
Abstract
Aims: The aim of this study was to determine the prognostic significance of periprocedural bleeding based on various definitions on 30-day and one-year all-cause mortality in patients undergoing routine or urgent percutaneous coronary intervention (PCI).
Methods and results: In this exploratory analysis of 25,107 patients enrolled in the three phase-3 CHAMPION trials, we assessed the prognostic impact of four bleeding scales (GUSTO, TIMI, ACUITY, and BARC) at 48 hrs. Follow-up all-cause mortality data were available at 30 days in all three trials, and at one year in CHAMPION PCI and CHAMPION PLATFORM. Bleeding rates within 48 hrs of PCI were variably identified by each clinical definition (range: <0.5% to >3.5%). Severe/major bleeding, measured by all bleeding scales, and blood transfusion requirement were independently associated with increased mortality at 30 days and one year after PCI (p<0.001 for all associations). Mild/minor bleeding was not independently predictive of one-year mortality (p>0.07 for all associations). Each bleeding definition demonstrated only modest ability to discriminate 30-day and one-year mortality (adjusted C-statistics range: 0.49 to 0.67).
Conclusions: Commonly employed clinical definitions variably identify rates of bleeding after PCI. Severe or major, but not mild or minor, bleeding is independently associated with increased 30-day and one-year mortality. These data may aid in selection of appropriate bleeding metrics in future clinical trials. CHAMPION PCI - ClinicalTrials.gov Identifier: NCT00305162; CHAMPION PLATFORM - ClinicalTrials.gov Identifier: NCT00385138; CHAMPION PHOENIX - ClinicalTrials.gov Identifier: NCT01156571.
Abbreviations
ACUITY: Acute Catheterization and Urgent Intervention Triage Strategy
BARC: Bleeding Academic Research Consortium
CHAMPION: Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition
FDA: Food and Drug Administration
GUSTO: Global Use of Strategies to Open Occluded Coronary Arteries
NCDR: National Cardiovascular Data Registry
NSTE-ACS: non-ST-segment elevation acute coronary syndrome
PCI: percutaneous coronary intervention
PS: propensity score
STEMI: ST-segment elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
Introduction
Bleeding complications related to percutaneous coronary intervention (PCI) continue to present major obstacles to contemporary interventional practice1. Periprocedural bleeding has emerged as an important marker of hospital quality, a performance metric, a target for bleeding avoidance strategies, and a key safety endpoint in clinical trials. Although consensus definitions have been proposed2, contemporary clinical trials continue to employ several traditional bleeding scales and the requirement for blood transfusions to define safety. As such, an appraisal of the relative prognostic significance of commonly employed clinical definitions of major periprocedural bleeding complications is required. We aimed to define the prognosis of periprocedural bleeding complications within 48 hrs using four different definitions and requirement for blood transfusion on 30-day and one-year all-cause mortality in the three phase-3 CHAMPION (Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition) trials.
Methods
THE CHAMPION PROGRAM
The study designs3 and primary results4-6 of each of the CHAMPION trials have been published previously. In brief, the CHAMPION trials4-6 were prospective, double-blind, double-dummy, randomised clinical trials evaluating cangrelor, an intravenous, rapidly acting, potent P2Y12 antagonist, compared with clopidogrel. The trials included patients undergoing elective or non-elective PCI for stable angina, non-ST-segment elevation acute coronary syndrome (NSTE-ACS), or ST-segment elevation myocardial infarction (STEMI). Patients who had received abciximab within five to seven days, or eptifibatide, tirofiban, or fibrinolytic therapy within 12 hours of randomisation were not eligible for enrolment. Receipt of an oral P2Y12 inhibitor within seven days of randomisation in CHAMPION PLATFORM and CHAMPION PHOENIX was not allowed, but clopidogrel at stable doses of ≤75 mg daily were permitted in CHAMPION PCI6. All patients provided informed consent for participation, and the institutional review boards or ethics committees at each participating site approved the trial protocol. Study participants were randomised to receive cangrelor (as a 30 μg/kg bolus followed by a 4 μg/kg/min infusion for ≥2 hours or for the duration of PCI, whichever was longer) or clopidogrel (300 mg or 600 mg at the beginning or end of PCI). All patients were administered aspirin (75-325 mg) and clopidogrel 75 mg daily during the first 48 hrs, after which dual antiplatelet therapy was determined by the individual operator. Periprocedural anticoagulation, choice of stent and access site, and sheath management protocol were similarly left to the discretion of the site investigators.
