Until recently, patients at high bleeding risk (HBR) were underrepresented in randomised trials of patients undergoing percutaneous coronary intervention (PCI). Lately, however, this important patient population has attracted much attention, with a number of completed and ongoing (NCT03023020; NCT03287167) randomised trials focusing specifically on HBR patients1,2,3,4. While this is a welcome development, the inclusion criteria in such trials have differed considerably. These differences are reflected in the marked variations in major bleeding rates across published trials1,2,3,4,5. Against this background, the Academic Research Consortium for High Bleeding Risk (ARC-HBR) recently proposed criteria to standardise the definition of HBR for use in clinical trial enrolment. Based on consensus, HBR was arbitrarily defined as a rate of Bleeding Academic Research Consortium (BARC) 3 or 5 bleeding of ≥4% or intracranial haemorrhage (ICH) of ≥1% at one year. A number of risk factors for bleeding were identified and classified as major or minor criteria based on these cut-offs. The presence of ≥1 major or ≥2 minor criteria was proposed to confer HBR status6.
In the current issue of EuroIntervention, Ueki et al report a validation of the proposed criteria and a comparison with the discrimination of two contemporary bleeding risk scores – the PRECISE-DAPT and PARIS scores7,8,9. The authors retrospectively analysed data from 12,121 consecutive patients enrolled in the Bern PCI registry who underwent PCI between 2009 and 2016, with independently adjudicated BARC bleeding and ischaemic events at one-year follow-up10.
The main finding was that the presence of ≥1 major or ≥2 minor criteria was associated with a BARC 3 or 5 bleeding rate of ≥4% at one year, whereas the presence of one minor criterion was associated with a one-year BARC 3 or 5 bleeding rate of <4%, with each additional bleeding criterion increasing the bleeding rate in a stepwise fashion. However, a minority of individual criteria were associated with higher or lower than expected bleeding rates when considered in isolation. The rate of BARC 3 or 5 bleeding was more than threefold higher in patients meeting ARC criteria for HBR compared with those who did not (6.4% vs 1.9%, p<0.001). Importantly, the rate of ischaemic events – measured according to the device-oriented composite endpoint (DOCE), comprising cardiac death, target vessel myocardial infarction, or target lesion revascularisation – was also higher in ARC-defined HBR compared with non-HBR patients (12.5% vs 6.1%, p<0.001). Finally, compared with the PRECISE-DAPT and PARIS risk scores, the ARC-HBR criteria were more sensitive but less specific at predicting BARC 3 or 5 bleeding events.
A validation study is a critical part of the evaluation of a consensus-based definition and the authors should be commended for undertaking this analysis in a timely manner. It provides evidence of the performance of the ARC-HBR consensus definition in a real-world population. While it broadly supports the one-year 4% cut-off in BARC 3 or 5 bleeding used to define HBR, it suggests that there may be a need for future adjustment of some criteria. It also confirms that patients at the highest risk of bleeding events also tend to be those at the highest risk of ischaemic/thrombotic events. This has important implications for the use of these criteria for decisions regarding the duration or intensity of antithrombotic therapy.
A number of limitations should be considered when interpreting the results. The study was single-centre in nature, which impacts on external validity. In addition, data on a number of the ARC-HBR criteria were not available. This limits internal validity. Moreover, while it is interesting to compare the performance of the ARC-HBR criteria with that of two risk scores for prediction of bleeding, it should be remembered that the ARC-HBR criteria were not intended for use as a quantitative bleeding risk score, rather as a tool to standardise inclusion criteria for enrolment in clinical trials of HBR patients. Indeed, individual criteria carry different bleeding risks and the definition does not take the differential bleeding risk of each criterion into account. Finally, and most importantly, in clinical practice, bleeding risk is dependent not only on patient characteristics at baseline, but also on the antithrombotic therapy regimen – e.g., choice of P2Y12 inhibitor, DAPT duration, use of dual versus triple therapy in patients taking oral anticoagulation (OAC) and compliance. The lack of information regarding antithrombotic therapies is an important limitation.
