Abstract
Background: The Academic Research Consortium – High Bleeding Risk (ARC-HBR) initiative defined conditions associated with percutaneous coronary intervention (PCI)-related bleeding.
Aims: We sought to further explore these HBR conditions in the setting of transcatheter aortic valve replacement (TAVR).
Methods: Patients from the SCOPE 2 trial were stratified by their bleeding risk status based on the ARC-HBR definitions. Baseline and procedural characteristics, as well as key clinical outcomes including Bleeding Academic Research Consortium (BARC) 3-5 bleeding, were compared in ARC-HBR positive (HBR+) and ARC-HBR negative (HBR−) patients.
Results: Of 787 patients randomised in SCOPE 2 and included in this study, 633 were HBR+ (80.4%). Compared with HBR− patients, those HBR+ were older and more frequently presented with diabetes, a history of coronary artery disease, atrial fibrillation, prior cerebrovascular accident, and a Society of Thoracic Surgeons predicted risk of 30-day mortality (STS-PROM) (4.9±2.9% vs 3.3%±2.1%; p<0.0001). In addition, HBR+ patients were more frequently on oral anticoagulation therapy. At 1 year, HBR+ patients had higher rates of all-cause death (12.4% vs 4.3%, respectively, risk difference 8.09%; 95% confidence interval [CI]: 3.76-12.41; p=0.0002); the rates of BARC 3-5 type bleeding were relatively high but not statistically different compared with HBR− patients (7.7% vs 6.1%, risk difference 1.67%; 95% CI: –2.72 to 6.06; p=0.46). Subgroup analyses for bleeding events showed no significant interaction in terms of STS-PROM score, age, or medications.
Conclusions: The ARC-HBR criteria failed to isolate a subgroup of patients at higher bleeding risk in TAVR patients from a randomised trial. These findings have potential implications, especially for the selection of post-TAVR antithrombotic regimens based on individual bleeding-risk profiles. Specific HBR criteria should be defined for TAVR patients.
Introduction
Transcatheter aortic valve replacement (TAVR) has proven to be an effective and minimally invasive procedure in patients suffering from severe aortic stenosis12. TAVR is an alternative to surgical aortic valve replacement (SAVR) for a large proportion of patients with severe aortic stenosis, especially for those who are older or present with intermediate or high risk for surgery34. Since the late 2000’s, TAVR has led to constant improvement in clinical outcomes with the development of techniques and technological ameliorations along with increased operator experience. Although less prevalent compared with SAVR, the risk of major bleeding after TAVR has been estimated to be as high as approximately 6% and is associated with a three-fold increase in one-year mortality567.
Unlike percutaneous coronary intervention (PCI)-related bleeding risk conditions that have been recently defined by an Academic Research Consortium - High Bleeding Risk (ARC-HBR) consensus document8, those associated with major bleeding remain insufficiently explored after TAVR. The Valve Academic Research Consortium (VARC)-3 consensus provided an overview of risk assessment after TAVR that included the definitions of bleeding, but the conditions that increased this risk were not the subject of this initiative9. Some investigations based on local series or national registries aimed to evaluate the predictors and outcomes of major bleeding in TAVR patients710. Peripheral vascular disease, end-stage renal disease, and coagulopathy have been identified to increase the rates of major bleeding.
Against this background, we aimed to stratify patients from the SCOPE 2 trial11, a randomised comparison of the ACURATE neo (Boston Scientific) and the CoreValve Evolut (Medtronic) in 796 high-risk TAVR patients, according to their bleeding risk status based on the ARC-HBR definitions and to compare an array of key clinical outcomes including Bleeding Academic Research Consortium (BARC) 3-5 type bleeding complications in ARC-HBR positive (HBR+) and ARC-HBR negative patients (HBR–).
Methods
Study design
The SCOPE 2 trial design and rationale along with the principal 1-year results have been reported previously11. In brief, SCOPE 2 was a multicentre, randomised, parallel-design, non-inferiority, open-label trial carried out in 23 tertiary heart valve centres in 6 countries. It compared the safety and effectiveness of transfemoral TAVR using the ACURATE neo versus the CoreValve Evolut in patients with symptomatic severe aortic stenosis deemed to be at increased risk for mortality with SAVR, as assessed by the local Heart Teams. Inclusion criteria were the presence of symptomatic aortic stenosis with aortic annulus diameters covered by the sizes of the ACURATE neo and CoreValve Evolut valves. Left ventricular ejection fraction (<20%), pre-existing prosthetic valves in the aortic and/or mitral positions, bicuspid or unicuspid valves, severe mitral regurgitation, and/or peripheral anatomy inappropriate for transfemoral implant due to size, disease and degree of calcification or tortuosity of the aorta or ilio-femoral arteries represented the main criteria for exclusion. Complete details of the inclusion and exclusion criteria have been reported11. The trial was conducted according to the Declaration of Helsinki and Good Clinical Practice and was approved by the investigational review board or research ethics committee at each participating centre. All participants gave their informed consent. Procedural recommendations were per standard of care. The mode of anaesthesia was selected according to local standard practice. Pre- and post-dilatation were performed at the operator’s discretion, although predilatation is recommended by the manufacturer of the ACURATE neo valve. Access site closure was performed according to local practice. Minimally required laboratory analyses included haemoglobin, creatinine, and high-sensitivity troponin values. Dual antiplatelet therapy (preferably with aspirin and clopidogrel) was recommended for at least 3 months, followed by single antiplatelet therapy. In patients with an indication for oral anticoagulation or who had undergone recent coronary stent implantation, combination regimens and their duration were given at the discretion of the operator. Clinical follow-up was performed at 30 days and 1 year.
