Abstract
Background: The identification of bleeding risk factors in patients undergoing percutaneous coronary intervention (PCI) is essential to inform subsequent management. Whether clinical presentation per se affects bleeding risk after PCI remains unclear.
Aims: We aimed to assess whether clinical presentation per se predisposes to bleeding in patients undergoing PCI and if the Academic Research Consortium (ARC) High Bleeding Risk (HBR) criteria perform consistently in acute (ACS) and chronic (CCS) coronary syndrome patients.
Methods: Consecutive patients undergoing PCI from the Bern PCI Registry were stratified by clinical presentation. Bleeding events at one year were compared in ACS versus CCS patients, and the originally defined ARC-HBR criteria were assessed.
Results: Among 16,821 patients, 9,503 (56.5%) presented with ACS. At one year, BARC 3 or 5 bleeding occurred in 4.97% and 3.60% of patients with ACS and CCS, respectively. After adjustment, ACS remained associated with higher BARC 3 or 5 bleeding risk (adjusted HR 1.21, 95% CI: 1.01-1.43; p=0.034), owing to non-access site-related occurrences, which accrued mainly within the first 30 days after PCI. The ARC-HBR score had lower discrimination among ACS compared with CCS patients, and its performance slightly improved when ACS was computed as a minor criterion.
Conclusions: ACS presentation per se predicts one-year major bleeding risk after PCI. The ARC-HBR score discrimination appeared lower in ACS than CCS, and its overall performance improved numerically when ACS was computed as an additional minor risk criterion.
Introduction
Patients undergoing percutaneous coronary intervention (PCI) for either acute (ACS) or chronic (CCS) coronary syndrome are at risk for haemorrhagic complications due to the invasive nature of the procedure and mandated use of antithrombotic therapy1,2. As bleeding events adversely affect patient outcomes, the identification of factors predisposing to heightened bleeding risk after PCI is essential for appropriate treatment selection3,4.
The bleeding risk might differ according to the clinical presentation. Patients undergoing PCI for ACS are known to experience a higher crude incidence of bleeding complications compared with CCS5,6. However, whether ACS presentation per se – rather than differences in clinical and procedural characteristics and antithrombotic medications – is associated with an excess of bleeding remains controversial1. The higher bleeding risk in ACS patients might be due to a pro-inflammatory state causing a transient impairment of haemostasis7, but may also result from differences in patient management and pharmacotherapy2,8. While some studies suggested that acute presentation might be independently associated with bleeding5,6,9,10, this association has not been confirmed in other reports11,12. On this basis, international guidelines recommend relying on clinical presentation for ischaemic but not bleeding risk stratification purposes2,8. Also, the recent framework proposed by the Academic Research Consortium (ARC) for High Bleeding Risk (HBR) did not assign incremental bleeding risk to ACS patients under the premise that the more aggressive antithrombotic therapy prescribed more than the acute presentation per se might explain previous findings1. To date, no validation of the ARC-HBR criteria in separately appraised ACS and CCS patients exists.
We therefore investigated – in a large cohort of contemporary all-comer PCI patients – whether ACS presentation per se is associated with higher bleeding risk and whether the ARC-HBR criteria provide a consistent risk stratification framework in patients presenting with or without ACS.
Methods
STUDY DESIGN AND PARTICIPANTS
All consecutive patients undergoing PCI at Bern University Hospital between February 2009 and December 2018 were prospectively entered in the Bern PCI Registry (NCT02241291). No exclusion criteria were applied. Participants provided written informed consent. Clinical characteristics and outcomes up to one year were collected prospectively. The prescription of dual antiplatelet therapy (DAPT) followed the recommendations of international guidelines. Detailed information on data collection is available in Supplementary Appendix 1. The study complied with the Declaration of Helsinki and was approved by the institutional ethics committee.
ASSESSMENT OF ARC-HBR CRITERIA
The fulfilment of each ARC-HBR criterion was assessed systematically according to the originally proposed definition (Supplementary Table 1). Age, haemoglobin, creatinine, platelet count, and anticipated use of long-term oral anticoagulation were collected prospectively. All other ARC-HBR criteria but non-deferrable major surgery on DAPT were evaluated retrospectively from electronic clinical records by trained investigators blinded to clinical outcomes. The ARC-HBR score was calculated by assigning one point to each adjudicated major criterion and 0.5 point to each adjudicated minor criterion. The inter-observer variability among different readers, as previously reported, indicated an excellent agreement for ARC-HBR criteria adjudication4.
CLINICAL ENDPOINTS
The primary study endpoint was Bleeding Academic Research Consortium (BARC) type 3 or 5 bleeding, which was further stratified by (i) bleeding location (i.e., access site and non-access site-related), (ii) time of onset (i.e., early and late events with landmark at 30 days), and (iii) presence (i.e., CCS vs ACS) and type of ACS (unstable angina, non-ST-segment elevation myocardial infarction [NSTEMI], and ST-elevation myocardial infarction [STEMI]). Secondary endpoints included BARC 2, 3 or 5, Thrombolysis In Myocardial Infarction (TIMI)-defined, and Global Use of Strategies to Open Occluded Arteries (GUSTO)-defined bleeding. A clinical events committee adjudicated endpoint events against original source documents. Outcomes were defined in accordance with standardised definitions of the Academic Research Consortium.
STATISTICAL ANALYSIS
Baseline characteristics are reported as means (standard deviations) or counts and percentages. For the ACS versus CCS comparison, p-values were obtained using a t-test, Fisher’s test or chi-square test, as appropriate. Time-to-event analyses and Kaplan-Meier event curves were used for outcomes. Analyses were performed at one year and with landmark analysis at 30 days. Univariate and multivariate Cox regression was carried out to investigate the association of clinical presentation and other clinical and treatment characteristics with bleeding. A p-value <0.2 was used to select variables for the multivariable model. We assessed the unadjusted incidence of BARC 3 or 5 events at one year associated with each ARC-HBR criterion in ACS and CCS, and the incidence for each ARC-HBR criterion adjusted for the absence of all other criteria. Harrell’s C-index was computed to evaluate the performance of the ARC-HBR criteria to predict BARC 3 or 5 bleeding. The discrimination of the ARC-HBR score after including ACS as an additional minor risk criterion was assessed. All analyses were performed using Stata Release 16.1 (StataCorp LP, College Station, TX, USA).
Results
POPULATION CHARACTERISTICS
A total of 17,339 consecutive patients were prospectively entered into the Bern PCI Registry. Overall, 518 participants were excluded because of incomplete haemoglobin (n=459) or creatinine (n=59) values, with a final study population of 16,821 patients, of whom 9,503 (56.5%) presented with ACS and 7,318 (43.5%) with CCS.