PERIPROCEDURAL BLEEDING EVENTS
Non-coronary artery bypass graft surgery-related bleeding was assessed by requirement for blood transfusion and by four scales (GUSTO [Global Use of Strategies to Open Occluded Coronary Arteries]7, TIMI [Thrombolysis In Myocardial Infarction]8, ACUITY [Acute Catheterization and Urgent Intervention Triage Strategy]9, and BARC [Bleeding Academic Research Consortium]2) at 48 hrs (Supplementary Table 1). Necessary clinical parameters to derive the BARC scale algorithmically were only available in the CHAMPION PHOENIX trial.
FDA BLEEDING EVENT REVIEW PROCESS
Bleeding events were reported by blinded investigators, but were not specifically adjudicated by a clinical events committee. However, during the regulatory approval process of cangrelor, the sponsor and the US Food and Drug Administration (FDA) reviewed bleeding events in a post hoc, unblinded manner in the CHAMPION PHOENIX trial. This post hoc review process identified a quantitatively greater number of events10. We take a closer look at whether this adjudication captures qualitatively more severe or meaningful bleeding events.
PRIMARY ENDPOINT
The primary endpoint for this analysis was all-cause mortality. Follow-up data were available at 30 days in all three trials, and at one year in two trials (CHAMPION PCI and CHAMPION PLATFORM).
STATISTICAL ANALYSIS
For descriptive purposes, baseline characteristics were compared in patients who did and did not require a blood transfusion within 48 hrs of PCI in the safety populations of the three CHAMPION trials of patients who underwent randomisation and received ≥1 dose of the assigned study drug. Subsequent modelling of prognostic associations with mortality was performed in patients who completed follow-up at 30 days or one year in the modified intention-to-treat populations of each trial (all patients who underwent index PCI and received the study drug).
Given the variable availability of follow-up data, bleeding scales, and post hoc review data across the three trials, we conducted two separate analyses. First, we determined the prognostic implications of periprocedural bleeding in CHAMPION PCI and CHAMPION PLATFORM on 30-day and one-year mortality. Second, we evaluated the prognostic significance of investigator-reported and post hoc ascertained bleeding events on 30-day mortality in CHAMPION PHOENIX. For each set of analyses, propensity scores (PS) were calculated for each patient based on logistic regression analysis accounting for the following covariate set known to influence bleeding or mortality: treatment randomisation (cangrelor vs. clopidogrel), age, sex, region of enrolment (US vs. non-US), indication for PCI (stable angina, NSTE-ACS, STEMI), diabetes mellitus, baseline anaemia (baseline haemoglobin <13 g/dL for men and <12 g/dL for women or baseline haematocrit <39% for men and <36% for women), or acquired thrombocytopaenia (baseline platelets ≥100,000/uL and post-baseline platelets <100,000/uL or >50% reduction from baseline). Crude and PS-adjusted logistic regression models were used to calculate odds ratios (OR) and 95% confidence intervals (CI) for the association between periprocedural bleeding, based on various clinical definitions, and mortality during follow-up. Patients who did not experience a bleed based on each individual bleeding scale or who did not require a blood transfusion served as the reference group. Crude and PS-adjusted C-statistics were calculated to determine the relative discriminatory power of each clinical definition of bleeding in predicting subsequent mortality. Sensitivity, specificity, accuracy, positive predictive value, and negative predictive value with respect to subsequent 30-day and one-year mortality were calculated for each of the bleeding scales.
