Abstract
The identification and management of patients at high bleeding risk (HBR) undergoing transcatheter aortic valve implantation (TAVI) are of major importance, but the lack of standardised definitions is challenging for trial design, data interpretation, and clinical decision-making. The Valve Academic Research Consortium for High Bleeding Risk (VARC-HBR) is a collaboration among leading research organisations, regulatory authorities, and physician-scientists from Europe, the USA, and Asia, with a major focus on TAVI-related bleeding. VARC-HBR is an initiative of the CERC (Cardiovascular European Research Center), aiming to develop a consensus definition of TAVI patients at HBR, based on a systematic review of the available evidence, to provide consistency for future clinical trials, clinical decision-making, and regulatory review. This document represents the first pragmatic approach to a consistent definition of HBR evaluating the safety and effectiveness of procedures, devices and drug regimens for patients undergoing TAVI.
The Academic Research Consortium (ARC) is a collaborative forum of clinical, scientific, industry and regulatory stakeholders founded in 2006 to develop and disseminate consensus definitions for pivotal clinical trials of medical devices1. The Valve Academic Research Consortium (VARC) is an ARC derivative initiated in 2010 and devoted to the field of heart valve interventions. Recently, the VARC-3 collaboration provided an update on emerging clinical research issues in transcatheter aortic valve intervention (TAVI), including a clarification and redirection of endpoint definitions for future clinical trials2. VARC-3 also provided an overview of risk assessment after TAVI that included definitions of bleeding, but factors contributing to this risk were not sufficiently discussed. Standardised bleeding definitions for cardiovascular clinical trials were first introduced in 2011 by the Bleeding Academic Research Consortium (BARC)3. In addition to the BARC bleeding definitions, the ARC introduced a consensus document on factors defining high bleeding risk (HBR) in percutaneous coronary intervention (PCI) patients in 20194.
Although less prevalent compared with after surgical aortic valve replacement, major bleeding remains a frequent serious adverse event after TAVI that has been consistently and independently associated with an increased risk of early and late mortality56, longer periprocedural hospitalisation, higher healthcare costs, and worse quality of life at 1 year78. Compared to PCI, TAVI is more invasive and typically applied to elderly patients with more comorbidities and concomitant disease such as atrial fibrillation that increase bleeding risk. Medical conditions and risk factors for bleeding related to PCI were defined by the ARC-HBR initiative in 20194, with validation of such definitions in several contemporary groups of PCI patients91011, but they remain insufficiently explored in the context of TAVI. A recent post hoc analysis of the SCOPE II trial demonstrated that patients with and without HBR, according to the ARC-HBR criteria for PCI, experienced similar rates of BARC Type 3 or 5 bleeding12, and similar findings were observed in a large Japanese registry13. The fact that HBR definitions for TAVI and PCI might differ is not surprising: in transradial PCI, most major bleeding is not access site-related, whereas in TAVI, access site and procedural bleeding are far more prevalent. Known predictors of major bleeding in TAVI patients are conspicuously absent from the ARC-HBR definitions for PCI; however, predictors of PCI-related bleeding have not demonstrated an adverse bleeding risk after TAVI. HBR criteria should, therefore, be defined in a way that is specific to TAVI patients, especially for risk assessment prior to the selection of the TAVI procedural strategy and for the selection of post-TAVI antithrombotic regimens based on individualised bleeding risk profiles.
Given the complexity of bleeding pathophysiology, clear and standardised classifications and definitions of bleeding predictors are essential to reporting the outcomes of studies on heart valve diseases. Though there is extensive literature on the risk of bleeding after such interventions, there is still a lack of uniform definitions and reporting. To better characterise the profile of HBR patients with valve disease, VARC-HBR, a new ARC initiative, was designed, combining the contributions of experts from the VARC, BARC and ARC-HBR groups, including worldwide physicians, regulators, and industry representatives. A kick-off meeting of the consortium, organised by the Cardiovascular European Research Center (CERC), was held in Barcelona, Spain, in August 2022. The meeting included representatives of the U.S. Food and Drug Administration and the Japanese Pharmaceuticals and Medical Devices Agency, as well as observers from the pharmaceutical and medical device industries. Two additional meetings took place in February and April 2023 in order to reach consensus on the criteria for the VARC-HBR definition and their relative significance.