Compared with CCS, ACS patients were on average three years younger (66.5±12.8 vs 69.6±10.5 years), were more often active smokers, presented less frequently with cardiovascular risk factors such as hypertension and diabetes, and were less likely to have a history of myocardial infarction, coronary revascularisation, peripheral artery disease, and atrial fibrillation (Table 1). ACS patients more frequently underwent femoral access, invasive haemodynamic support, and staged PCI, but less often left main, bypass graft, or multivessel intervention (Supplementary Table 2). The ACS cohort was more likely to receive potent P2Y12 inhibitors, glycoprotein IIb/IIIa antagonists, and proton pump inhibitors, but less likely to receive oral anticoagulants or triple antithrombotic therapy (Supplementary Table 3).
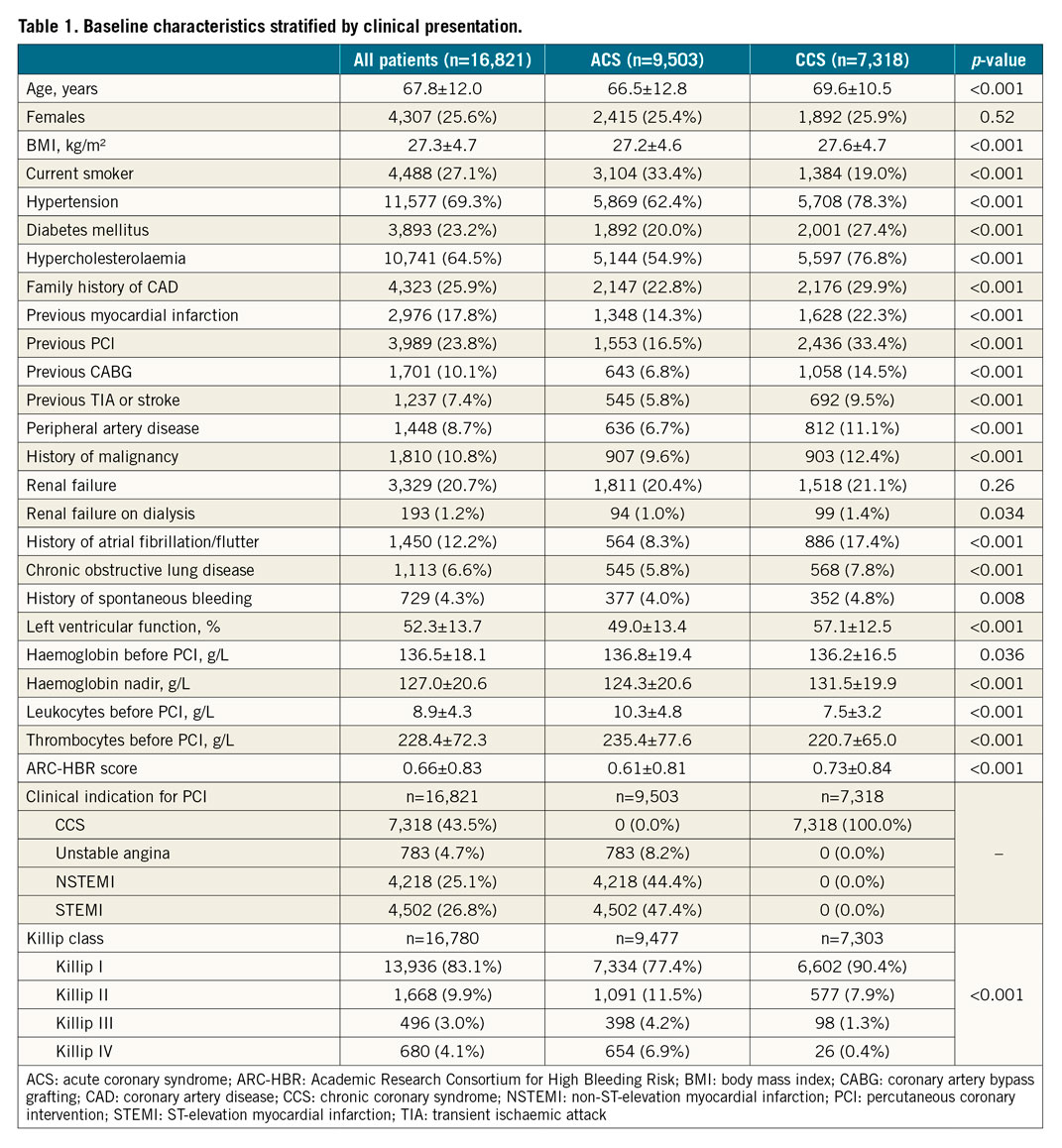
CLINICAL OUTCOMES AT ONE-YEAR FOLLOW-UP
At one year, BARC 3 or 5 bleeding occurred in 427 (4.97%) patients with ACS and 248 (3.60%) patients with CCS (unadjusted hazard ratio [HR] 1.41, 95% confidence interval [CI]: 1.20-1.64; p<0.001) (Table 2, Figure 1). At multivariable analysis, after adjusting for clinical, procedural, and treatment imbalances, ACS presentation remained independently associated with a higher risk of BARC 3 or 5 bleeding at one year (adjusted HR [adjHR] 1.21, 95% CI: 1.01-1.43; p=0.034) (Table 2).