Continuous variables are presented as mean±standard deviation (SD) or as median (interquartile range), and compared using Student’s t-tests or Wilcoxon rank-sum tests, as appropriate. Categorical variables are presented as n (%) and compared using chi-squared testing or Fisher’s exact tests, as appropriate; p<0.05 was considered statistically significant. All statistical analyses were performed using SAS software, version 9.3 (SAS Institute, Cary, NC, USA).
Results
Across the safety populations of the three CHAMPION trials, we characterised the baseline profiles of 25,107 patients (Supplementary Table 2). Patients who required blood transfusions within 48 hrs (n=160, 0.6%) were older (69.2±10.9 years vs. 63.0±11.2 years), female (59.4% vs. 27.6%), enrolled from the USA (56.9% vs. 43.4%), were more likely to present with an acute coronary syndrome, and had higher rates of comorbidities (diabetes mellitus, heart failure, peripheral artery disease; all comparisons p≤0.004). Although rates of glycoprotein IIb/IIIa inhibitor use were higher in patients who did compared with those who did not require a blood transfusion (12.6% vs. 5.8%; p=0.002), planned clopidogrel loading dose, utilisation of other periprocedural anticoagulants, and stent type did not differ by blood transfusion requirement (p>0.10 for all comparisons).
POOLED PATIENT-LEVEL ANALYSIS OF CHAMPION PLATFORM AND CHAMPION PCI
Within 48 hrs after PCI in the modified intention-to-treat populations, 35 patients (0.3%) experienced GUSTO-defined severe/life-threatening bleeding, 45 (0.3%) experienced TIMI-defined major bleeding, 501 (3.6%) experienced ACUITY-defined major bleeding, and 118 (0.8%) required blood transfusions. All-cause mortality occurred in 163 (1.2%) patients at 30 days and in 487 (3.5%) patients at one year after PCI. Mortality in patients who had experienced severe or major periprocedural bleeding events based on GUSTO, TIMI, and ACUITY scales were 25.7%, 20.0%, and 5.2%, respectively, at 30 days, and 25.7%, 20.5%, 9.1%, respectively, at one year (Table 1). Patients who required blood transfusions experienced a 30-day mortality rate of 11.9% and a one-year mortality rate of 19.1%. After accounting for key covariates included in the PS, severe or major periprocedural bleeding, measured by all bleeding scales, and blood transfusion requirement were independently associated with increased mortality at 30 days and one year after PCI (p<0.001 for all bleeding definitions) (Table 1). Although TIMI minor bleeding was independently associated with 30-day mortality (adjusted OR 4.12; 95% CI: 1.58-10.73; p=0.004), this association was no longer significant at one year. GUSTO mild and ACUITY minor bleeding were not independently predictive of subsequent mortality at either time point. From 30 days to one year, there were no additional deaths observed in patients who experienced periprocedural GUSTO-defined severe/life-threatening or TIMI-defined major bleeding events. In contrast, rates of mortality from 30 days to one year continued to rise in patients who experienced periprocedural ACUITY-defined major bleeding (5.2% to 9.1%) and in patients who required a blood transfusion (11.9% to 19.1%) (Table 1).
