
The obligate period of antiplatelet therapy after percutaneous coronary intervention (PCI) continues to be one of the most important considerations in the treatment of coronary artery disease. In a population that is ageing and with increasing incidence of atrial fibrillation, renal dysfunction, diabetes, and obesity, there are competing and overlapping risks for bleeding events and thrombotic events. At the same time, the newer-generation drug-eluting stents have significantly lower rates of stent thrombosis, and a shorter duration of antiplatelet therapy may become more commonplace.
The PARIS and PRECISE-DAPT scores have been developed to predict bleeding in patients who have undergone PCI1,2. The PARIS risk score was developed from a cohort of 4,190 patients and validated in ADAPT-DES. The score takes into account age, body mass index (BMI), smoking, anaemia, chronic kidney disease, and the use of triple therapy. The bleeding risk score had a C-statistic of 0.64 for BARC 3 or 5 (major) bleeding1. The PRECISE-DAPT score was developed in 14,963 patients with PCI pooled from eight trials. Similar to PARIS, the PRECISE-DAPT score includes age and renal function, though it does not include BMI, smoking, or any patients on anticoagulation, but instead includes haemoglobin, white blood cell count, and prior bleeding. In the PLATO validation cohort, PRECISE-DAPT showed a C-statistic of 0.70 for TIMI major or minor bleeding and a C-statistic of 0.66 in BERN-PCI2.
In this issue of EuroIntervention, Abu-Assi et al have conducted an insightful analysis of the performance of the PARIS and PRECISE-DAPT scores in predicting bleeding among 1,926 patients with in-hospital PCI and discharged on DAPT from the University Hospital Complex of Vigo3.
They found that the PARIS risk score classified significantly more patients as intermediate risk for bleeding than the PRECISE-DAPT score (43% vs. 20%), while the PRECISE-DAPT score classified significantly more patients as high risk (39% vs. 15%). Both scores performed modestly with respect to BARC type 2, 3, or 5 bleeding (C-statistic=0.61 for PRECISE-DAPT and 0.63 for PARIS). Both scores performed somewhat better with respect to BARC type 3 or 5 (major) bleeding (C-statistic for both=0.73).
One of the most important strengths of this study is to validate these two newer bleeding risk scores in a “real-world” PCI patient population rather than in a trial population. The study population is older, had more chronic kidney disease, lower BMI, and higher rates of triple therapy use compared with the PARIS study. This cohort had nearly double the proportion of patients receiving triple therapy than PARIS, while PRECISE-DAPT had none. Indeed, triple therapy was the most important predictor of major bleeding events other than anaemia. Though not the focus of this analysis, these data provide yet another reason to try to avoid triple therapy.
The authors wisely chose to categorise bleeding events in this study by BARC criteria and to conduct a separate analysis for major bleeding alone, compared with major and minor bleeding4. To argue in favour of including minor bleeding events, it was shown in an analysis of REPLACE-2 that using too stringent a definition of bleeding endpoints could underrepresent this cause of late mortality5. On the other hand, in a more recent analysis of the performance of BARC, TIMI, GUSTO, and ACUITY bleeding events in the pooled CHAMPION trials, it was shown that only major bleeding events had any effect on thirty-day or one-year mortality6.
As with other bleeding risk scores, validation in retrospective cohorts has inherent limitations. In addition, this study population includes only patients who had initially presented with acute coronary syndromes, receiving differing anticoagulation and antiplatelet therapies. Nearly one third of patients had received a bare metal stent, and over 70% of patients who received PCI for ACS in this study were treated with clopidogrel rather than ticagrelor or prasugrel. These factors do somewhat limit the generalisability of the results in more current practice, though similar limitations are evident with the other available risk scores.
The modestly strong predictive performance of these risk scores is on a par with prior risk scores: the CRUSADE bleeding score, developed from approximately 72,000 patients with NSTEMI, had a C-statistic of 0.71 in the validation cohort7. CRUSADE does not take age into account. The REACH bleeding score was developed from approximately 68,236 patients with coronary disease, largely in outpatients with stable coronary disease. While there is granularity in this score with regard to the number of antiplatelets and the presence or absence of systemic anticoagulation, the score does not take into account weight or chronic kidney disease. When validated in CHARISMA, it had a C-statistic of 0.648.
Despite these limitations, the use of risk scores has been shown to be superior to clinical judgement and is recommended in international guidelines. As electronic medical records and point-of-care decision-making tools become increasingly available and sophisticated, the possibility of personally tailored antithrombotic therapy becomes ever more real. Yet the competing risks of bleeding and thrombosis after PCI are not static over time. In an elegant analysis of HORIZONS-AMI, it was shown that the relative risk of thrombosis and bleeding may change from day to day - with the highest and most dynamic bleeding and thrombosis risks in the first thirty days after PCI9. In addition to individual patient characteristics, procedural factors such as stent size, access site, periprocedural antithrombotic therapy, and selection of oral antiplatelet agents have been shown to affect significantly the bleeding and thrombosis profile over time. Ideally, when a patient presents with an acute or stable coronary syndrome, the bleeding risk could be appropriately assessed up front by evidence-based scores such as the ones described in this issue, allowing measures to be taken to mitigate this risk (Figure 1). Future studies are needed to understand better the relative risk of bleeding versus thrombosis over time in different populations, and prospective studies are needed to optimise the use of risk score-based individualised post-PCI care.
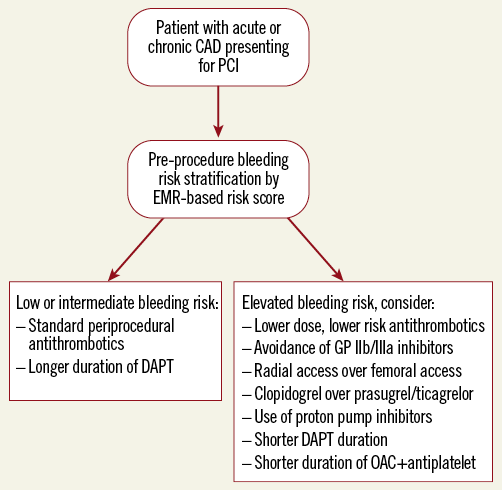
Figure 1. Suggested algorithm for the assessment of bleeding risk in patients undergoing PCI. CAD: coronary artery disease; DAPT: dual antiplatelet therapy; EMR: electronic medical record; GP: glycoprotein; OAC: oral anticoagulant
Conflict of interest statement
D. Bhatt discloses the following relationships - Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott); Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda. B. Peterson has no conflicts of interest to declare.