Landmark TAVI clinical trials have used heterogeneous bleeding definitions that may challenge the comparison of bleeding rates among studies. The rates of periprocedural and non-periprocedural bleeding in contemporary trials are provided in Supplementary Appendix 1 and Supplementary Table 161415161718192021222324. In addition, bleeding rates in contemporary trials in TAVI patients with or without a clinical indication of oral anticoagulation are available in Supplementary Appendix 2 and Supplementary Table 225262728293031. There are few data on factors that promote HBR in TAVI patients. Given the advanced age and comorbidities of these patients, HBR criteria as defined in the literature of PCI are frequently observed, but these criteria do not discriminate the risk of BARC Type 3 or 5 bleeding (Supplementary Appendix 3)123233. The risk of bleeding after TAVI varies over time. A recent report from 10 clinical studies indicated that the increase in mortality risk associated with major bleeding was observed both at 30-day and at 1-year follow-up34. An assessment for bleeding risk should be encouraged prior to the procedure, but also at 30 days after TAVI, as early severe bleeding may be associated with higher rates of 1-year bleeding events.
Defining the VARC-HBR criteria
The VARC-HBR task force agreed to define a “very high” bleeding risk as a BARC 3-5 bleeding risk at 1 year of ≥8%, a “high” bleeding risk as a BARC 3-5 risk of ≥4% and <8%, and a “moderate” bleeding risk as a BARC 3-5 risk of <4%.
The cutoff value of 8% for BARC 3-5 bleeding was based on the consensus of the participants, considering that 1-year major bleeding rates in recent TAVI trials, which largely excluded intermediate- and high-risk patients, were ≤8% and that, in TAVI trials enrolling all-comer patients, 1-year BARC Type 3-5 bleeding rates were in the range of 7% to 9% (e.g., 7.2% in SCOPE II, 8.0% in POPular TAVI, and 8.5% in ENVISAGE-TAVI AF where patients required long-term oral anticoagulation [OAC] for atrial fibrillation). These rates were mitigated when drug combinations and drug intensity were reduced. The bleeding rates observed in trials published between 2010 and 2016 largely exceeded 10%, but this was most likely related to the effects of operator learning curves, outdated technologies, and a highly selected population of very high-risk patients deemed not suitable for surgery, which represent a smaller proportion in current real-world practice.
VARC-HBR DEFINITION
Twenty-one clinical, anatomical, or procedural criteria were identified as major or minor by consensus, supported by published evidence (Table 1).
Patients are considered at very high risk of bleeding if at least two major or three minor criteria are met, at high risk if one major or two minor criteria are met, and at moderate risk if only one minor criterion is met. It is recognised that the coexistence of increasing numbers of risk factors for bleeding is associated with a stepwise increase in the risk of BARC 3-5 bleeding; therefore, as opposed to the ARC-HBR definition, the proposed consensus-based definition takes into account three levels of risk to better characterise the bleeding risk of patients undergoing TAVI. The risk stratification is proposed as a three-level scale, since sufficient data are not currently available to create a point-based score considering the relative weight of each criterion.
The proposed consensus-based definition considers the available evidence for patients at HBR undergoing TAVI and is pragmatic for application to clinical trials supporting clinical practice recommendations and regulatory review. The criteria establishing the definition are discussed below, categorised as patient-, anatomy-, or procedure-related factors (Figure 1). Associated BARC 3-5 bleeding rates at 1 year are provided when available. Since periprocedural and non-periprocedural bleeding risks have different risk factors, some predictors may only apply in the early term. Therefore, the participants decided to consider periprocedural and non-periprocedural bleeding events that impact both clinical outcomes and patient survival, with a clear identification of factors impacting mostly periprocedural or non-periprocedural bleeding risk, or both (Figure 1).
Table 1. Major and minor criteria for HBR at the time of TAVI.
