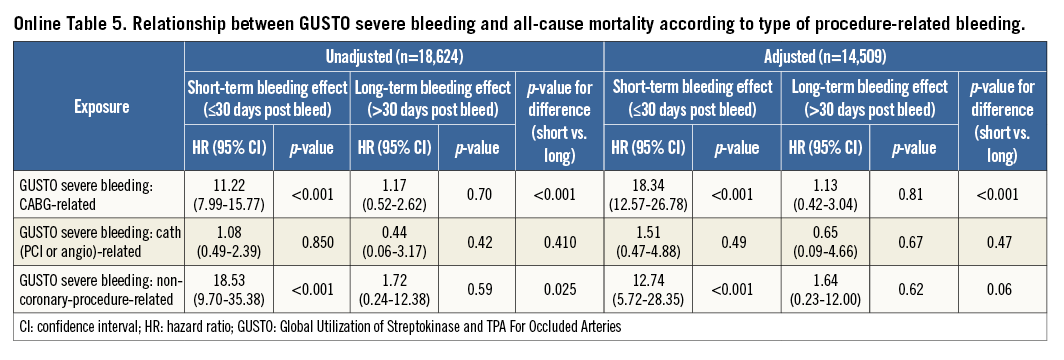
The previous analyses were repeated using the GUSTO and TIMI classifications for bleeding and yielded similar results (Online Figure 2, Online Figure 3, Online Table 2-Online Table 5).
Relationship between PLATO bleeding and mortality according to severity
At least one minor bleeding event was reported in 879 (4.7%) patients: 811 had only one minor bleeding event, 65 had two minor bleeding events, and three patients had three or more minor bleeding events. Among these 879 patients (with minor bleeding events), 158 also presented with a major bleed at some point during the study. The association of minor bleeding with short-term mortality was less marked than that of major bleeding (respectively, adjusted HR 2.12, 95% CI: 1.37-3.28 vs. HR 9.07, 95% CI: 7.33-11.23; p<0.001). The relationship between major and minor bleeding and long-term mortality was similarly low (respectively, adjusted HR 1.25, 95% CI: 0.91-1.72 vs. HR 1.57, 95% CI: 1.06-2.33; p=0.39).
Relationship between bleeding and mortality according to study drug
The association of major bleeding with mortality was similar in patients treated with clopidogrel or ticagrelor for both short-term mortality (clopidogrel: HR 9.05, 95% CI: 6.68-12.26; ticagrelor: HR 9.50, 95% CI: 7.22-12.51; p-value for interaction=0.81) and long-term mortality (clopidogrel: HR 1.02, 95% CI: 0.62-1.68; ticagrelor: HR 1.51, 95% CI: 1.02-2.25; p-value for interaction=0.22).
Discussion
In the present analysis based on the PLATO trial population, major bleeding, regardless of the definition used, was associated with a marked increase in short-term but not long-term mortality. There was, however, substantial variation according to the type of bleeding: spontaneous bleeding was associated with both short-term and long-term mortality whereas procedure-related bleeding was associated only with short-term mortality. Finally, compared with major bleeding, minor bleeding was associated with a modest increase in short-term mortality and similar long-term mortality.
It is well established that bleeding has detrimental consequences on subsequent outcomes1-3,10-15. However, the present analysis is based on a large contemporary cohort in which modern antithrombotic treatments and techniques were employed. This study provides a systematic analysis of the short- and long-term effects of bleeding, covering the full spectrum of bleeding types (spontaneous, PCI- or angiogram-related, CABG-related, non-coronary-procedure-related major bleeding), severities (major, minor), definitions (PLATO, GUSTO, TIMI), ACS types (STEMI, non-STEMI), and management approaches (medical, PCI, CABG).