ARC-HBR criteria
Some of the ARC-HBR criteria needed to be modified or were not available because they were either not captured in the electronic data capture or represented criteria for exclusion in the trial, as summarised in Supplementary Table 1. This approach has been followed in other validation studies12131415. Major and minor ARC-HBR criteria applied in the current study are as follows: age ≥75 years (minor); oral anticoagulant or novel oral anticoagulant at discharge (major); estimated glomerular filtration rate (eGFR) <30 ml/min (major) and eGFR ≥30, <60 ml/min (minor); baseline haemoglobin <11 g/dL (major), and 11-12.9 g/dL for men and 11-11.9 g/dL for women (minor); thrombocytes at index procedure <100×109/L (major); non-steroidal anti-inflammatory drugs (NSAID) at discharge (minor); active cancer in the past 12 months (major); previous intracranial bleeding (major); any ischaemic stroke at any time not meeting the major criterion (minor). Patients were at HBR if at least one major criterion or two minor criteria were met10. An overall ARC-HBR score was calculated by adding 1 point for any major criterion and 0.5 for any minor criterion.
Outcomes
The primary endpoint was major or life-threatening bleeding (BARC type 3 or 5) at 12 months12. Other key clinical outcomes were the occurrence of death, stroke, hospitalisation for valve-related symptoms or worsened chronic heart failure, myocardial infarction, new permanent pacemaker implantation, and any arrhythmia responsible for haemodynamic disorders at 30 days and at 1 year. All patients were followed up to 12 months. The definitions of all endpoints have been reported previously10. An independent committee adjudicated events after a review of original source documents.
Statistical analysis
Discrete variables are expressed as percentages with frequencies and were compared by the χ2 or Fisher’s exact tests. Continuous variables are reported as mean±standard deviation and were compared by t-test if normally distributed or the Wilcoxon rank sum test for a non-parametric distribution. Event rates were based on Kaplan-Meier estimates in time-to-first-event analyses if mortality was part of the endpoint, or on cumulative incidence functions with the delta method for the estimation of the standard error, taking mortality as competing risk into account otherwise. Hazard ratios (HR) with 95% confidence intervals (CI) were determined by Cox regression analysis, and event rates were compared with the log-rank or Gray’s test, respectively. The day of the procedure was taken as day 0. For patients without a procedure, the day of randomisation was taken as day 0. Interaction testing was performed to determine whether the relative risk of BARC 3 or 5 type bleeding and mortality measures at 12 months varied by age, number of anti-thrombotic medications, Society of Thoracic Surgeons (STS) score, and presence of oral anticoagulants at the time of the procedure. A landmark analysis was performed for BARC 3 or 5 type bleeding from 30 days to 12 months of follow-up according to presence or absence of oral anticoagulants (OAC) at day 30. Wolbers’s adaptation of Harrell’s C-statistic for survival data was used to describe the prediction accuracy of HBR for major bleeds16. The C-index was 0.52. All analyses were performed in the intention-to-treat population. A two-sided p-value <0.05 was considered significant. All statistical analyses were performed with SAS software (Version 9.4, SAS/STAT version 15.1; SAS Institute Inc.).
Results
Between April 2017 and April 2019, 796 patients with symptomatic severe aortic stenosis were randomised to ACURATE neo (n=398) versus CoreValve Evolut (n=398). Due to missing values, nine patients could not be classified as high or low bleeding risk patients. Of the remaining 787 patients, 633 patients (80.4%) were stratified as high bleeding risk (HBR+) according to the ARC-HBR criteria.
Baseline characteristics
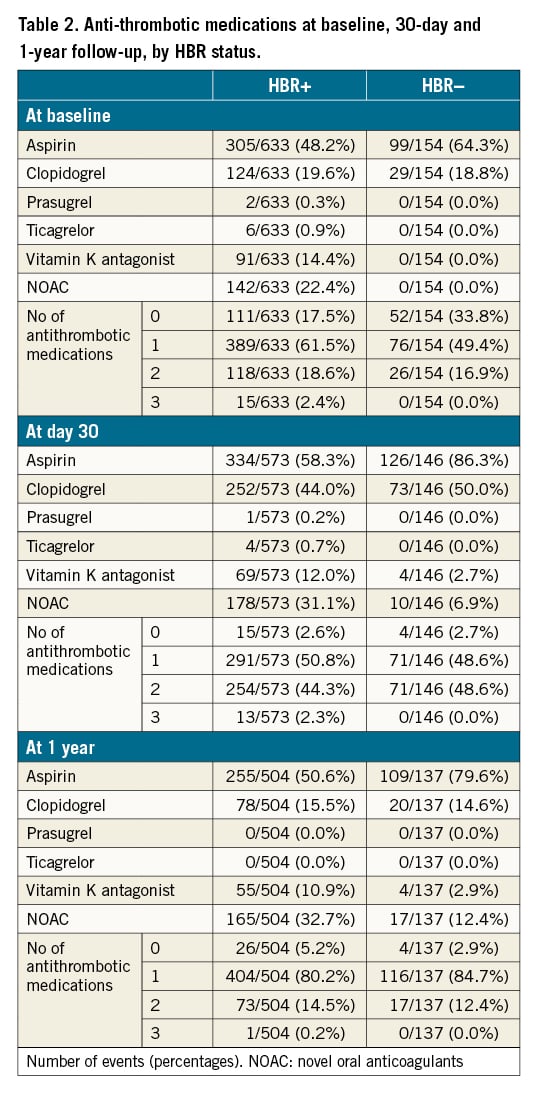
The main baseline characteristics of the study population are shown in Table 1. In brief, HBR+ patients were older and carried less favourable medical conditions. Compared to HBR– patients, they were more frequently diabetic and more frequently had a history of permanent pacemaker implantation, atrial fibrillation and/or a prior cerebrovascular accident. Their estimated STS predicted risk of mortality (STS-PROM) at 30 days was higher (4.9%±2.9% vs 3.3%±2.1%; p<0.0001). The distribution of medical conditions leading to HBR+ status is depicted in Figure 1. HBR+ patients were more frequently on OAC therapy (vitamin K antagonists [VKA]) or novel oral anticoagulants (NOAC), but the total number of antithrombotic medications was similar between groups (Table 2).