CHAMPION PHOENIX
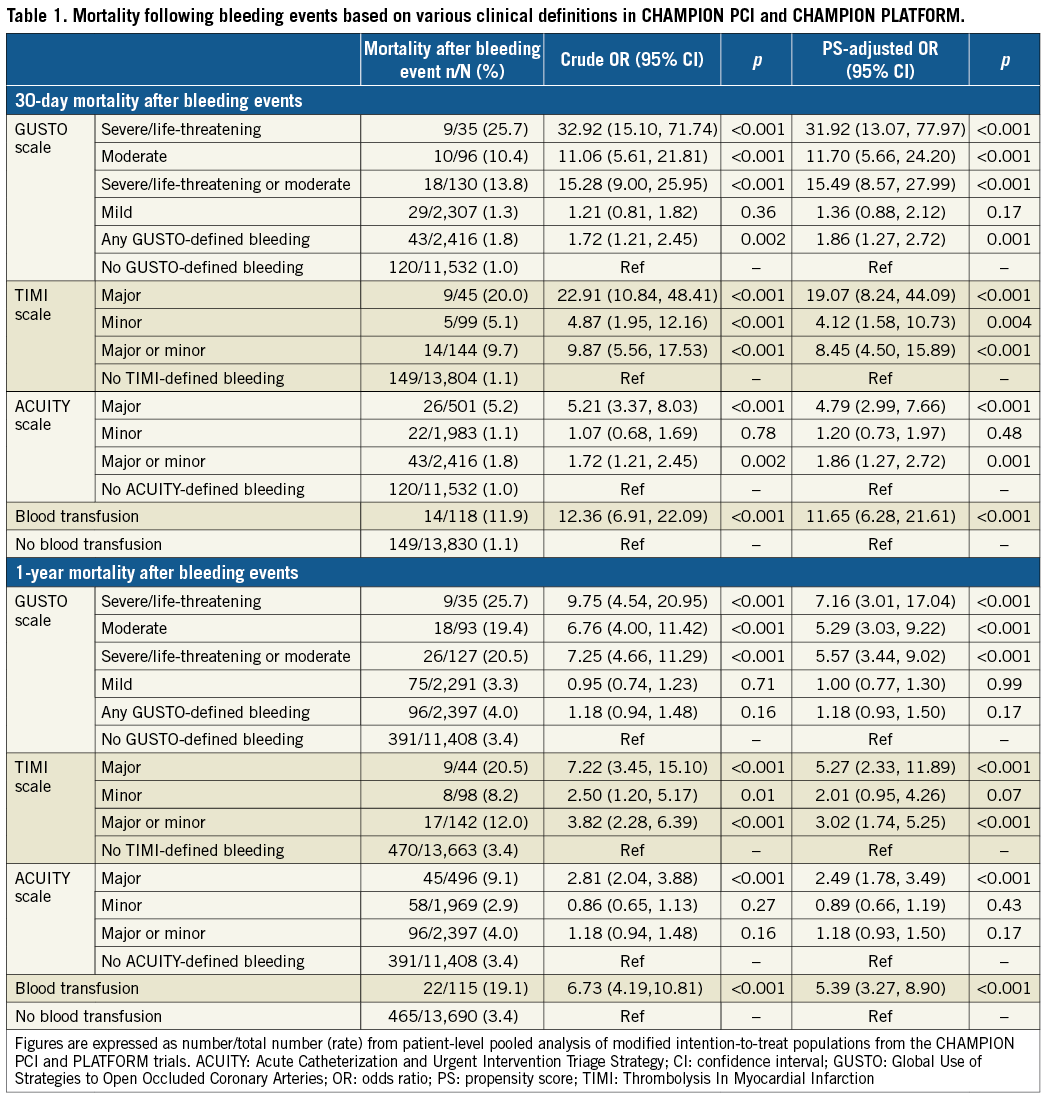
Investigator-reported rates of periprocedural bleeding within 48 hrs (Figure 1), overall 30-day mortality (n=115, 1.1%), and mortality rates in patients experiencing severe or major bleeding complications were similar in the CHAMPION PHOENIX trial to the other two CHAMPION trials (Figure 2, Supplementary Table 3). Consistently, severe or major periprocedural bleeding, based on the GUSTO, TIMI, and ACUITY scales, and blood transfusion requirement were independently associated with 30-day mortality (p≤0.02 for all bleeding scales), while mild or minor bleeding by each of these scales did not independently predict mortality. Of the 35 patients who experienced BARC type 3 bleeding events within 48 hrs, three (8.6%) died within 30 days. BARC type 3b bleeding within 48 hrs of PCI was independently associated with 30-day mortality (adjusted OR 16.82; 95% CI: 3.19-88.74; p=0.001). BARC type 1 and type 2 bleeding was not independently associated with mortality, and there were no deaths reported in patients who experienced BARC type 3a and type 3c bleeding.