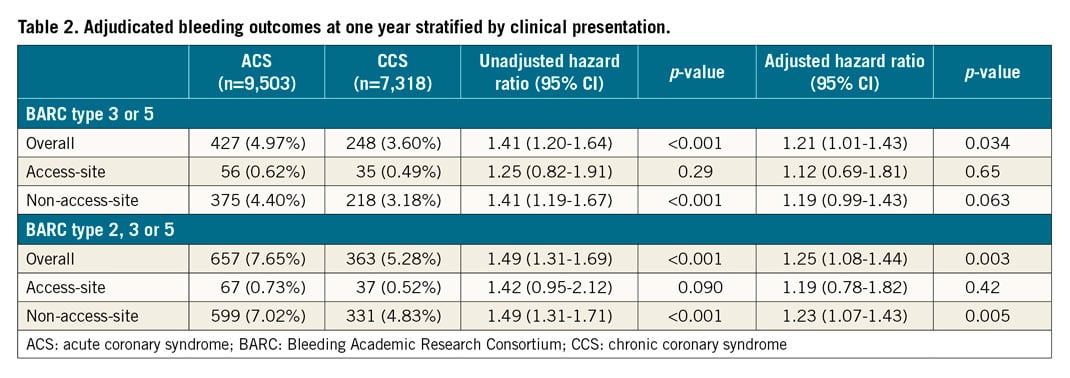
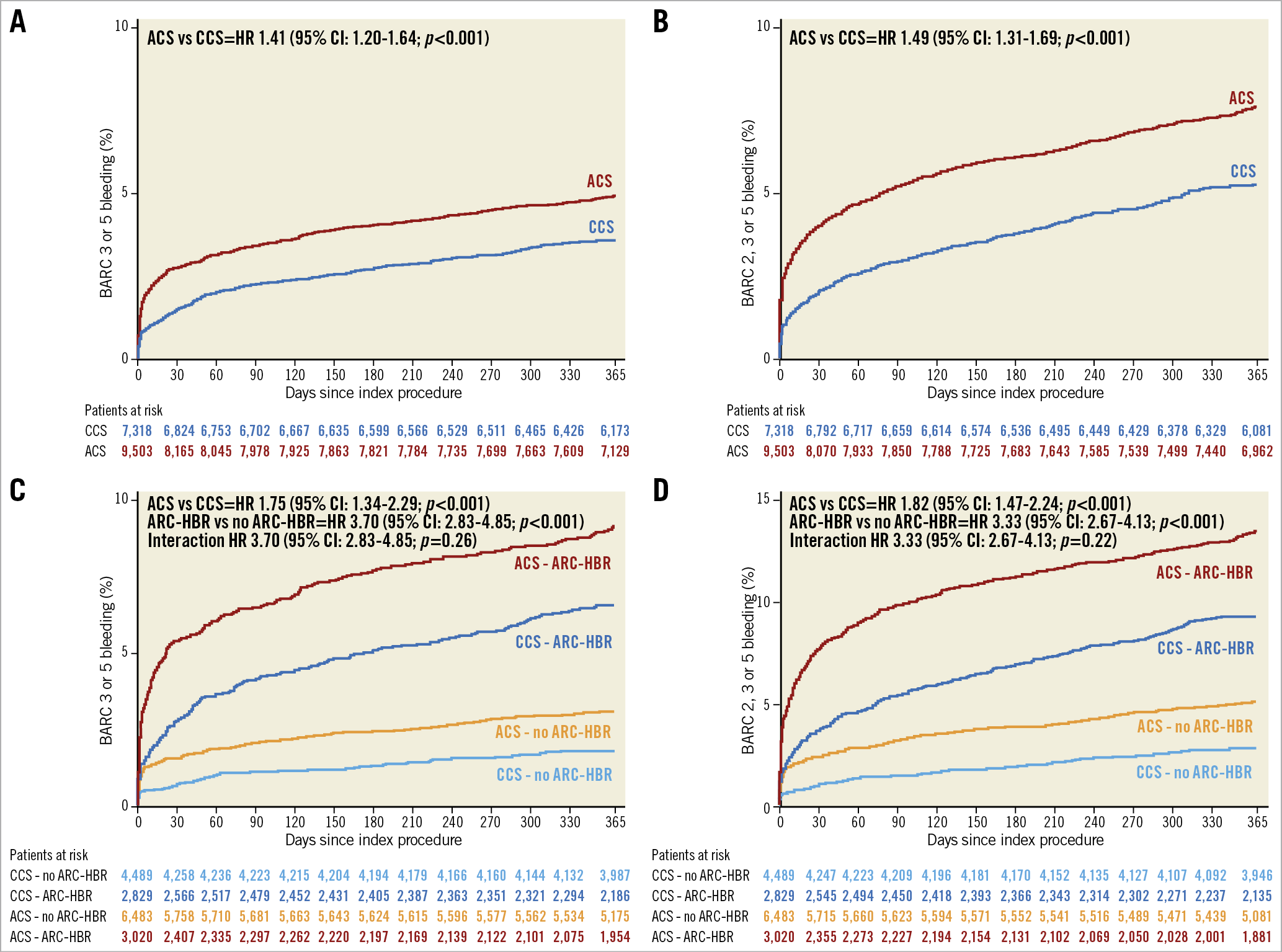
Figure 1. Kaplan-Meier event curves for BARC-defined bleeding at one year by clinical presentation. BARC 3 or 5 and BARC 2, 3 or 5 events in ACS (red) versus CCS (blue) (A & B), and further stratified by the ARC-HBR definition (C & D). ACS: acute coronary syndrome; BARC: Bleeding Academic Research Consortium; CCS: chronic coronary syndrome; HR: hazard ratio
BARC 2, 3 or 5 bleeding events at one year occurred in 657 (7.65%) ACS and 363 (5.28%) CCS patients (unadjusted HR 1.49, 95% CI: 1.31-1.69; p<0.001), and were independently predicted by ACS presentation after adjustment (adjHR 1.25, 95% CI: 1.08-1.44; p=0.003) (Table 2).
At sensitivity analyses, performed by adding “years of PCI” (i.e., 2009, 2010, etc.) as covariate in the multivariable model to explore a possible time effect on outcomes, the results remained largely consistent for both BARC type 3 or 5 (adjHR 1.27, 95% CI: 1.07-1.51; p=0.007) and type 2, 3 or 5 bleeding events (adjHR 1.22, 95% CI: 1.02-1.45; p=0.016).
ACS patients bled more than CCS patients also when the TIMI and the GUSTO scales were assessed (Supplementary Table 4). The incidence of intracranial or fatal bleeding did not differ. The rates of death, ischaemic, and composite outcomes are reported in Supplementary Table 4. Other bleeding risk factors at univariate and multivariate analyses are shown in Supplementary Table 5-Supplementary Table 8.
BLEEDING EVENTS STRATIFIED BY LOCATION, TIME OF ONSET, AND ACS SUBTYPES
The one-year incidence of access site and non-access site-related BARC 3 or 5 bleeding was 0.62% and 4.40% in the ACS group and 0.49% and 3.18% in the CCS group, respectively (Table 2). The corresponding figures for BARC 2, 3 or 5 bleeding were 0.73%, 7.02%, 0.52%, and 4.83%, respectively. After adjustment, ACS presentation remained independently associated with greater non-access site-related type 2, 3 or 5 bleeding (adjHR 1.23, 95% CI: 1.07-1.43; p=0.005), but with limited and inconclusive evidence for non-access site-related type 3 or 5 occurrences (adjHR 1.19, 95% CI: 0.99-1.43; p=0.063). Clinical presentation did not emerge as an independent predictor of access site-related bleeding (Table 2).