A detrimental effect of bleeding on short-term outcomes has already been described in patients with an ACS. In the Global Registry of Acute Coronary Events (GRACE) registry, Moscucci et al described an association between major bleeding and hospital death1. The effect of major bleeding on long-term outcome is much more controversial. Based on the analysis of several randomised trials published in the early 2000s, Eikelboom et al2 described a strong association between bleeding and death, with a higher risk of death in the first 30 days in patients with bleeding (HR 5.37) and a weaker risk after 30 days (HR 1.54). Rao et al3 reported a similar short- and long-term effect of severe bleeding in an analysis pooling data from several randomised trials conducted in the 1990s. More recently, Ndrepepa et al14 found that bleeding was the strongest correlate of 30-day mortality after PCI, whereas for the increased risk long-term mortality was mediated mostly by cardiovascular risk factors. Kikkert et al11 reported that after a serious bleed (vs. no bleed) the subsequent risk of death was not significantly increased beyond one month. Based on an analysis of the TRITON-TIMI 38 trial, Hochholzer et al10 found an association between serious bleeding and a higher risk of death. However, the risk was no longer increased beyond 40 days. Conversely, based on the CRUSADE registry, Lopes et al12 described that, in elderly patients, in-hospital major bleeding was associated with short-, intermediate-, and long-term mortality. Similarly, an association between bleeding defined according to the BARC classification and one-year mortality was described in several cohorts13,15. In the present analysis, we found a higher risk of death in the first 30 days following major bleeding (adjusted HR 9.28) but no association between major bleeding and long-term mortality.
The impact of minor bleeding on mortality is controversial. Giugliano et al16 found that, in the ExTRACT-TIMI 25 trial, minor bleeding was not associated with short-term mortality. Rao et al, in a pooled analysis, found a modest but significant impact of GUSTO mild and moderate bleeding3. Another pooled analysis from Mehran et al17 found a more pronounced effect of TIMI minor bleeding on mortality. In the present analysis, we observed that minor bleeding had a modest effect on both short- and long-term mortality; yet long-term risk was not significantly different from long-term risk following a major bleed. However, minor bleeding events were relatively rare in the PLATO study, and it is difficult and probably clinically irrelevant to analyse minor bleeds separately from major bleeding events.
A differential effect of bleeding according to the site of bleeding has been suggested previously. In an analysis combining the populations from several randomised trials evaluating bivalirudin, it was found that the effect of non-access-site bleeding on one-year mortality was twofold that of access-site bleeding. The effect of access-site bleeding on mortality was, however, still significant6. An analysis of the Multicenter Evaluation of Single High-Dose Bolus Tirofiban Versus Abciximab With Sirolimus-Eluting Stent or Bare-Metal Stent in Acute Myocardial Infarction (MULTISTRATEGY) trial found a higher 12-month risk of myocardial infarction or death associated with non-access-site-related bleeding but no association with access-site-related bleeding18. Kikkert et al19 drew similar conclusions after analysis of a cohort of 2,002 STEMI patients undergoing PCI. However, in a pooled analysis of 14,180 patients from seven randomised trials, both access- and non-access-site bleeds were independently associated with increased one-year mortality, even if non-access-site bleeding was a stronger correlate20. Finally, the STEEPLE trial21 did not find any effect of access-site haematoma ≥5 cm on mortality.
The relationship between bleeding and mortality in patients undergoing CABG is one of the most controversial: a recent analysis from the TRITON trial found an increased rate of bleeding but a reduced mortality in patients undergoing CABG who were treated with prasugrel compared with those on clopidogrel22. We found a stronger short-term effect on mortality for spontaneous bleeding, no effect for PCI- or angiogram-related bleeding, and an intermediate effect for CABG-related and non-coronary-procedure-related bleeding. Only spontaneous bleeding had an effect on long-term mortality. As it is descriptive, the present analysis does not provide any explanation for the association between spontaneous bleeding and long-term mortality. Beyond a direct effect of bleeding, the higher baseline risk of patients with spontaneous bleeding seems likely to play a role. Despite the use of multiple adjustments in the analysis, residual confounding cannot be excluded, particularly as some important covariates (such as history of cancer) were not captured in PLATO. Another possible reason could be that bleeding can lead to reduction or even discontinuation of antithrombotic treatment and delay or preclude invasive management, exposing the patient to an increased risk of ischaemic events.
In the current bleeding classifications, and more specifically in the Bleeding Academic Research Consortium bleeding classification23, only CABG-related bleeding events are individualised, with the rest of the classifications depending on the intensity of bleeding23. In the present analysis, the type of bleeding is at least as important as the intensity of bleeding. Based on this observation, bleeding type should be taken into consideration in future revisions of bleeding classifications.