| Major | Minor |
|---|---|
| Age >90 years | |
| BMI <20, cachexia (except for Asian patients) | |
| End-stage CKD (eGFR <30 mL/min), dialysis | |
| Liver cirrhosis with portal hypertension | |
| Active stage III and IV malignancies | |
| Haemoglobin <11 g/dL | |
| Severe baseline thrombocytopaenia (platelet count <50×109/L) | Moderate baseline thrombocytopaenia (platelet count ≥50 and <100×109/L) |
| Previous intracranial haemorrhage | |
| Moderate or severe ischaemic stroke (National Institutes of Health Stroke Scale score ≥5 on presentation) in the past 6 months | |
| Chronic bleeding diathesis, coagulopathy, Heyde’s syndrome | |
| Spontaneous (non-intracranial) bleeding requiring hospitalisation or transfusion in the past 6 months (or at any time if recurrent) | First spontaneous (non-intracranial) bleed requiring hospitalisation or transfusion >6 and <12 months before TAVI |
| Need for long-term OAC combined with at least one antiplatelet agent | Need for long-term OAC |
| Need for DAPT/concurrent PCI | |
| Non-deferrable major surgery | |
| Sheath-to-femoral artery ratio >1 | |
| Severe calcifications and tortuous iliac and/or femoral arteries (peripheral artery disease) | |
| Non-transfemoral access | |
| Immediate conversion to open heart surgery | |
| BMI: body mass index; CKD: chronic kidney disease; DAPT: dual antiplatelet therapy; eGFR: estimated glomerular filtration rate; OAC: oral anticoagulation; PCI: percutaneous coronary intervention; TAVI: transcatheter aortic valve implantation | |
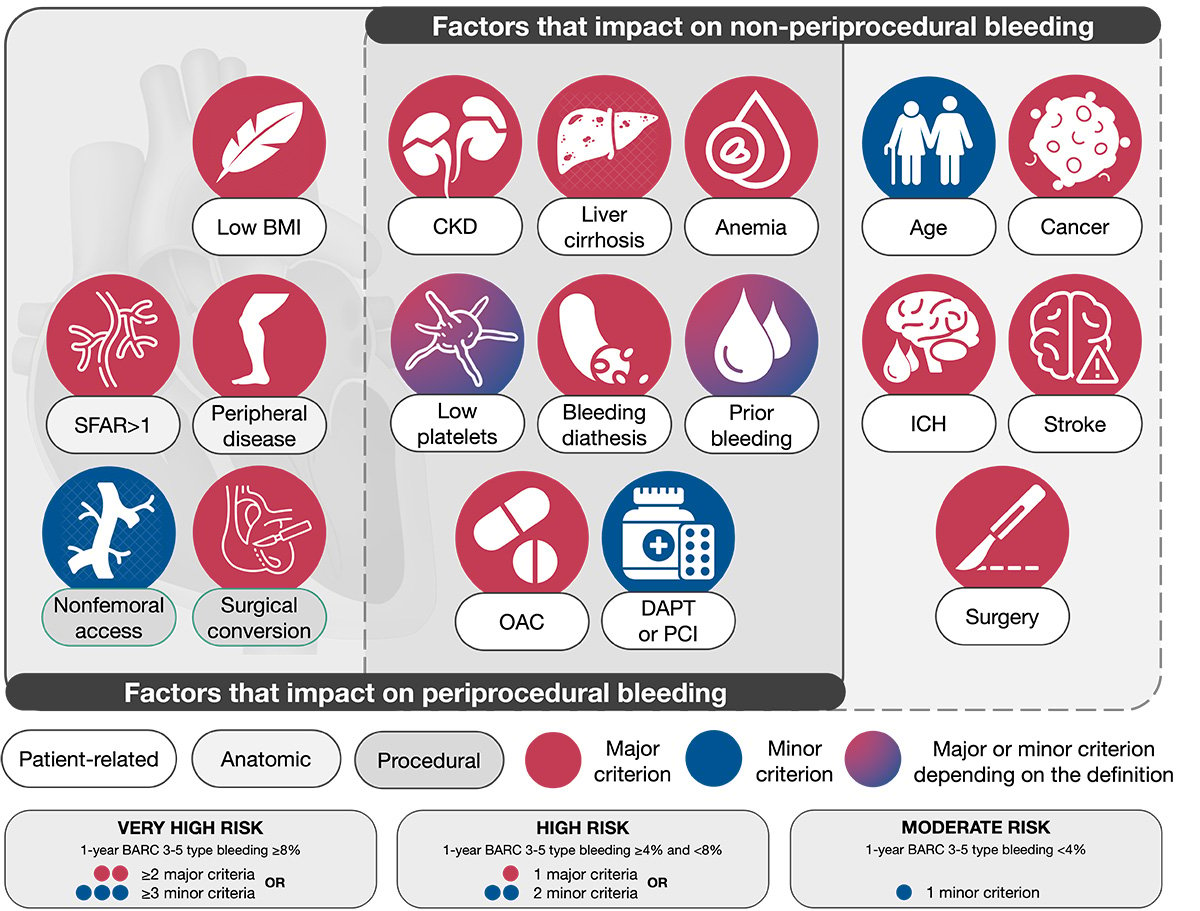
Figure 1. VARC-HBR criteria. BARC: Bleeding Academic Research Consortium; BMI: body mass index; CKD: chronic kidney
disease; DAPT: dual antiplatelet therapy; ICH: intracranial haemorrhage; OAC: oral anticoagulation; PCI: percutaneous
coronary intervention; SFAR: sheath-to-femoral artery ratio; VARC-HBR: Valve Academic Research Consortium for High
Bleeding Risk
PATIENT-RELATED FACTORS
AGE
Age ≥90 years is considered a minor VARC-HBR criterion (Table 1). Although the age and surgical risk of patients undergoing TAVI have decreased over the last decade, many patients are still over 8035. In PARTNER 3, the rate of BARC 3-5 bleeding was 3.6%, whereas it was 10.4% in PARTNER 2, where patients were 10 years older on average1719. Indeed, elderly patients undergoing TAVI have more comorbidities and coexisting risk factors compared to younger patients, including comorbidities that require long-term OAC and other conditions that require antiplatelet therapy17. Advanced age has generally persisted as an independent predictor of bleeding after adjustment for coexisting risk factors in current bleeding risk scores for patients undergoing TAVI. However, machine learning analysis did not identify age as a significant clinical variable for the 6-item PREDICT-TAVR score (available at: https://predict-tavr.shinyapps.io/dynnomapp/)33.
LOW BODY MASS INDEX