At landmark analysis, the excess of BARC bleeding among ACS patients occurred mainly within the first 30 days after PCI, but not thereafter, and was largely driven by a twofold higher risk of non-access site-related events (Table 3).
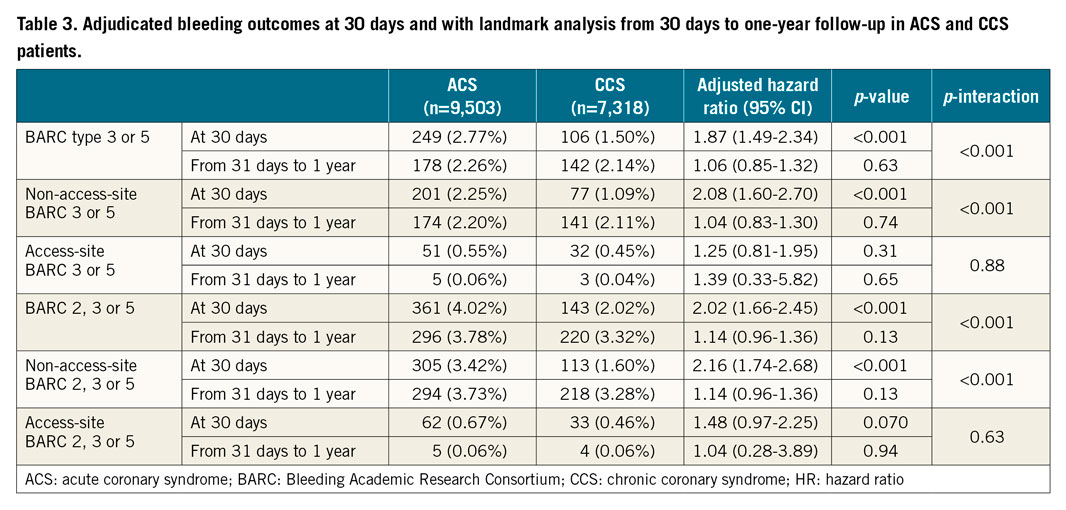
After adjustment, we found a gradient of BARC 3 or 5 bleeding risks across ACS subtypes, being the highest among STEMI patients (adjHR 1.92, 95% CI: 1.59-2.31; p<0.001), intermediate among NSTEMI patients (adjHR 1.26, 95% CI: 1.04-1.53; p=0.019), and negligible in unstable angina patients (adjHR 1.10, 95% CI: 0.74-1.66; p=0.63) (Figure 2). A similar graded-risk profile was noted for BARC 2, 3 or 5 bleeding. Clinical characteristics, procedural factors, and medications in patients presenting with unstable angina, NSTEMI, and STEMI are detailed in Supplementary Table 9-Supplementary Table 11.
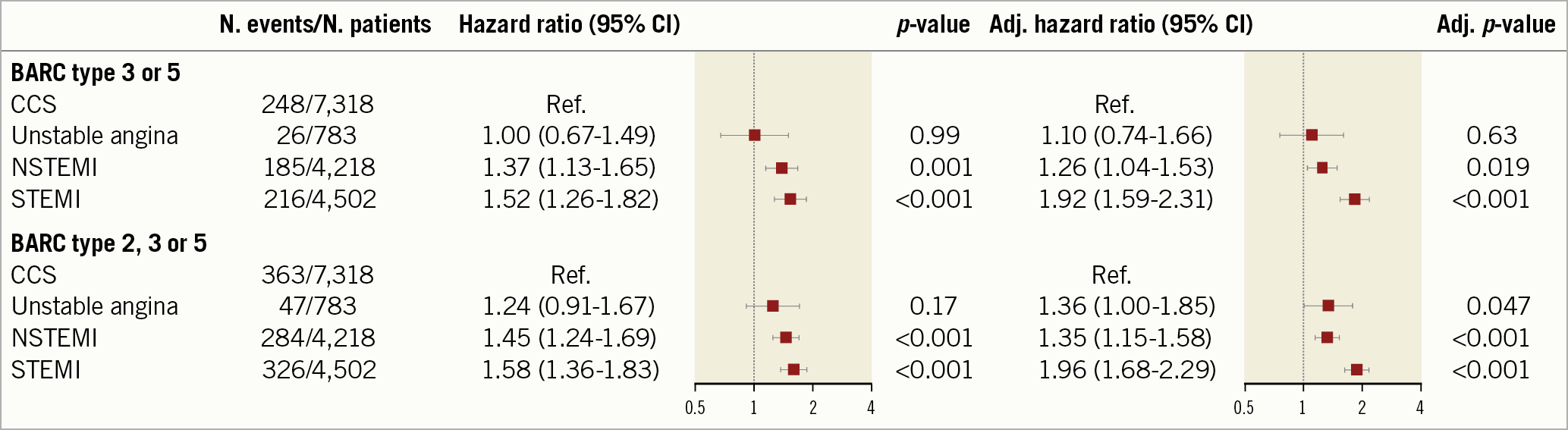
Figure 2. Adjudicated BARC-defined bleeding at one year by ACS subtype. Unadjusted (left) and adjusted (right) hazard ratio for BARC 3 or 5 and BARC 2, 3 or 5 bleeding. CCS is the reference. BARC: Bleeding Academic Research Consortium; CCS: chronic coronary syndrome; NSTEMI: non-ST-elevation myocardial infarction; STEMI: ST-elevation myocardial infarction
ARC-HBR CRITERIA IN PATIENTS WITH ACS AND CCS
Thirty-one percent (n=3,020) of ACS and 39% (n=2,869) of CCS patients qualified as HBR defined by the presence of at least one major or two minor ARC-HBR criteria. The risk of BARC-defined bleeding was significantly higher in patients who fulfilled versus those who did not fulfil the ARC-HBR definition for both ACS and CCS groups (Figure 1).
Severe anaemia, thrombocytopaenia, and recent surgery or trauma were more frequently met criteria among ACS patients, whereas advanced age, oral anticoagulants, chronic kidney disease (CKD), mild anaemia, prior stroke, and use of non-steroidal inflammatory drugs or corticosteroids were more common in CCS patients (Supplementary Figure 1A).
The unadjusted risks of BARC 3 or 5 bleeding for each ARC-HBR criterion by clinical presentation are displayed in Supplementary Figure 2. At multivariate analysis, after adjusting for each ARC-HBR criterion but the one considered, greater BARC 3 or 5 bleeding risk was found among ACS compared with CCS patients with no ARC-HBR criterion as well as those with moderate CKD, or recent spontaneous bleeding and also trended higher in patients with advanced age (Figure 3).