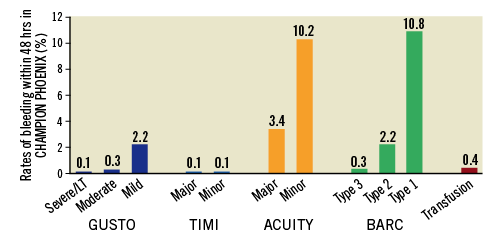
Figure 1. Rates of investigator-reported periprocedural bleeding events within 48 hrs in the CHAMPION PHOENIX trial. ACUITY: Acute Catheterization and Urgent Intervention Triage Strategy; BARC: Bleeding Academic Research Consortium; GUSTO: Global Use of Strategies to Open Occluded Coronary Arteries; LT: life-threatening; TIMI: Thrombolysis In Myocardial Infarction
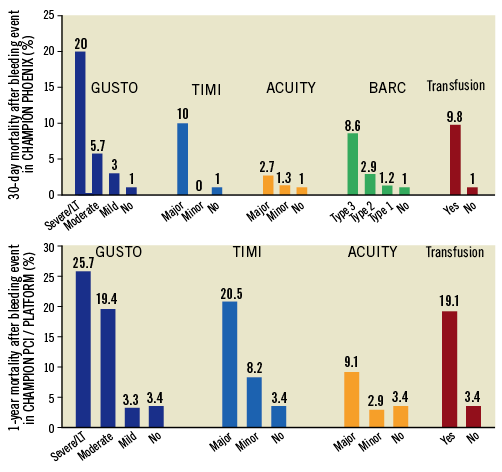
Figure 2. Take-home Figure. Rates of mortality in patients who experienced bleeding events within 48 hrs of percutaneous coronary intervention. Thirty-day mortality in the CHAMPION PHOENIX trial is displayed in the top panel, and one-year mortality in the CHAMPION PCI and CHAMPION PLATFORM trials is displayed in the bottom panel. ACUITY: Acute Catheterization and Urgent Intervention Triage Strategy; BARC: Bleeding Academic Research Consortium; GUSTO: Global Use of Strategies to Open Occluded Coronary Arteries; LT: life-threatening; TIMI: Thrombolysis In Myocardial Infarction
POST HOC BLEEDING EVENTS REVIEW
The sponsor and the US FDA undertook an independent review of bleeding events in the CHAMPION PHOENIX trial in a post hoc, unblinded manner (Supplementary Table 3). This process identified quantitatively greater bleeding events; however, these were predominantly mild/minor in severity (GUSTO mild bleeding: 235 investigator-reported events vs. 1,390 post hoc review events; and TIMI minor bleeding: 12 investigator-reported events vs. 43 post hoc review events). Mortality at 30 days following post hoc GUSTO mild bleeding and TIMI minor bleeding was 1.4% and 2.3%, respectively, and these post hoc events were not independently associated with 30-day mortality (p=0.15 for GUSTO mild bleeding, and p=0.38 for TIMI minor bleeding). There were numerically more GUSTO severe/life-threatening and TIMI major bleeds that were identified in post hoc review, but overall rates of bleeding events in these categories remained low. There was no quantitative or qualitative difference in bleeding events based on the ACUITY scale and blood transfusion requirement after post hoc review (Supplementary Table 3).
DISCRIMINATORY POWER AND PREDICTIVE ACCURACY
Each of the clinical definitions of bleeding demonstrated only modest discriminatory power in predicting subsequent short- and long-term mortality (Table 2). PS-adjusted C-statistics ranged from 0.54 to 0.67 for 30-day mortality and 0.53 to 0.63 for one-year mortality in the CHAMPION PCI and CHAMPION PLATFORM trials, and 0.49 to 0.67 for 30-day mortality in CHAMPION PHOENIX (Table 2). Sensitivity (patients who died after a bleeding event/total deaths) was low across bleeding scales, but best for ACUITY major (0.18) with respect to 30-day mortality in CHAMPION PCI and CHAMPION PLATFORM. Specificity (patients who survived without a bleeding event/all survivors) and negative predictive value (patients who survived without a bleeding event/all patients who did not experience a bleeding event) were high for all bleeding definitions at both time points, ranging from 0.96 to 1.00. Accuracy (patients who survived without a bleeding event and patients who died after a bleeding event/all patients) was high across bleeding definitions at both time points, ranging from 0.93 to 0.99 (Supplementary Table 4).