Limitations
The present analysis presents some limitations. First, the observation of an association between bleeding and a higher mortality rate does not necessarily imply causality. Bleeding events may only be a marker of higher baseline risk. This is confirmed when analysing the baseline characteristics of patients showing older age, higher TIMI risk scores, and higher prevalence of diabetes. Furthermore, because of the time-dependent nature of post-baseline bleeding, many events or changes to patients could have occurred between randomisation and bleed that we are unable to account for. However, higher mortality persisted after multiple adjustments and, even if we cannot fully control for all confounders, bleeding can be plausibly perceived as directly affecting mortality. Second, this descriptive analysis of the effect of bleeding does not provide direct information about the optimal procedural or pharmacological strategies to manage ACS. A differential effect of bleeding according to antiplatelet treatment has been described24. In the present analysis, we did not observe any difference in the effect of bleeding in patients on clopidogrel compared with patients on ticagrelor. However, in this study we analysed the separate impact of bleeding on mortality; the overall impact of a given antithrombotic agent on mortality is the net result of lives saved by preventing ischaemic events and lives lost by causing fatal bleeding. The relative frequency of the two is often very different. Some procedural aspects, such as the use of radial access for PCI in STEMI patients, have been shown to be associated with improved survival. However, data on the radial or femoral approach were not collected in the PLATO trial.
Conclusion
Major bleeding is associated with high subsequent mortality in patients with an ACS. However, this association is much stronger in the first 30 days and is strongest for spontaneous (as opposed to procedure-related) bleeding. These observations should be taken into consideration when analysing the balance of risk and benefit of therapies for ACS.
| Impact on daily practice Bleeding events in patients with an acute coronary syndrome are relatively frequent and appear to be associated with a poor prognosis. In the present analysis based on the PLATO trial population, major bleeding, regardless of the definition used, was associated with a marked increase in short-term but not long-term mortality. There was, however, substantial variation according to the type of bleeding: spontaneous bleeding was associated with both short-term and long-term mortality whereas procedure-related bleeding was associated only with short-term mortality. When detailing the relationship between type of procedure-related bleeding and all-cause mortality, it appeared that CABG- and non–coronary-procedure-related bleeding were only associated with a higher short-term mortality, whereas PCI- and angiography-related bleeding were not associated with either short-term or long-term mortality These observations should be taken into consideration when analysing the balance of risk and benefit of therapies for ACS. |
Acknowledgements
Editorial support was provided by Sophie Rushton-Smith, PhD (MedLink Healthcare Communications), and was funded by the authors. Publication management was provided by Ebba Bergman, PhD (Uppsala Clinical Research Center), and was funded by AstraZeneca.
Conflict of interest statement
G. Ducrocq has received speaker’s and/or consulting fees from AstraZeneca and Lilly. R. Becker is a scientific advisory board member for Regado Biosciences, Daiichi-Sankyo, Portola, and Boehringer-Ingelheim. C. Cannon has received research grants/support from Accumetrics, Arisaph, AstraZeneca, Boehringer-Ingelheim, CSL Behring, Essentialis, GlaxoSmithKline, Janssen, Merck, Regeneron, Sanofi, and Takeda, is on advisory boards for Bristol-Myers Squibb, Lipimedix, and Pfizer (funds donated to charity), and holds equity in Automedics Medical Systems. R. Harrington has received advisory board member fees from Baxter, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo, Gilead, Johnson & Johnson, The Medicines Company, Medtronic, Merck, and WebMD, and grants from AstraZeneca, Bristol-Myers Squibb, The Medicines Company, Merck, and Portola Pharmaceuticals, and has ownership interest in Adverse Events and MyoKardia. C. Held reports receiving institutional research grants from AstraZeneca, Merck, GlaxoSmithKline, Roche and Bristol-Myers Squibb, and advisory board member honoraria from AstraZeneca. A. Himmelmann reports being an employee of AstraZeneca. R. Lassila is an advisory board member for AstraZeneca, Bayer, Boehringer-Ingelheim, Pfizer, Novo Nordisk and LEO Pharma. R. Storey has received research grants from AstraZeneca and Merck, research support from Accumetrics, honoraria from AstraZeneca, Accumetrics and Medscape, and consultancy fees from AstraZeneca, Correvio, Accumetrics, Sanofi-Aventis, Regeneron, PlaqueTec, Roche, Daiichi Sankyo and The Medicines Company. L. Wallentin has received research grants from AstraZeneca, Merck & Co, Boehringer-Ingelheim, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, is a consultant for Abbott, Merck & Co, Regado Biosciences, Athera Biotechnologies, Boehringer-Ingelheim, AstraZeneca, GlaxoSmithKline, and Bristol-Myers Squibb/Pfizer, and has received lecture fees from AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, honoraria from Boehringer-Ingelheim, AstraZeneca, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, and travel support from AstraZeneca, Bristol-Myers Squibb/Pfizer and GlaxoSmithKline. P. Steg has received research grants (to INSERM U1148) from Servier and Sanofi, is a speaker or consultant (including steering committee, DMC and CEC memberships) for Amarin, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo-Lilly, GlaxoSmithKline, Medtronic, Merck Sharp & Dohme, Novartis, Otsuka, Pfizer, Sanofi, Servier, The Medicines Company and Vivus, and is a stockholder in Aterovax. The other authors have no conflicts of interest to declare.