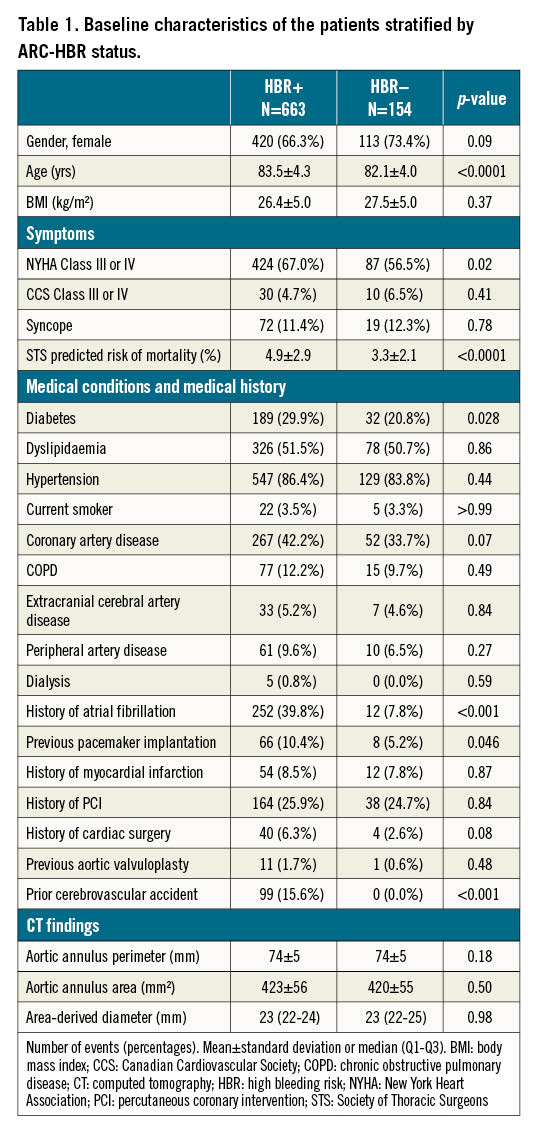
Figure 1. Distribution of medical conditions leading to HBR+ status. CVA: cardiovascular accident; eGFR: estimated glomerular filtration rate; F/M: female/male; HBR: high bleeding risk; NOAC: novel oral anticoagulants; TIA: transient ischaemic attack
Procedural characteristics and immediate outcome
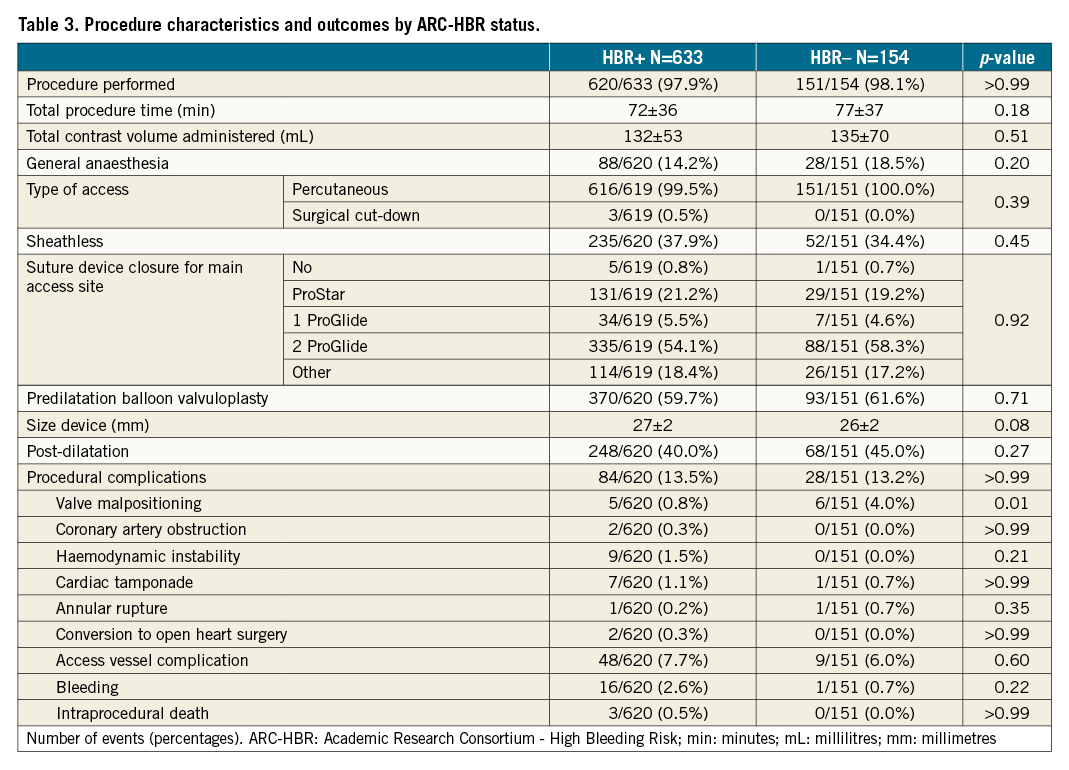
The procedural characteristics did not vary significantly between HBR+ and HBR– patients (Table 3). A suture-mediated closure device was used in >80% of the patients. The immediate complication rates were similar between the two groups (13.4% vs 13.3%; p>0.99).
Outcomes at 30 days and 12 months
Estimates of clinical outcomes at 30-day follow-up are shown in Table 4. BARC 3 or 5 type bleeding did not differ significantly between HBR+ and HBR– patients. HBR+ patients had higher rates of composite all-cause death and disabling stroke (4.8% vs 1.3%, risk difference 3.50%; 95% CI: 1.00-5.99; p=0.0061).