A body mass index (BMI) <20 is considered a major VARC-HBR criterion (Table 1). In order to reflect ethnical specificities, the group of participants decided to exclude Asian patients with a BMI <20 and no obvious frailty, who should not be considered at HBR. Vascular complications and BARC 3-5 bleeding are more frequent in low-BMI patients36373839. Although BMI ≤20 should be considered a frailty marker, clinical frailty is not routinely used in clinical practice due to the cumbersome nature of its assessment. An easily measurable surrogate, a product of BMI and serum albumin (i.e., modified BMI), has been shown to be associated with increased events, including severe bleeding events and mortality at 1 year38. Due to a lack of consensus on how frailty is best assessed and the paucity of data demonstrating a causative role in bleeding in patients undergoing TAVI, the use of a frailty score was not selected as a criterion. Nevertheless, the inclusion of advanced age and coexisting VARC-HBR criteria may account, to some degree, for frailty.
CHRONIC KIDNEY DISEASE
Severe or end-stage chronic kidney disease (CKD, defined as an estimated glomerular filtration rate [eGFR] <30 mL/min or patients requiring dialysis) is considered a major VARC-HBR criterion (Table 1). Severe CKD is an important factor for severe bleeding after TAVI40, and the bleeding risk increases incrementally with worsening CKD41424344. The reduced clearance of certain antithrombotic medications, related anaemia and inherent platelet dysfunction contribute to explaining the increased risk of CKD patients45. In the majority of studies, eGFR <30 mL/min in isolation places patients in the highest quartile for bleeding risk, whereas milder CKD is associated with a slightly to moderately increased bleeding risk42464748. In the PREDICT-TAVR score, creatinine clearance was one of the six strongest elements for identifying bleeding risk at 30 days after TAVI33.
LIVER CIRRHOSIS WITH PORTAL HYPERTENSION
The presence of cirrhosis with portal hypertension is considered a major VARC-HBR criterion (Table 1). The reported prevalence of cirrhosis in patients undergoing TAVI in the USA is 3%, and this increased 3-fold between 2003 and 201464950. The bleeding risk in patients with chronic liver disease may be related to impaired haemostasis (resulting from coagulation factor deficiency, thrombocytopaenia, platelet dysfunction, or increased fibrinolysis)51 or to oesophageal varices in the presence of portal hypertension. Data from the National Inpatient Sample (NIS) registry (n=34,752) from the years 2015 to 2018 showed liver disease to be an independent predictor of in-hospital major bleeding in patients undergoing TAVI (adjusted odds ratio [OR] 1.96, 95% confidence interval [CI]: 1.61-2.39)49. In addition, 1% of patients had in-hospital gastrointestinal bleeding after TAVI, and the presence of liver disease was associated with one of the highest odds of having a gastrointestinal bleed52. Among 2,401 patients who underwent TAVI within the randomised cohorts and continued access registries in the PARTNER trial and who survived to 30 days, severe liver disease was more frequently associated with the presence of late bleeding complications (5.0%) compared to an absence (2.4%) (p=0.09)6. In a recent meta-analysis, of 1,476 patients undergoing TAVI, 41% were affected by severe chronic liver disease. In this report, in-hospital major bleeding was 9.25%53. Although Child-Pugh and Mayo End-Stage Liver Disease criteria were used as exclusion criteria in some TAVI trials, such scores have been validated for predicting mortality in end-stage liver disease but not for predicting bleeding risk5455.