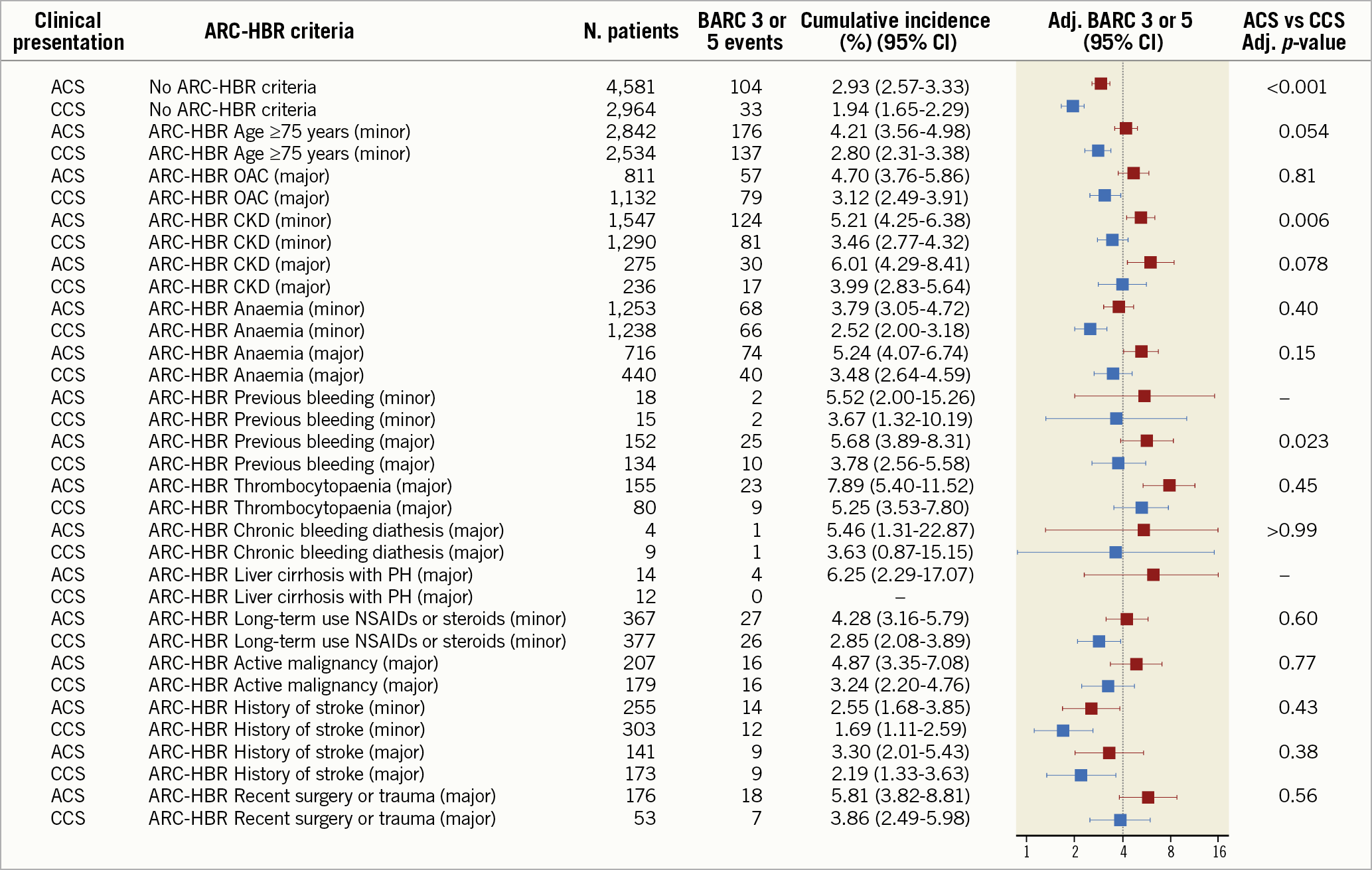
Figure 3. Adjusted incidence of BARC 3 or 5 bleeding at one year for minor and major ARC-HBR criteria in ACS (red) and CCS (blue). The estimates of the main effects model, adjusted cumulative incidence in case all other criteria are set to absent. Dotted line: 4% bleeding incidence expected in the presence of a major ARC-HBR criterion. ACS: acute coronary syndrome; Adj: adjusted; BARC: Bleeding Academic Research Consortium; CCS: chronic coronary syndrome; CKD: chronic kidney disease; NSAIDs: non-steroidal anti-inflammatory drugs; OAC: oral anticoagulation; PH: portal hypertension
ARC-HBR SCORE IN PATIENTS WITH ACS AND CCS
Mean ARC-HBR score was lower among ACS than CCS patients (0.61±0.81 vs 0.73±0.84; p<0.001), and distributed differently between study groups (Supplementary Figure 1B). For each 0.5-point increase in the ARC-HBR score from 0 to ≥2.5 points, a stepwise increase in the risk of one-year BARC 3 or 5 events was observed in both groups (Figure 4, Supplementary Figure 3). Compared with CCS, the incidence of BARC 3 or 5 events was higher among ACS patients with a score ≤1, but not for higher score values (Figure 4).