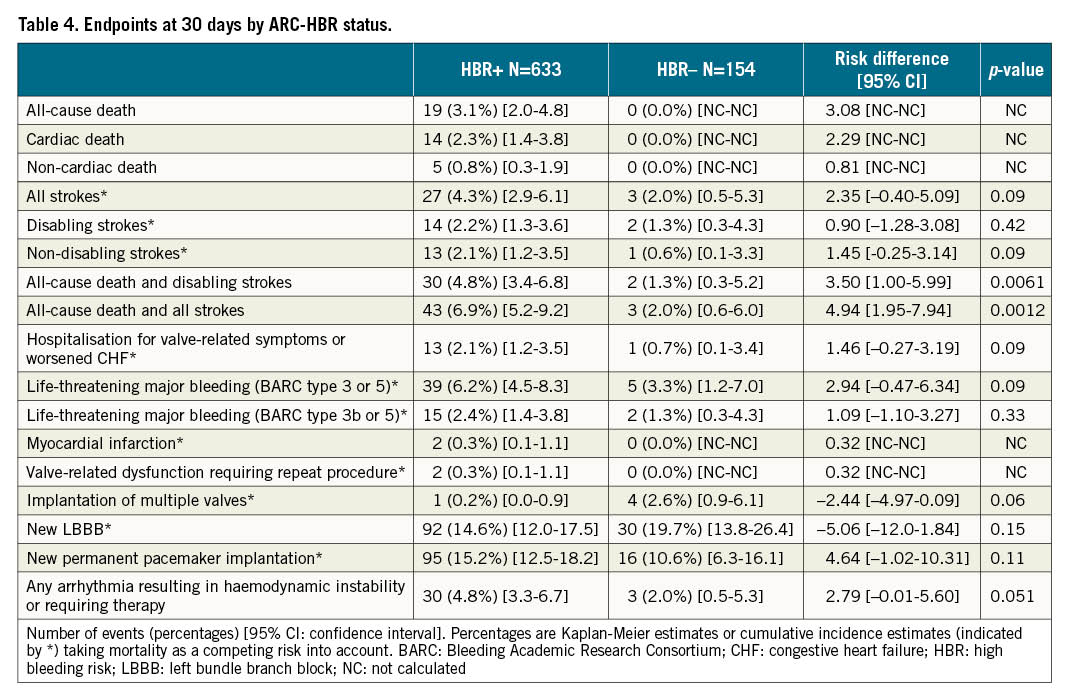
At 12 months, the rates of all-cause death were higher in HBR+ compared to HBR– patients (Table 5). However, the rates of BARC 3 or 5 type bleeding were not statistically different between groups (7.7% vs 6.1%, risk difference 1.67%; 95% CI: -2.72 to 6.06; p=0.46) (Central illustration, Figure 2). Compared to HBR– patients, HBR+ had more frequent access site-related BARC 3 or 5 type bleeding (3.0% vs. 0.7%, risk difference 2.36%; 95% CI: 0.49-4.23; p=0.0133). However, the rates of non-access site related BARC 3 or 5 type bleeding were not statistically different between groups (4.6% vs 4.8% for HBR+ and HBR– patients, respectively, risk difference –0.17%; 95% CI: –3.99 to –3.65; p=0.93).
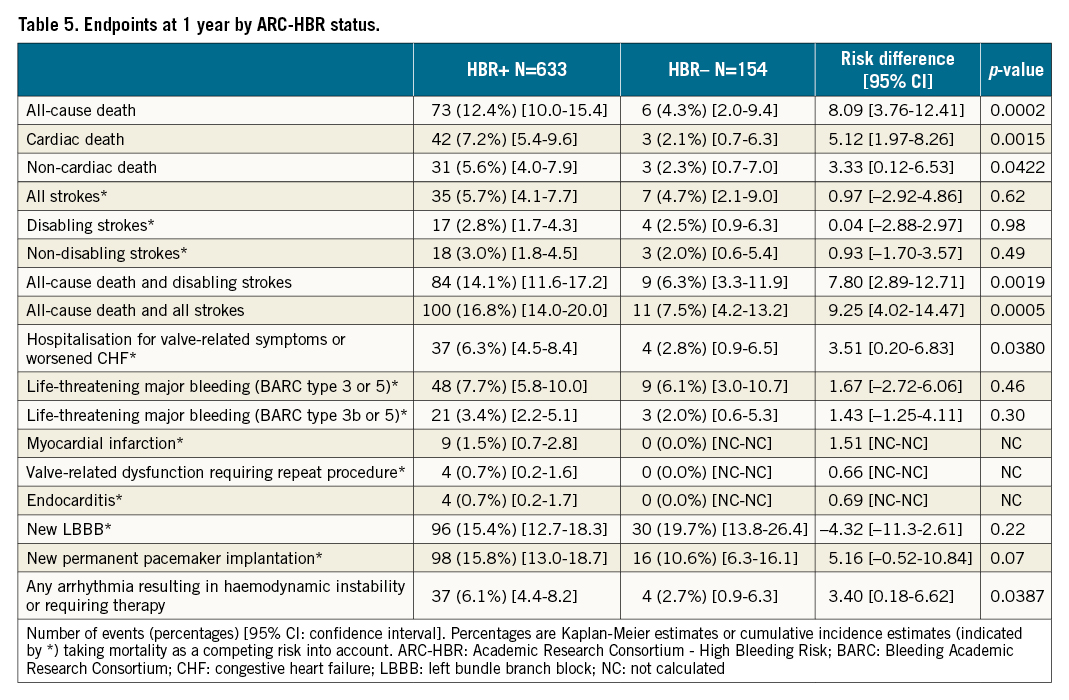
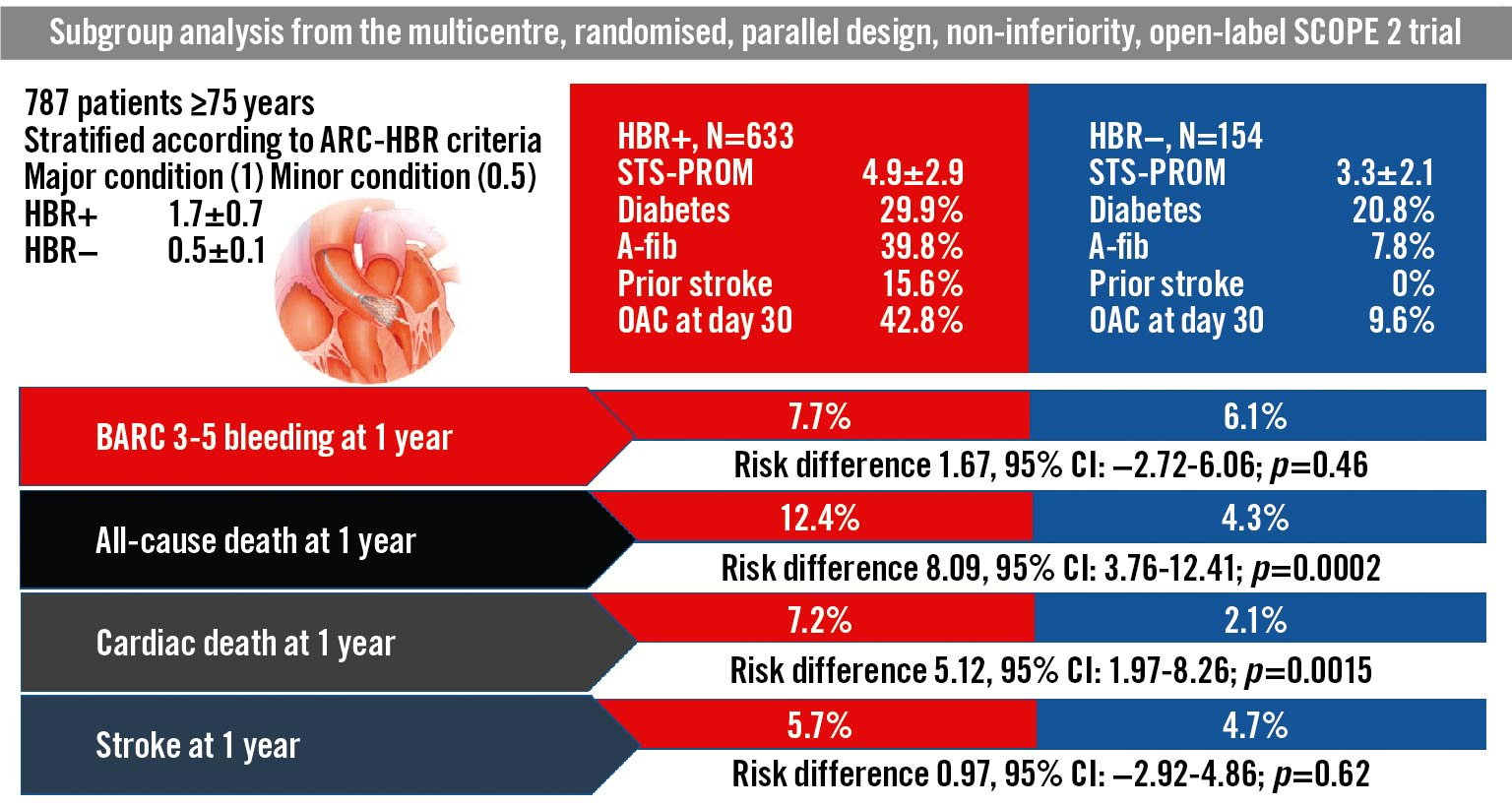