Discussion
In this exploratory analysis of ~25,000 patients undergoing PCI in the three phase-3 CHAMPION trials, we highlight a number of key findings: 1) severe/major periprocedural bleeding events, defined by several different bleeding scales, and need for blood transfusion are independently associated with mortality up to one year after PCI; 2) mild/minor bleeding events based on all bleeding definitions are not independently associated with excess late mortality; 3) post hoc and unblinded adjudication does not appear to change blood transfusion requirement or the number or type of bleeding events defined by the ACUITY scale; and 4) all bleeding scales demonstrated modest ability to discriminate mortality after PCI. Our primary findings were first demonstrated in a pooled, patient-level analysis of the CHAMPION PCI and CHAMPION PLATFORM trials, and then independently validated in the larger, robust, and more recent CHAMPION PHOENIX experience.
VARIABLE RATES AND PROGNOSIS BY CLINICAL BLEEDING DEFINITION
Our pooled data from the CHAMPION trials are consistent with both trial-based and registry-based analyses exploring this clinical question; however, these data provide several novel insights. GUSTO severe/life-threatening and TIMI major bleeding events are associated with an upfront mortality risk at 30 days that exceeds 20%. Indeed, although GUSTO severe/life-threatening bleeding events are rare, these complications are associated with the highest odds of subsequent mortality. However, no further deaths were identified in patients who experienced severe or major bleeding based on GUSTO or TIMI scales from 30 days to one year. This preferential prediction of early mortality is consistent with prior experiences11,12 and may be related to inclusion of life-threatening complications, including intracranial haemorrhage and bleeds of haemodynamic significance, in clinical definitions of bleeding events. In contrast, ACUITY-defined major bleeding captures a greater number of bleeding events. Early mortality risk associated with ACUITY major bleeding events was more modest (~5-10% at 30 days), but continued to accrue up to one year, a finding consistent with data from the ACUITY trial13. Blood transfusions may have more relevance in real-world practice given that they are readily identifiable, require limited adjudication, benefit from clinician familiarity, and appear to be independently associated with short- and long-term post-PCI mortality. The ACUITY bleeding scale and requirement for blood transfusion may identify patients with greater medical comorbidities, thus potentially contributing to the gradually accruing mortality risk over time. Our data also serve to validate the consensus BARC hierarchical grading scheme in patients undergoing PCI for a broad spectrum of indications, even beyond acute coronary syndrome14-16. We also demonstrate in this contemporary experience that mild or minor bleeding events do not carry independent prognostic value.
All contemporary bleeding definitions display only modest ability to discriminate subsequent mortality. These bleeding definitions were not developed with the intention to predict mortality, and the overall mortality rates in the CHAMPION experience were low. Furthermore, our models were not able to account for other potential hazards of periprocedural bleeding, including discontinuation or modification of chronic antiplatelet therapies. As such, these validated bleeding scales should continue to be used in contemporary clinical trials, and variation in their relative frequency and associated clinical impact may have important implications in trial design, endpoint selection, and sample size estimation.
IMPACT OF POST HOC REVIEW ON BLEEDING SEVERITY
The US FDA’s request to re-review bleeding data in the CHAMPION PHOENIX trial allows a unique look at the role of this process on the number and severity of identified bleeds, albeit in a post hoc and unblinded manner. Post hoc review captures quantitatively a greater number of events defined by the GUSTO and TIMI scales, but these identified events appear mild or minor in severity with low overall prognostic impact on mortality. Greater event identification via post hoc review may have been facilitated by more rigorous application of predefined clinical definitions of bleeding by reviewers and consideration of specific laboratory data and other adjunctive information not necessarily included on case report forms. There was no appreciable impact of the review process on the quantitative or qualitative capture of bleeding events defined by the ACUITY scale or by requirement for blood transfusion. Although routine adjudication in contemporary cardiovascular trials for all relevant endpoints remains controversial and may add to the cost and complexity of the trial17,18, our experience supports its application when feasible to quantify overall bleeding risk adequately.