ACTIVE ADVANCED STAGE MALIGNANCY (STAGE III, IV)
Active malignancy (excluding non-melanoma skin cancer) is considered a major VARC-HBR criterion (Table 1). Active malignancy is defined as a diagnosis within the previous 12 months or ongoing active cancer treatment (surgery, radiotherapy, chemotherapy, or immunotherapy). Cancer that is considered to be in complete remission or that requires only maintenance therapy (e.g., tamoxifen for breast cancer) is not considered active. The reported prevalence of active cancer in patients undergoing TAVI was 5.6% in Japan and 4% in the USA and European Union; this increased 7-fold between 2008 and 20165658. Active cancer was associated with a higher rate of periprocedural major bleeding (8.6% vs 3.1% for patients with or without cancer; p<0.0001), despite a similar rate of major vascular complications. In contrast, most of the reports indicate that severe bleeding and associated rates of mortality are similar between cancer and non-cancer patients undergoing TAVI5859606162. The likely mechanisms for this finding are the impossibility to differentiate active malignancies from a history of malignancy and the variability in cancer type and stage63, as “bleeding cancers” accounted for very low rates in some cohorts (i.e., 3.2% of colorectal and 3.6% of urinary and bladder cancers in reports from the US National Readmission Database)57. Besides the presence of a potentially bleeding tumour, patients with advanced cancer are often anaemic, have thrombocytopaenia, and clotting diathesis, which place them at higher risk of both bleeding and thromboembolic complications63.
ANAEMIA
A haemoglobin (Hb) level <11 g/dL is considered a minor VARC-HBR criterion (Table 1). Preoperative anaemia defined by World Health Organization criteria (Hb <13 g/dL in men and <12 g/dL in women) is frequently encountered in patients undergoing TAVI, with a reported prevalence of 57% in the Rotterdam64 and 59% in the Hamburg registries65. Preoperative anaemia, a marker of chronic bleeding and impaired haemostasis, correlates with the risk of 30-day life-threatening bleeding in patients undergoing TAVI, and this risk increases incrementally from 5% to 8% in patients with mild to severe anaemia66. In addition, baseline anaemia is a predictor of impaired 3-year survival after TAVI, and its effect is surpassed by the adverse impact of periprocedural complications65. Despite their lower risk of mortality at 1 year compared to men, women more frequently have baseline anaemia and a higher risk of vascular/bleeding complications and mortality at 30 days67. Anaemia is an important part of the essential frailty toolset, a practical frailty scale that also includes physical weakness, cognitive impairment, and malnutrition. Compared to other frailty scores, it is a more robust predictor of adverse outcomes after TAVI or surgical aortic valve replacement68. This frailty scale is an independent predictor of bleeding and red cell transfusion early after TAVI68 and is also associated with a greater risk of late (up to 2 years) bleeding events, even after adjusting for age, sex, and other clinical covariates69. The detrimental effect of bleeding complications on survival after TAVI has been reported66570, and a postprocedural Hb drop resulting from bleeding, inflammation, and haemodilution has been associated with an increased incidence of acute kidney injury and 1-year mortality66.