Central illustration. Subgroup analysis of the SCOPE 2 trial population according to ARC-HBR criteria. A-fib: atrial filbrillation; ARC: Academic Research Consortium; CI: confidence interval; HBR: high bleeding risk; OAC: oral anticoagulants; STS-PROM: Society of Thoracic Surgeons predicted risk of mortality
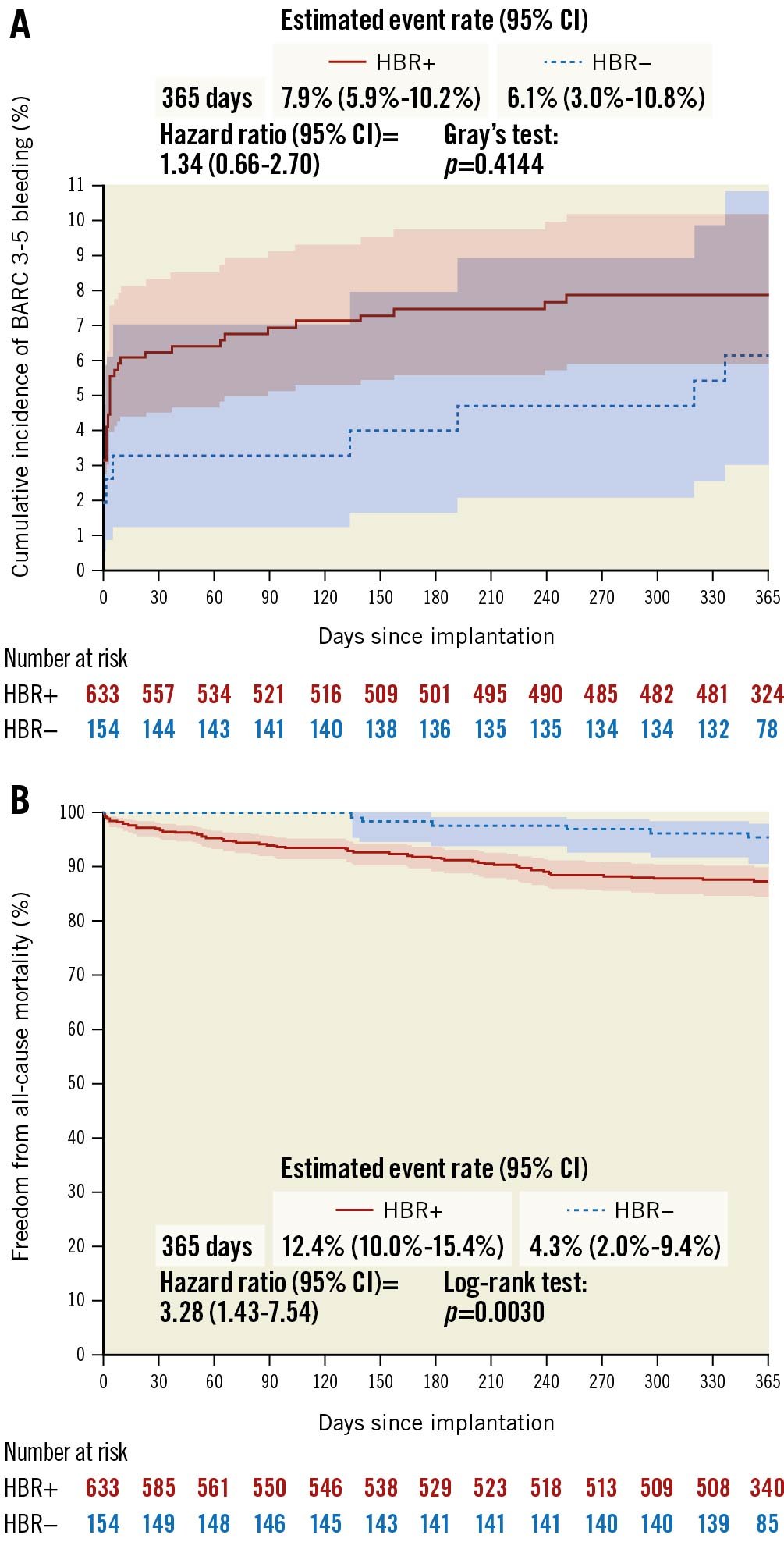
Figure 2. Cumulative incidence curves. A) BARC 3-5 bleeding and B) freedom from all-cause mortality in HBR+ and in HBR– patients. BARC: Bleeding Academic Research Consortium; CI: confidence interval; HBR: high bleeding risk
Subgroup analyses for bleeding events showed no significant interaction with patients’ age (Figure 3). In addition, a landmark analysis conducted according to the presence or absence of OAC at day 30 to determine the risk of BARC 3-5 bleeding up to one year showed no difference between HBR+ and HBR– patients (Table 6). In the subgroup analysis for mortality at 12 months (Figure 4) a significant difference for intermediate-risk (STS 5-8) and older patients (81-85 and >85 years) was observed.