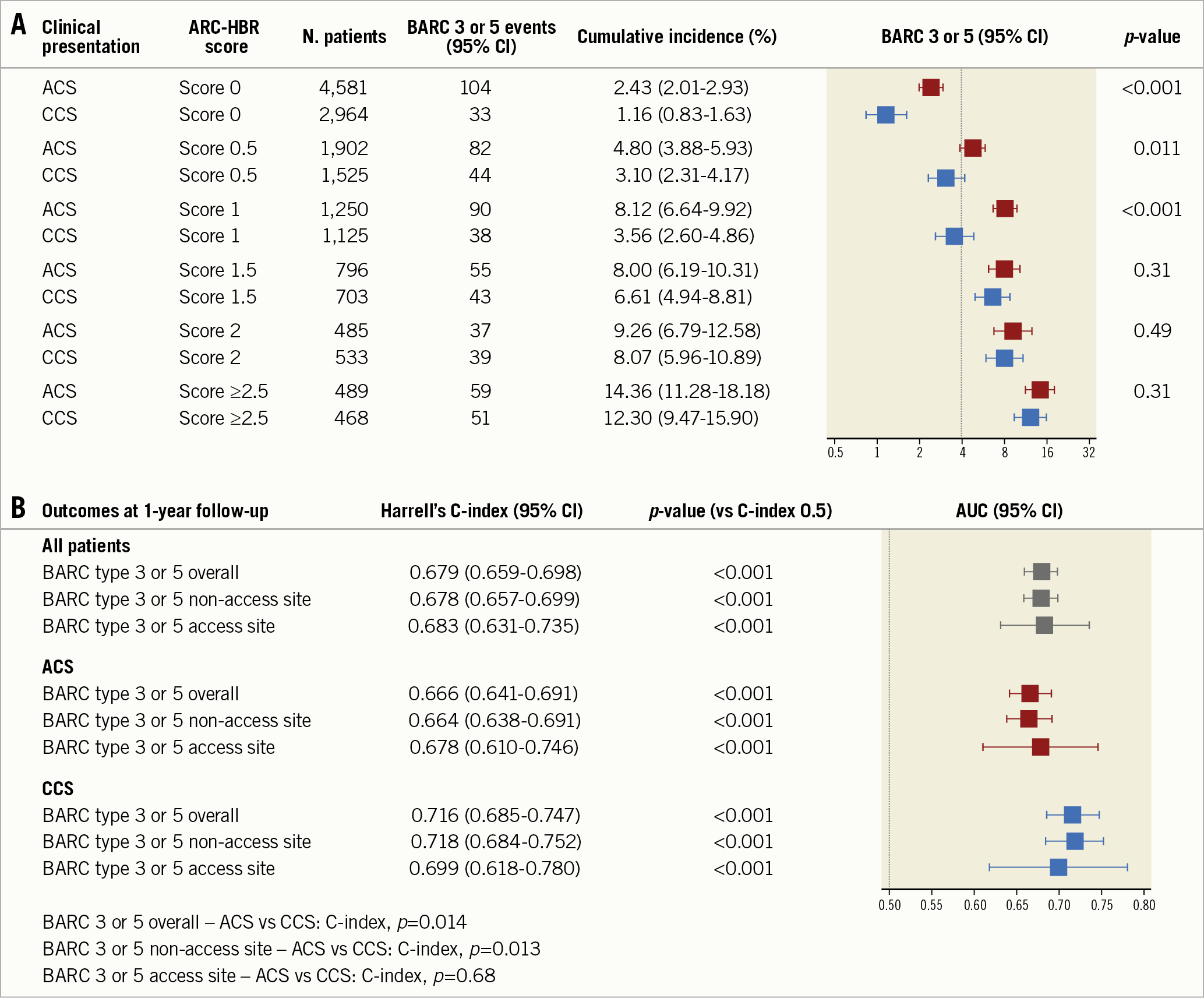
Figure 4. Adjudicated BARC 3 or 5 bleeding at one year by ARC-HBR score. Incidence of BARC 3 or 5 bleeding for score values from 0 to ≥2.5 in ACS (red) and CCS (blue) patients. The dotted line indicates a 4% incidence of bleeding, expected in the presence of one major or two minor ARC-HBR criteria (A). Harrell’s C-index of the ARC-HBR score for the prediction of BARC 3 or 5 bleeding (B). ACS: acute coronary syndrome; AUC: area under the curve; BARC: Bleeding Academic Research Consortium; CCS: chronic coronary syndrome; CI: confidence interval
The discrimination of the ARC-HBR score was lower among ACS compared with CCS patients with respect to BARC 3 or 5 bleeding (C-indices: 0.666 [95% CI: 0.641-0.691] vs 0.716 [95% CI: 0.685-0.747]; p=0.014). In the overall study population, the ARC-HBR score showed moderate discrimination to predict BARC 3 or 5 events (C-index: 0.679 [95% CI: 0.659-0.698]), which slightly increased when ACS was included as an additional minor risk criterion (C-index: 0.692 [95% CI: 0.673-0.711]) (Figure 4). On the other hand, the ARC-HBR score performance significantly diminished when the originally proposed binary ARC-HBR definition was adopted (C-index: 0.639 [95% CI: 0.621-0.658]).
Discussion
We investigated the impact of clinical presentation on centrally adjudicated and prospectively collected bleeding events at one year and the ARC-HBR stratified performance in bleeding risk prediction in a large and unselected population of 16,821 PCI patients. The main findings can be summarised as follows (Central illustration):
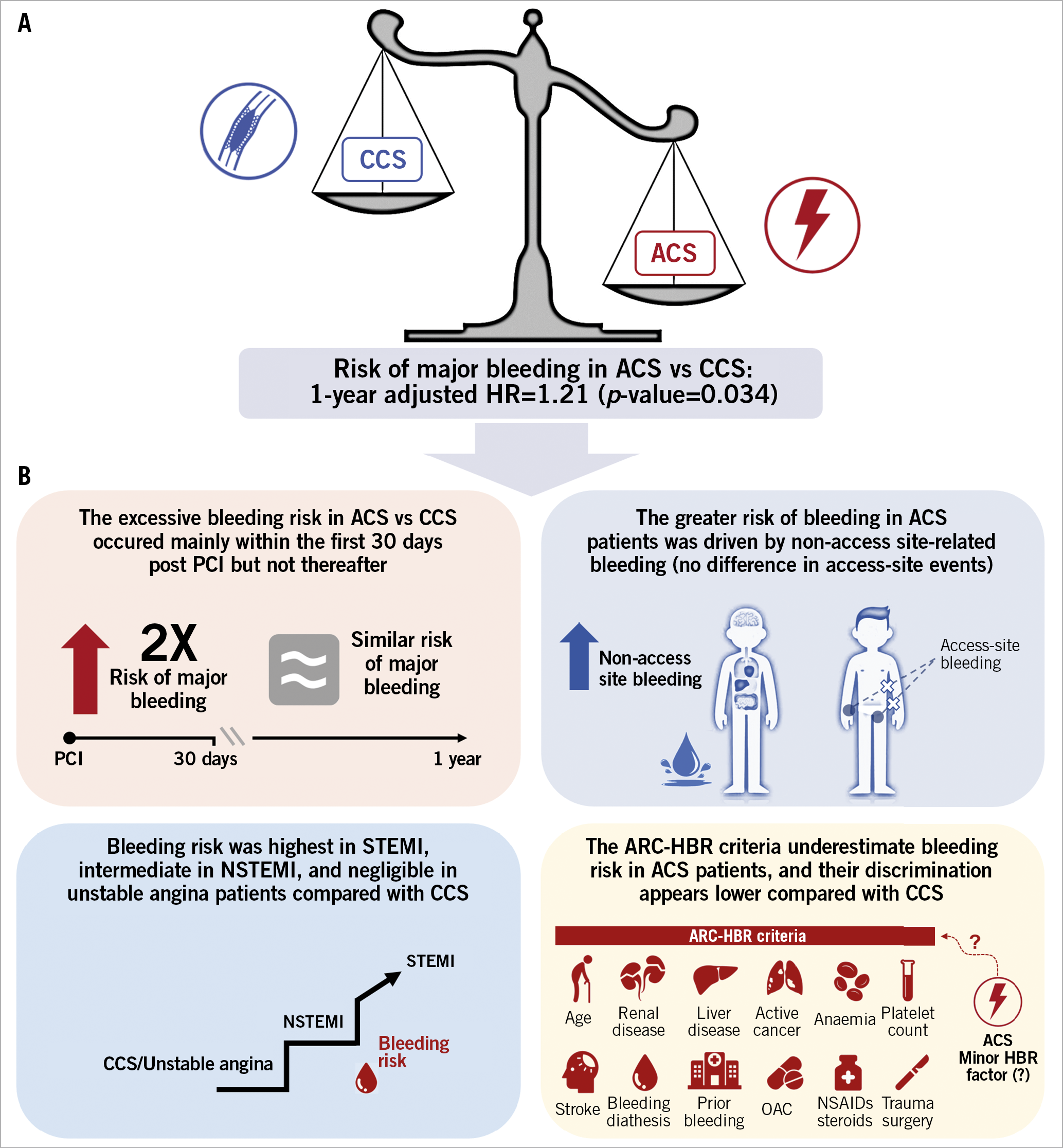
Central illustration. Clinical presentation and bleeding risk after PCI. ACS presentation per se predisposes to excessive bleeding risk compared with CCS (A) that is driven largely by non-access-site complications occurring mainly in the first 30 days. The bleeding risk was highest among STEMI, intermediate among NSTEMI, and minimal in unstable angina. ACS per se might qualify as a minor criterion in future iterations of the ARC-HBR framework (B). ACS: acute coronary syndrome; BARC: Bleeding Academic Research Consortium; CCS: chronic coronary syndrome; HR: hazard ratio; NSTEMI: non-ST-segment elevation myocardial infarction; OAC: oral anticoagulation; STEMI: ST-segment elevation myocardial infarction
1) ACS presentation per se, after extensive multivariable adjustment, remained associated with greater BARC type 3 or 5 and type 2, 3 or 5 bleeding risks.
2) The excess of bleeding among ACS patients was driven by non-access site-related events, which accrued mainly within the first 30 days and was highest among STEMI patients, intermediate among NSTEMI, and minimal for unstable angina patients.
3) Compared with CCS, ACS patients were less likely to qualify as high bleeding risk according to the ARC-HBR definition and presented lower ARC-HBR scores, mainly due to less frequent fulfilment of the advanced age and oral anticoagulation criteria.
4) ACS patients bled more than CCS patients across multiple ARC-HBR criteria. After multivariate adjustment, based on all ARC-HBR criteria but the one considered, ACS patients continued to show higher BARC 3 or 5 bleeding risk if they fulfilled none, moderate CKD or recent bleeding criteria among the ARC-HBR criteria as well as if their ARC-HBR score was ≤1.
5) The ARC-HBR score discrimination was lower among ACS compared with CCS patients. The inclusion of ACS as an additional minor risk criterion slightly improved the performance of the score in the overall study cohort.