Study limitations
There are certain limitations to our work. Despite multivariate accounting for potential covariates, measured or unmeasured factors related to periprocedural bleeding events may continue to confound the estimation of subsequent mortality. We did not incorporate time-updated clinical parameters, including changes in antithrombotic therapy in our baseline analytical models. Our models did not account for access site since this information was not routinely collected in the CHAMPION PCI and CHAMPION PLATFORM trials. Metrics of bleeding were not prospectively adjudicated in the CHAMPION trials, and follow-up in CHAMPION PHOENIX was limited to 30 days. Although data regarding the severity and grading of bleeding complications were collected, locations of bleeding and their relation to the access site were not tabulated. The BARC bleeding scale was developed and validated after the completion of CHAMPION PCI and CHAMPION PLATFORM and, as such, was only available in CHAMPION PHOENIX. Spontaneous bleeding events after 48 hrs were not captured. Severe or major criteria for certain bleeding definitions include fatal or near-fatal events, and thus may confound our analytic approach. Our findings may not be generalisable beyond the scope of the CHAMPION programme inclusion criteria, and newer potent P2Y12 inhibitors, such as prasugrel and ticagrelor, were not used in the three trials. Patients at increased risk of severe bleeding (e.g., recent intracranial haemorrhage) were not eligible for participation.
Conclusions
In this exploratory analysis of ~25,000 patients enrolled in a global contemporary PCI programme, severe or major periprocedural bleeding within 48 hrs of PCI was independently associated with subsequent mortality, especially soon after the index bleeding event. Bleeding scales variably identify rates of severe complications after PCI. Bleeding, as defined by the GUSTO and TIMI scales, predicts upfront mortality risk within 30 days. The GUSTO scale is highly specific and captures bleeding events with the greatest prognostic impact. The ACUITY bleeding scale and blood transfusion requirement continue to predict mortality risk up to one year post PCI and may not require specific adjudication. Our data provide further validation of the BARC scale as it independently predicts 30-day mortality after PCI. On a trial level, in weighing the optimal bleeding scale for an individual study, trialists will need to carefully account for the relative operating characteristics, discriminatory power, and prognostic implications of bleeding scales in power estimates and trial designs. On a patient level, tailored, individualised decision making regarding antithrombotic selection, intensity, and duration should be influenced not only by the risks of bleeding events, but also by their relative clinical implications on subsequent mortality.
| Impact on daily practice In this exploratory analysis of patient-level data from the three phase-3 CHAMPION trials, four commonly employed clinical definitions variably identify rates of bleeding after PCI, but appropriately risk stratify patients with respect to subsequent 30-day and one-year mortality. Bleeding, as defined by the GUSTO and TIMI scales, predicts upfront mortality risk within 30 days. The GUSTO scale is highly specific and captures bleeding events with the greatest prognostic impact. The ACUITY scale and blood transfusion requirement continue to predict mortality risk up to one year post PCI and may not require specific adjudication. These data from a global contemporary PCI programme may aid in selection of appropriate bleeding metrics in future clinical trials. |
Acknowledgements
We would like to thank Steven E. Elkin, MS, and Debra Bernstein, PhD, of The Medicines Company for their statistical support, along with Yuyin Liu, MS, and Lanyu Lei, MS, of the Harvard Clinical Research Institute for their independent verification of the analyses. Data analysis was also provided by FMD K&L Inc., funded by Chiesi USA, Inc., which currently markets cangrelor. Harvard Clinical Research Institute received funding from The Medicines Company and from Chiesi USA, Inc. for these analyses. A full list of the CHAMPION investigators can be found in Bhatt DL et al, Intravenous platelet blockade with cangrelor during PCI4; Harrington RA et al, Platelet inhibition with cangrelor in patients undergoing PCI6; and Bhatt DL et al, Effect of platelet inhibition with cangrelor during PCI on ischemic events5.