Supplementary data
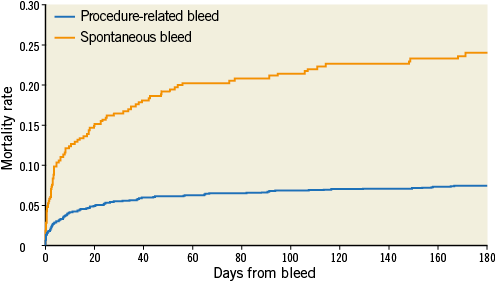
Online Figure 1. Time from bleed to mortality or censoring according to spontaneous vs. procedure-related bleeding. Patients are classified according to their first bleeding after randomisation.
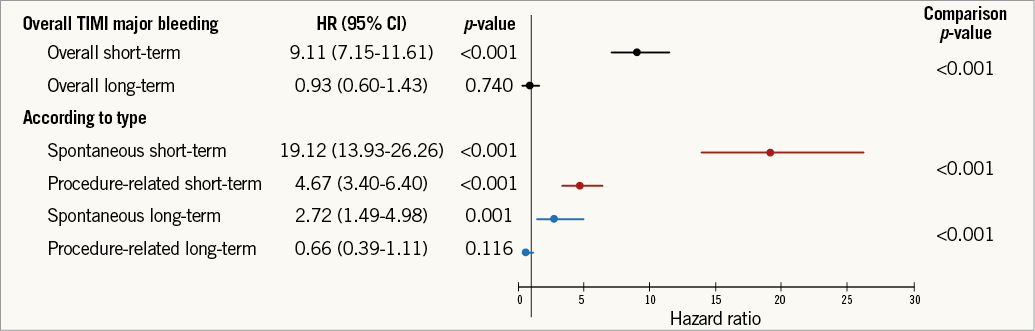
Online Figure 2. Relationship between TIMI major bleeding and mortality according to bleeding type. Adjusted hazard ratio, 95% confidence interval, and relevant comparison p-values are shown. TIMI: Thrombolysis In Myocardial Infarction. Adjustment model includes: age, region, history of myocardial infarction, non-haemorrhagic stroke, peripheral artery disease, diabetes mellitus, congestive heart failure, baseline heart rate, systolic blood pressure, Killip class, time from symptom onset to randomisation, white blood cell count, haemoglobin concentration, electrocardiographic changes at entry, ST-segment depression, elevated troponin, and final ACS diagnosis.
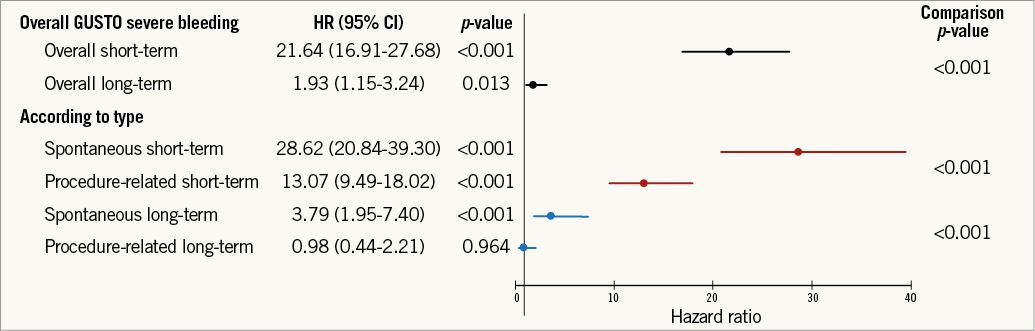
Online Figure 3. Relationship between GUSTO bleeding and mortality according to bleeding type. Adjusted hazard ratio, 95% confidence interval, and relevant comparison p-values are shown. GUSTO: Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. Adjustment model includes: age, region, history of myocardial infarction, non-haemorrhagic stroke, peripheral artery disease, diabetes mellitus, congestive heart failure, baseline heart rate, systolic blood pressure, Killip class, time from symptom onset to randomisation, white blood cell count, haemoglobin concentration, electrocardiographic changes at entry, ST-segment depression, elevated troponin, and final ACS diagnosis.