The finding that anticipated long-term use of OAC – a major criterion per the ARC-HBR definition – was associated with a one-year BARC 3 or 5 bleeding rate of only 2.5% is surprising. In randomised trials evaluating antithrombotic therapy regimens in patients taking OAC after PCI or acute coronary syndrome, observed rates of BARC 3 or 5 bleeding (or Thrombolysis In Myocardial Infarction [TIMI] major or minor bleeding, which closely aligns with it) at one year were consistently >4% with strategies of a vitamin K antagonist (VKA) plus DAPT, VKA plus a P2Y12 inhibitor, non-VKA OAC (NOAC) plus DAPT and NOAC plus a P2Y12 inhibitor, except when reduced-dose NOAC was used in the latter group10,11,12,13,14,15. In this respect, this finding is difficult to interpret in light of the lack of information on antithrombotic strategies in OAC patients.
On the other hand, moderate chronic kidney disease (CKD) in isolation – a minor criterion per the ARC-HBR definition – was associated with a higher than expected one-year BARC 3 or 5 bleeding rate of 4.8% in the data set of Ueki et al. A potential explanation may be the co-existence of unknown confounders that were criteria in the ARC-HBR definition but were not collected in the registry (e.g., bleeding diathesis). Alternatively, the bleeding risk of patients with moderate CKD may be underestimated by the ARC-HBR criteria. Bleeding risk continues to increase with worsening renal parameters, and it is possible that the cut-off for a minor criterion chosen by the ARC-HBR is too low. In this respect, as more data become available, the criteria for CKD may need to be recalibrated.
Other criteria used by Ueki et al were defined differently from the definitions used by the ARC-HBR. In fact, this may well explain why they were associated with lower than expected BARC 3 or 5 bleeding rates. For example, when considering ischaemic stroke, the authors included any stroke as a major criterion, whereas ARC-HBR included only moderate or severe ischaemic stroke occurring within the previous six months. Moreover, this was based on data supporting an ICH rate of ≥1% rather than a BARC 3 or 5 bleeding rate of ≥4% in such patients. Ultimately, complete validation of all of the proposed criteria will only be possible with prospective data collection.
A number of the findings of Ueki et al confirm the findings of other validation studies. The 39% prevalence of ARC-defined HBR patients is similar to that observed in all-comer PCI registries from Japan and the USA (43% and 44%, respectively)16,17. Consistent with randomised trials in HBR patients, the most commonly fulfilled HBR criteria in these validation studies were age ≥75 years, OAC, anaemia, and CKD. Against this, OAC represented a relatively smaller proportion of each of the real-world HBR cohorts (10.5%, 8.2%, and 18.5%, respectively) compared with 36-39% in randomised trials in HBR patients1,2,3. It is notable that the importance of OAC as a predictor of bleeding was also called into question in the Japanese study, where warfarin was used in all cases16. All three validation studies showed that patients who met the ARC criteria for HBR had more comorbidities, higher cardiovascular risk, and more complex cardiovascular disease compared with those who did not. Finally, the finding of a threefold increase in major bleeding and a doubling in ischaemic/thrombotic events was consistent across the studies (Figure 1).