Funding
The CHAMPION Program was supported by The Medicines Company.
Conflict of interest statement
M. Vaduganathan is supported by the NHLBI T32 postdoctoral training grant (T32HL007604). R. Harrington discloses the following relationships – advisory board: Evidint, Regado, Scanadu; honoraria: Amgen, Daiichi-Lilly, Gilead Sciences, Gilead Sciences Inc., Janssen R&D, Medtronic, Merck, Novartis Corporation, The Medicines Company, Vida Health, Vox Media, WebMD; other: American Heart Association; research funding: AstraZeneca, BMS, CSL Behring, GSK, Merck, Portola, Sanofi Aventis, The Medicines Company; ownership interest: Element Science, MyoKardia. G. Stone discloses the following relationships – honoraria: Boston Scientific, InspireMD, Atrium, Eli Lilly – Daiichi Sankyo partnership, AstraZeneca. P. Steg discloses the following relationships – research funding (to INSERM U1148): Sanofi, Servier; speaking or consultant fees: Amarin, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers-Squibb, CSL-Behring, Daiichi-Sankyo, GlaxoSmithKline, Janssen, Lilly, Novartis, Pfizer, Regeneron, Roche, Sanofi, Servier, The Medicines Company; stock ownership: Aterovax. C. Gibson discloses the following relationships – honoraria: The Medicines Company. C. Hamm discloses the following relationships – honoraria: AstraZeneca, Sanofi Aventis, Lilly; research funding: AstraZeneca, The Medicines Company. M. Price discloses the following relationships – honoraria: AstraZeneca, Merck & Co., Accriva Diagnostics, The Medicines Company. R. Lopes received consulting fees from Bayer Corporation US (Bayer AG/Bayer in Japanese subsidiaries), Boehringer Ingelheim, Merck, Pfizer, and Portola; research funds and consulting fees from Bristol-Myers Squibb and GlaxoSmithKline; and research funds from Guidant Corporation. S. Leonardi reports no relationships relevant to the contents of this paper to disclose. E. Deliargyris was an employee of The Medicines Company at the time of the analysis and remains a shareholder. J. Prats was an employee of The Medicines Company at the time of the analysis and reports consulting fees from Chiesi USA. K. Mahaffey’s financial disclosures can be viewed at http://med.stanford.edu/profiles/kenneth-mahaffey. H. White discloses the following relationships – honoraria: AstraZeneca, Sirtex and Acetelion; research funding: Sanofi-Aventis, Eli Lilly, National Health Institute, GlaxoSmithKline, Merck Sharpe & Dohme, Omthera Pharmaceuticals, Pfizer, Elsai, Dalgen Products, and AstraZeneca. D. Bhatt discloses the following relationships – advisory board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; board of directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; chair: American Heart Association Quality Oversight Committee; data monitoring committees: Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); research funding: Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi (including for serving as co-Chair and co-PI of the CHAMPION trials), Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company (including for serving as co-Chair and co-PI of the CHAMPION trials); royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); site co-investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott); trustee: American College of Cardiology; unfunded research: FlowCo, Merck, PLx Pharma, Takeda.
Supplementary data
Supplementary Table 1. Definitions of severe or major periprocedural bleeding.
Supplementary Table 2. Baseline characteristics of patients who did and did not require blood transfusions within 48 hrs in the safety populations of the three CHAMPION trials.
Supplementary Table 3. Mortality following investigator-reported and post hoc ascertained bleeding events based on various clinical definitions in CHAMPION PHOENIX.
Supplementary Table 4. Test characteristics of clinical definitions of periprocedural bleeding events in predicting subsequent mortality.
To read the full content of this article, please download the PDF.