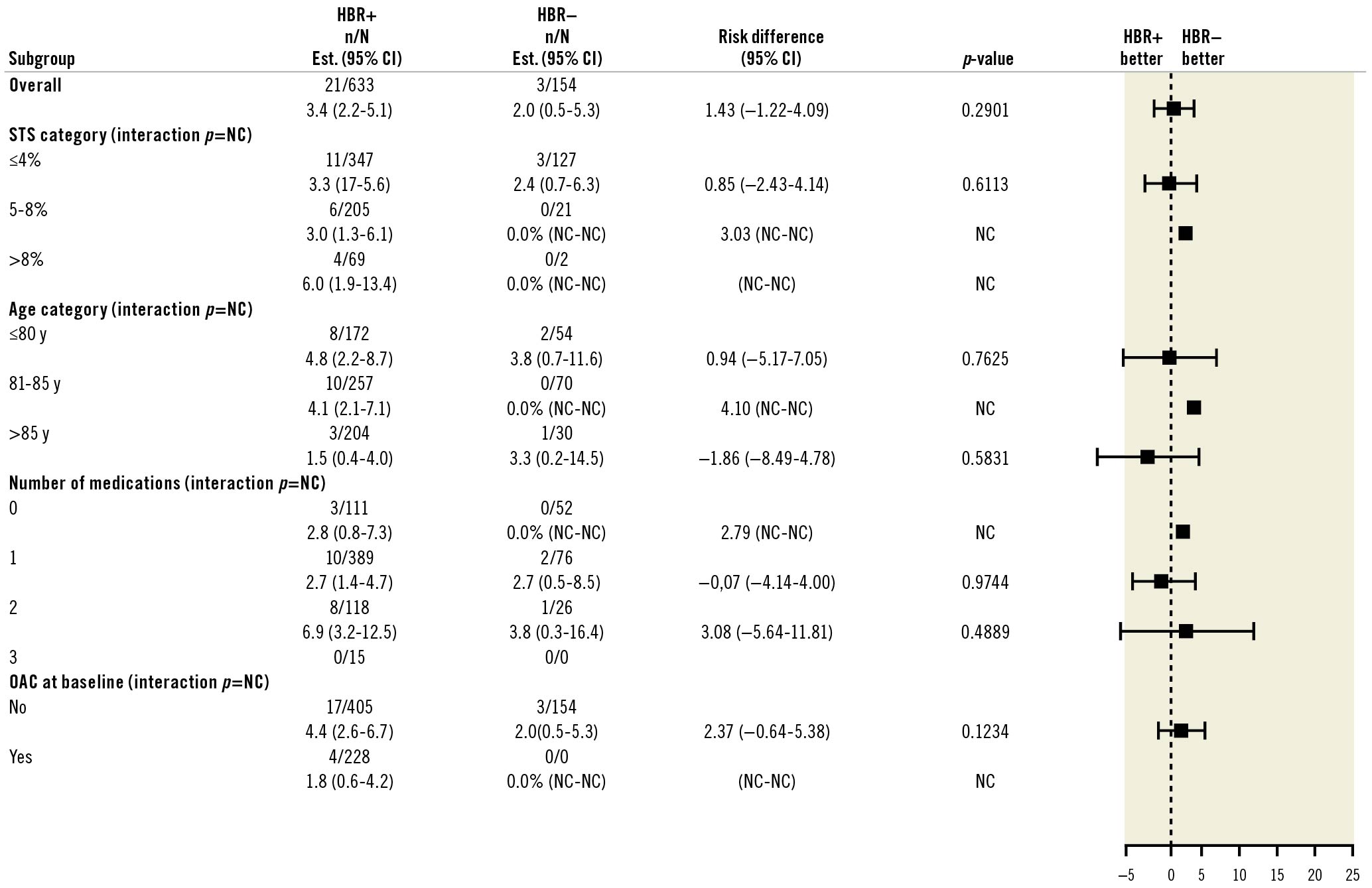
Figure 3. BARC 3-5 bleeding at one year by subgroups. CI: confidence interval; HBR: high bleeding risk; OAC: oral anticoagulants; NC: not calculated; STS: Society of Thoracic Surgeons
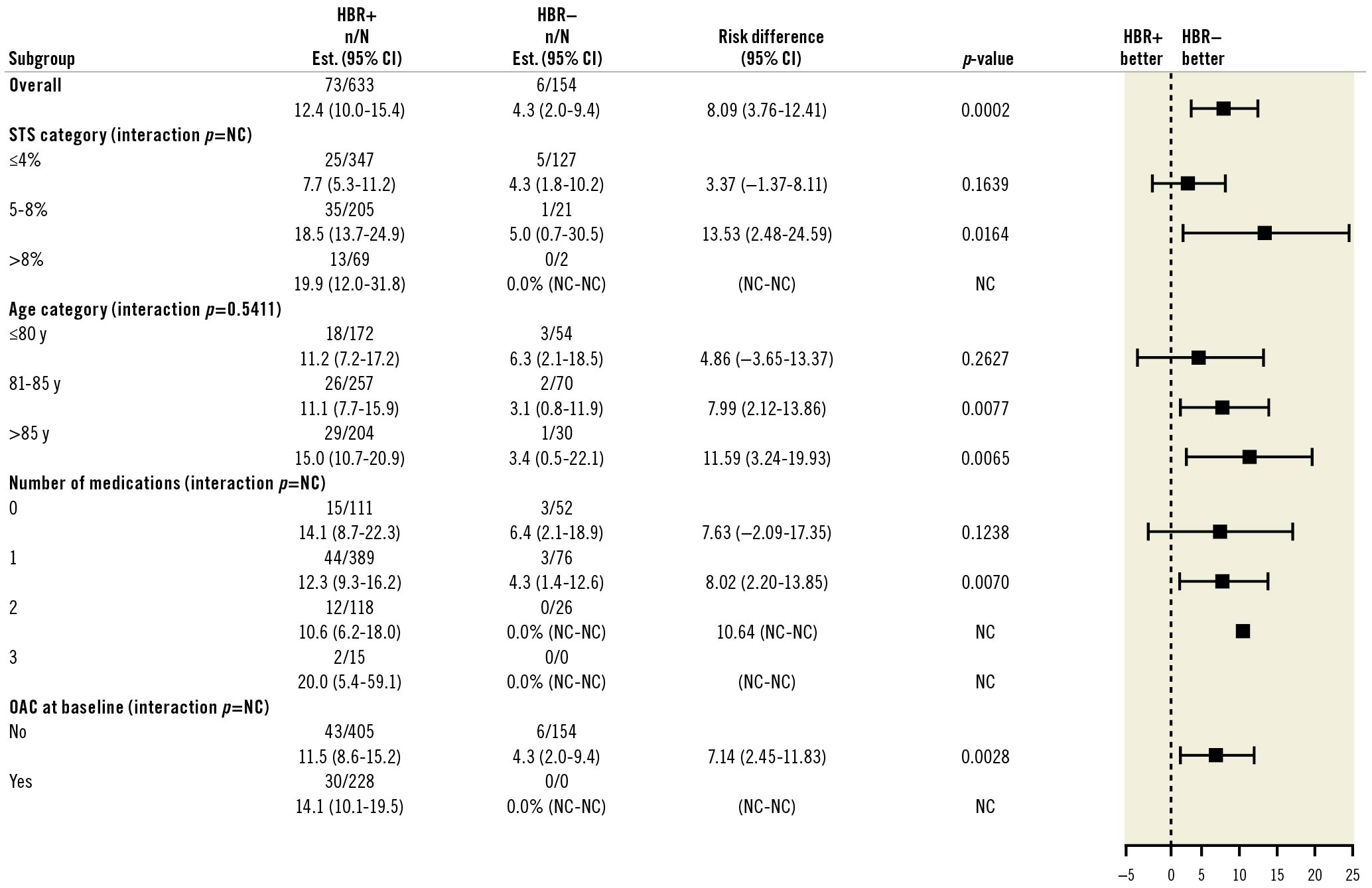
Figure 4. Mortality at one year by subgroups. CI: confidence interval; HBR: high bleeding risk; OAC: oral anticoagulants; NC: not calculated; STS: Society of Thoracic Surgeons
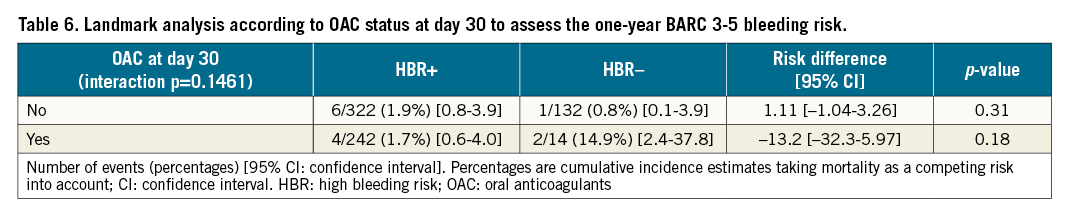
Patients with BARC 3 or 5 type bleeding had a 27.4% rate of death at 12 months, an almost three-fold increase (p<0.0052) compared to patients who did not experience BARC 3 or 5 type bleeding during follow-up (9.6%). Further, the rate of death at 12 months in HBR+ patients was 30.9% after BARC type 3 or 5 bleeding and 11.0% in the absence of severe bleeding events. In HBR– patients, it was 11.1% and 3.9%, respectively.
Discussion
The present analysis of the SCOPE 2 trial indicates that HBR+ patients, identified based on ARC-HBR standards for PCI, had higher rates of death and stroke at 12 months, but similar rates of BARC 3 or 5 type bleeding compared to HBR– patients. These findings suggest that ARC-HBR criteria defined in a PCI population are not relevant to discriminate an increased bleeding risk in TAVR patients. A contributing role of age is likely, as TAVR patients are on average 15 to 20 years older than patients undergoing PCI. An age cut-off of 75 years, as defined by the ARC-HBR definitions for PCI to indicate a minor criterion, is probably not adequate for