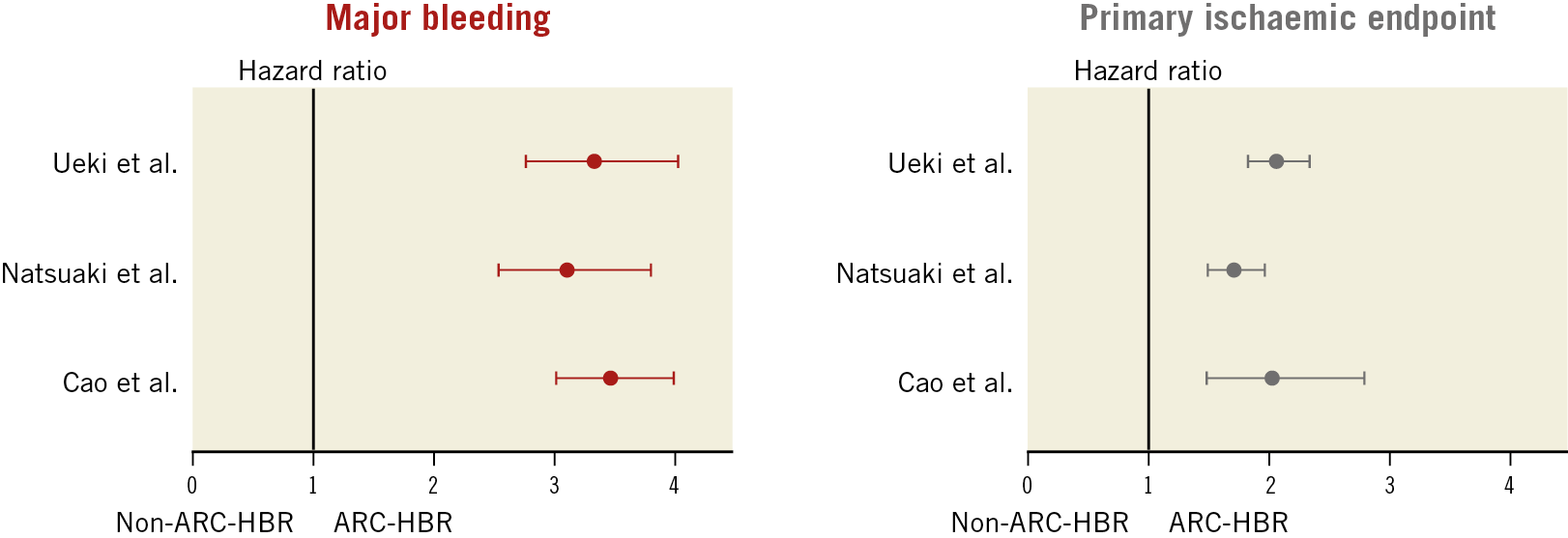
Figure 1. Primary bleeding and ischaemic endpoints in HBR versus non-HBR patients according to the ARC-HBR criteria in validation studies in real-world PCI registries. In the studies of Ueki et al9, Natsuaki et al16, and Cao et al17, the primary bleeding endpoints were BARC 3 or 5, GUSTO moderate or severe, and study-defined major bleeding, respectively, and the primary ischaemic endpoints were the device-oriented clinical endpoint (DOCE; the composite of cardiac death, target vessel myocardial infarction, or target lesion revascularisation), the composite of myocardial infarction or ischaemic stroke, and post-discharge myocardial infarction, respectively. Hazard ratios and 95% confidence intervals were calculated by the authors when they were not provided by the investigators. ARC-HBR: Academic Research Consortium for High Bleeding Risk
Overall, the analysis of Ueki et al is a valuable contribution to the literature on risk stratification of patients according to bleeding risk at baseline. Recognising the characteristics that cluster in patients at HBR is an important first step in optimising the treatment of these patients. Ultimately, however, validation that these factors predict bleeding risk is only a stepping-stone. Generating evidence that specific interventions improve the overall outcomes of these patients will require dedicated, prospective evaluation in clinical trials enrolling patients meeting these criteria. As seen in the analysis, the critical obstacle to real progress is the frequent coincidence of both high bleeding risk and high ischaemic/thrombotic risk within the same patient. This is the challenge that personalised medicine aims to tackle.
Conflict of interest statement
P. Urban reports consulting honoraria from Biosensors, honoraria for CEC and DSMB activities from Edwards Lifesciences and Terumo, and is a shareholder of the Center for European Research in Cardiovascular medicine (CERC) and of MedAlliance. R. Colleran has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.