Clinical presentation is deemed critical to guide ischaemic risk stratification by international guidelines in view of the higher risk of coronary events following ACS and the potential benefit accrued with intensified and prolonged antithrombotic therapy compared with CCS2,8. However, whether it should also inform bleeding risk assessment and encourage the adoption of bleeding avoidance strategies remains unclear.
Our analysis from a large contemporary cohort of consecutive PCI patients provides evidence that ACS presentation per se carries enhanced major and actionable bleeding risks within one year after PCI. The higher hazard associated with ACS was largely due to bleeding events unrelated to vascular access. As shown here, and consistent with many previous observations, non-access site-related bleeding events are much more prevalent than access site-related occurrences after PCI and have been associated with a roughly twofold greater mortality risk compared with access-site bleeding13. Therefore, our novel findings carry major implications for clinical practice.
Previous studies of PCI populations consistently reported a higher crude rate of bleeding complications – ranging from twofold to fourfold – in ACS versus CCS patients5,6,9,10,11,12,14. However, when potential confounders were adjusted for, ACS presentation emerged as an independent predictor of bleeding in some studies5,6,9,10 but not in others11,12. This apparent inconsistency might be due to relevant differences among studies in terms of patient characteristics, follow-up duration (i.e., in-hospital, 30-day, or long-term), antithrombotic regimen (i.e., extensive versus selective use of glycoprotein IIb/IIIa antagonists), as well as bleeding definition, location, and severity5,6,9,10,11,12. The relatively low number of patients and/or events in some studies may have hampered extensive multivariable adjustment.
Our analysis of a large, unselected, and contemporary PCI cohort shows that ACS presentation per se represents an independent determinant of spontaneous bleeding. Compared with CCS patients, the bleeding hazard in ACS was driven by an excess of events occurring in the first 30 days after the intervention. This observation might partly explain why clinical presentation emerged as an independent predictor of events in scores modelling short-term bleeding risk after PCI (i.e., REPLACE-2/ACUITY/HORIZONS, NCDR-CathPCI Registry 1, NCDR-CathPCI Registry 2)6,9 but not in those focusing on long-term occurrences (i.e., PARIS, PRECISE-DAPT)15,16.
Our analysis also suggests the existence of a graded risk across ACS subtypes. Compared with CCS, the risk of bleeding was not different in patients presenting with unstable angina, whereas an NSTEMI or STEMI presentation conferred a 26% and 92% increase in the adjusted hazard, respectively. These findings are consistent with previous algorithms including cardiac biomarkers and/or ST-segment changes as independent predictors of post-PCI bleeding3,5,6,9,17,18.
Acute presentation is a well-established determinant of ischaemic risk after PCI2,8. Our data add to this evidence that patients with ACS are also more prone to bleed and should therefore be carefully screened and managed to mitigate the concomitant high risk of ischaemic and bleeding complications. Given that the use of potent antithrombotic therapies is essential in ACS to prevent recurrent coronary events2, every effort should be made to identify and act promptly on modifiable bleeding risk factors (i.e., anaemia, falls/trauma, use of NSAIDs or corticosteroids) in the acute setting – particularly within the first 30 days after STEMI.