TAVR patients. Indeed, the rate of major or life-threatening bleeding was 3.6% in PARTNER 3 (mean age 73.3±5.8 years) and 10.4% in PARTNER II, where patients were older (81.5±6.7 years)34. Factors that increase the risk of bleeding after TAVR include a high prevalence of chronic kidney disease, liver disease, peripheral vasculopathy, acquired thrombocytopenia, colonic malignancy, and acquired reversible von Willebrand factor deficiency1718192021. Since PCI is most frequently performed via the radial approach with smaller sheaths and catheters, the presence of peripheral vasculopathy is notably absent from ARC-HBR criteria while the size, presence of calcifications, and tortuosity of the iliac and femoral arteries are strongly associated with TAVR-related vascular complications and bleeding. More recently, Navarese et al have developed a 6-item algorithm comprising blood haemoglobin and serum iron concentrations, oral anticoagulation and dual antiplatelet therapy, common femoral artery diameter, and creatinine clearance that was able to identify patients at high risk of bleeding within 30 days after TAVR19. The role of anti-thrombotic medications is crucial in determining the rate of bleeding in these patients. However, we found that despite having more frequent use of OAC and being stratified according to bleeding-associated conditions, HBR+ patients had similar rates of severe bleeding compared to HBR–, which highlights the fact that important conditions reflecting the specificity of a TAVR population are missing in the ARC-HBR definition.
In the present study, approximately 7% of patients who underwent TAVR had BARC 3 or 5 type bleeding at one year, which is consistent with recent reports57. A previous study reported that life-threatening bleeding after TAVR, as defined by the VARC criteria, occurred in approximately 15 to 20% of TAVR procedures22. Our estimate of major bleeding complications in TAVR was 7.2%, which we believe to be a more contemporary estimate and could be further ameliorated using a single antithrombotic agent (e.g., a VKA or an NOAC in patients with atrial fibrillation, or aspirin in patients without) if PCI is not concurrently performed4.
Major bleeding or vascular complications have decreased as TAVR technology evolves into smaller device and sheath sizes2223, and bleeding complications are inconsistent in the early literature24. However, major bleeding is still associated with a three-fold increase in one-year mortality following TAVR567. Patients referred to TAVR are elderly, frail and at risk for both bleeding and ischaemic complications71725. Careful evaluation of risk and benefit is warranted to identify the optimal antithrombotic regimen, as major late bleeding complications are associated with an increased risk of mortality5. Our data support and strengthen a previous report26 suggesting that ARC-HBR criteria are not suitable for TAVR patients. Further studies and initiatives are warranted to determine the conditions qualifying HBR for the specific subset of patients requiring percutaneous valvular interventions.
Limitations
The present analysis has several limitations. The present report describes a post hoc subgroup analysis according to bleeding risk in the SCOPE 2 trial. Outcomes were assessed among ARC-HBR+ and ARC-HBR– patients. As per trial protocol, the current subgroup analysis should be considered exploratory and hypothesis-generating as the trial did not meet its primary objective and was not powered for either comparative assessment of bleedings nor for this patient stratification. In addition, some of the ARC-HBR criteria were not captured in the trial. Most uncaptured medical conditions (cirrhosis and all severe coagulation conditions, active cancer with bad prognosis) were criteria for exclusion in the SCOPE 2 trial, and some of them (brain malformations) are very rare in this TAVR population. In addition, ischaemic strokes and transient ischaemic attacks were classified as minor criteria in the absence of timing of the events. We assumed that this would not significantly impact the determination of the patients’ groups.
Conclusion
ARC-HBR criteria defined for PCI patients did not identify a subset of TAVR patients at increased rates of BARC 3 or 5 bleeding in the SCOPE 2 trial. Specific HBR criteria should be defined for TAVR patients. These findings are clinically relevant and have potential important implications, especially for the selection of post-TAVR antithrombotic regimens based on individual bleeding risk profiles.
Impact on daily practice
Severe bleeding after TAVR is associated with increased morbidity and mortality. As opposed to PCI-related risks of bleeding that have been defined by an ARC initiative, the conditions leading to severe bleeding after TAVR remain insufficiently explored. The present report confirms and strengthens the fact that conditions stratifying high bleeding risk criteria in a PCI population are not relevant to qualify high bleeding risk in the subset of valvular patients requiring percutaneous intervention. High bleeding risk criteria should be defined in a way that is specific to TAVR patients. A dedicated initiative is warranted.
Acknowledgments
The authors are grateful to Mrs. Dupic for her skillful assistance in reviewing the present manuscript.
Guest editor
This paper was guest edited by Franz-Josef Neumann, MD; Department of Cardiology and Angiology II, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Funding
The SCOPE 2 Trial is an investigator-initiated trial, funded by CERIC.
Conflict of interest statement
P. Garot is the medical Director and a shareholder of CERC. M-C. Morice is the CEO and a shareholder of CERC and a minor shareholder of Electroducer. C. Tamburino has received speaker fees from Medtronic. S. Bleiziffer has received speaker fees from Boston Scientific and Medtronic. M. Cunnington has received speaker and consultant fees from Medtronic. A. Wolf is a proctor for Boston Scientific, Edwards Lifesciences, and Medtronic. M. Barbanti has received consultant fees from Edwards Lifesciences. P. Pagnotta is a proctor for Boston Scientific, Cardia, and Gore Medical. F. Bedogni has received personal fees from Abbott Vascular, Boston Scientific, Medtronic, Meril Life Sciences, and Terumo. E. Van Belle has received personal fees from Philips/Volcano. M. Vasa-Nicotera is a proctor for Boston Scientific and Medtronic. A. Chieffo has received speaker fees from Abiomed, Abbott Vascular, Biosensors, Cardinal Health, and Magenta, and consultant fees from Abiomed, Abbott Vascular, Biosensors, Cardinal Health, and Magenta. K. Bogaerts has received consultant fees from the Cardiovascular European Research Center. C. Hengstenberg is a proctor for Boston Scientific; reports institutional grants from Boston Scientific; has received speaker fees from Boston Scientific; and has received consultant fees from Boston Scientific. D. Capodanno has received speaker fees from AstraZeneca, Biosensors, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Menarini, Pfizer, and Sanofi, and consultant fees from Abbott Vascular, Amgen, AstraZeneca, and Bayer. The other authors have no conflicts of interest to declare. The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; consultancy fees paid to his institution from Boehringer Ingelheim; and grant support from Bayer Healthcare, Boston Scientific, Biotronik, Edwards Lifesciences, GlaxoSmithKline, Medtronic, and Pfizer.
Supplementary data
To read the full content of this article, please download the PDF.