This is the first study validating the ARC-HBR criteria in ACS and CCS patients separately. The ARC-HBR consensus did not include the acute presentation among the HBR criteria because the increased bleeding risk associated with ACS was deemed to be related to the more aggressive antiplatelet therapy and inconsistent evidence from the literature. Our analysis suggests that the ARC-HBR score discrimination was lower in ACS compared with CCS, mainly because of an underestimation of the bleeding risk among patients at a lower score. After extensive adjustment, including type and duration of antithrombotic therapy, ACS presentation appears to affect bleeding risk independently by acting somewhat as a minor HBR criterion. This consideration derives from the observation that the risk of bleeding is higher in ACS than CCS patients but still below the proposed 4% risk threshold for one-year major bleeding when assessed in isolation. Of note, this effect seems to be dissipated at scores ≥1.5, when the presence of multiple HBR criteria might prevail on the risks associated with ACS per se.
Our previous analysis showed that most of the so-called minor criteria identify in isolation patients at HBR and should be regarded as major more than minor criteria4. Our current analysis adds to the previous one by suggesting that ACS might qualify as a truly minor criterion in future iterations of the proposed ARC-HBR nomenclature. This consideration is further supported by the observation that the discrimination ability of the ARC-HBR score slightly increases when ACS is included as an additional minor risk criterion.
The causes underlying a higher bleeding risk in ACS patients can only be speculated upon. Inflammation and haemostasis are known to be tightly intertwined as platelets, leukocytes, and interleukins are involved in both mechanisms7,19. The development of a pro-inflammatory state in ACS patients might lead to a pro-haemorrhagic state, which could partly explain the excess in bleeding complications – the so-called inflammatory bleeding7. This hypothesis potentially matches our findings which show a more pronounced effect in the early post-PCI phase and in STEMIs – both featuring a more prominent inflammatory response19. The possible role of inflammation is also supported by prior studies reporting an independent association between white blood cell count and bleeding risk after PCI16,20. Differences in patient management may also partly account (despite multivariable adjustments) for bleeding predisposition in ACS. As prompt mechanical reperfusion is critical in ACS2,8, bleeding risk assessment is usually incomplete before or shortly after the intervention (i.e., laboratory values not yet available). In addition, even if bleeding risk factors are known upfront, a timely PCI remains mandatory in ACS, giving little or no chance to address concomitant risk conditions adequately. Conversely, in CCS patients, careful assessment of bleeding risk is feasible, and PCI can be postponed pending the correction of modifiable factors predisposing to bleeding. Our analysis supports these considerations, showing that severe anaemia and history of recent trauma/surgery were less prevalent in the CCS than in the ACS cohort.
Altogether, our findings lend support to the concept that clinical presentation might integrate the bleeding risk stratification in addition to other validated risk factors such as those included in the ARC-HBR document, yet carrying a truly minor impact on risk prediction.
Limitations
Our results have several limitations. First, this is a single-centre study and may suffer from limited generalisability. However, the clinical characteristics of our study groups and the incidence of bleeding are consistent with those reported in multicentre cohorts3,6,9,12. Despite extensive adjustment, which was made possible by the large number of bleeding events accrued, we cannot exclude the effect of residual confounders, including more intense antithrombotic therapies in ACS than in CCS patients. Therefore, replication of our findings in other data sets remains desirable. Proton pump inhibitors were prescribed in about 40% of patients in our cohort. However, the prescription rate is overall consistent with other large contemporary registries of patients treated with DAPT for coronary artery disease21. Among the ARC-HBR criteria, eight were adjudicated retrospectively, which may potentially have resulted in an underestimation of their prevalence. Finally, one single major ARC-HBR criterion, planned post-PCI surgery, was not evaluated.
Conclusions
ACS presentation per se confers higher bleeding risk compared with CCS, largely owing to non-access-site complications that occur mainly in the first 30 days and seem more pronounced among STEMI patients. The ARC-HBR framework performed less well among ACS patients due to bleeding risk underestimation among those with no, one, or two minor criteria or a single major criterion. However, ACS presentation did not confer in isolation an HBR status and might therefore qualify as a minor criterion in future iterations of the ARC-HBR framework.
|
Impact on daily practice Acute coronary syndrome presentation per se predisposes to excessive bleeding risk after PCI compared with chronic coronary syndrome, even after adjusting for clinical and procedural factors. The higher risk of bleeding in acute coronary syndrome patients is largely due to non-access-site complications occurring mainly in the first 30 days, which seems more pronounced in the setting of STEMI. Clinical presentation can affect the risk of bleeding events after PCI and should therefore inform risk stratification and clinical decision making in daily practice. |
Guest Editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Funding
This study was supported by the Department of Cardiology at Bern University Hospital, Bern, Switzerland.
Conflict of interest statement
S. Stortecky reports grants from Edwards Lifesciences, Medtronic, Abbott Vascular, and Boston Scientific, and personal fees from Boston Scientific/BTG, and Teleflex, outside the submitted work. T. Pilgrim reports grants and personal fees from Biotronik, and Boston Scientific, personal fees from HighLife SAS, and other from Medtronic, outside the submitted work. D. Capodanno reports personal fees from AstraZeneca, Bayer, Sanofi, Boehringer, Daiichi Sankyo, and Biosensors, outside the submitted work. P. Urban reports other from CERC (CRO in Massy, France), during the conduct of the study, other from MedAlliance, and personal fees from Edwards Lifesciences, and Biosensors, outside the submitted work. L. Räber reports research grants to the institution by Abbott, Biotronik, Boston Scientific, Medis, Sanofi, and Regeneron, and speaker/consultation from Abbott, Amgen, AstraZeneca, Canon, Sanofi, and Vifor. S. Windecker reports research and educational grants to the institution from Abbott, Amgen, BMS, Bayer, Boston Scientific, Biotronik, Cardinal Health, CardioValve, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson&Johnson, Medtronic, Querbet, Polares, Sanofi, Terumo, and Sinomed. S. Windecker serves as unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Sinomed, V-Wave and Xeltis, but has not received personal payments from pharmaceutical companies or device manufacturers. He is also member of the steering/executive committee group of several investigator-initiated trials that receive funding from industry without impact on his personal remuneration. S. Windecker is an unpaid member of the Pfizer Research Award selection committee in Switzerland. M. Valgimigli reports personal fees from AstraZeneca, grants and personal fees from Terumo, personal fees from Alvimedica/CID, Abbott Vascular, Daiichi Sankyo, Opsens, Bayer, CoreFLOW, Idorsia Pharmaceuticals Ltd, Universität Basel | Dept. Klinische Forschung, Vifor, Bristol Myers Squibb SA, iVascular, and Medscape, outside the submitted work. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis, consultancy fees paid to his institution from Boehringer Ingelheim, and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