THROMBOCYTOPAENIA
Severe baseline thrombocytopaenia (platelet count <50×109/L) is considered a major VARC-HBR criterion, while moderate baseline thrombocytopaenia (platelet count ≥50 and <100×109/L) is considered a minor VARC-HBR criterion (Table 1). Baseline thrombocytopaenia refers to thrombocytopaenia that is present before TAVI and is distinct from acquired thrombocytopaenia. The reported prevalence of baseline thromboÂcytopaenia in patients undergoing TAVI is 20% to 40%71727374, and the incidence of post-TAVI thrombocytopaenia ranges from 69% to 87%757677. Patients with thrombocytopaenia are underrepresented in randomised trials of TAVI, and those who are enrolled generally have no more than mild thrombocytopaenia, because a platelet count of <100×109/L is a common exclusion criterion. Thrombocytopaenia is a risk factor for both bleeding and ischaemic complications. In an analysis from the NIS database, 9.3% of patients had in-hospital major or life-threatening bleeding, and patients presenting with baseline thrombocytopaenia had higher adjusted rates of such bleeding events (OR 1.47, 95% CI: 1.36-1.59)52. In another report from the same database, in the propensity-matched cohort, patients with baseline thrombocytopaenia had higher rates of vascular complications and severe bleeding requiring blood transfusion, compared to those without thrombocytopaenia71. Notably, the bleeding risk appears to be proportional to the degree of thrombocytopaenia7478. An analysis of the Japanese multicentre OCEAN-TAVI registry including patients with baseline thrombocytopaenia undergoing TAVI (n=2,588) showed increased rates of BARC 3-5 bleeding at 3 years in patients with baseline mild thrombocytopaenia (7.3% vs 3.6%, adjusted hazard ratio [HR] 2.10, 95% CI: 1.36-2.21) and moderate/severe thrombocytopaenia (14.1% vs 3.6%, adjusted HR 2.66, 95% CI: 1.35-4.88; p=0.006), compared to those without thrombocytopaenia74.
PREVIOUS INTRACRANIAL HAEMORRHAGE
Previous intracranial haemorrhage (ICH) at any time is considered a major VARC-HBR criterion (Table 1). In the SWEDEHEART registry, approximately 1% of patients undergoing TAVI reported a prior ICH79. A medical history of previous ICH was present in 2.7% of the patients with postprocedural haemorrhagic stroke compared to 0.4% in patients without a haemorrhagic stroke. Patients with a prior ICH were also prone to more frequent non-cerebral major bleeding events (0.7% vs 0.4% for patients without a major bleeding event). In the METHYSTROKE study (ClinicalTrials.gov: NCT02972008), 26% of unselected patients requiring TAVI had preprocedural cerebral microbleeds. Within 3 days after TAVI, a total of 40% of the patients had a cerebral microbleed, with 23% of these patients exhibiting new haemorrhagic lesions. Associations with a new postprocedural cerebral microbleed included a history of previous bleeding (including gastrointestinal and cerebral bleeding; p=0.03), a longer procedure (p=0.02), and a higher total dose of heparin (p=0.03)80.
STROKE
A moderate or severe ischaemic stroke (National Institutes of Health Stroke Scale score ≥5 on presentation) within 6 months prior to TAVI is considered a major VARC-HBR criterion (Table 1). The reported prevalence of a history of stroke in patients undergoing TAVI in the USA from 2011 to 2017 is ~15%81. The authors reported that patients with prior stroke were more prone to have a post-TAVI recurrent stroke, but the rates of bleeding were not captured in this analysis. In the ENVISAGE-TAVI AF trial, 17% of patients had a history of cerebrovascular events, and the rate of post-TAVI life-threatening bleeding was ~2 per 100 person-years, which was higher than the 1.5 per 100 person-years observed in the GALILEO trial, where only 5.2% of the patients had a history of stroke2631.
CHRONIC BLEEDING DIATHESIS, COAGULOPATHY, HEYDE’S SYNDROME
The presence of a clinically significant chronic bleeding diathesis is considered a major VARC-HBR criterion (Table 1). Chronic bleeding diatheses include inherited or acquired conditions known to be associated with increased bleeding risk such as platelet dysfunction, von Willebrand factor (vWF) disease, inherited or acquired clotting factor deficiencies or acquired antibodies to clotting factors8283. For the purpose of the current VARC-HBR definition, thrombocytopaenia is discussed separately. Data on bleeding rates after TAVI in patients with bleeding diatheses are scarce, because such patients have generally been excluded from trials. The most important and reliable predictor of bleeding in patients with bleeding diatheses is a personal history of bleeding, which may be assessed with a bleeding questionnaire84. Aortic stenosis is associated with acquired type 2A vWF disease85. Heyde’s syndrome refers to the association between aortic valve stenosis and gastrointestinal bleeding from angiodysplasia85. Among patients undergoing TAVI, Godino et al reported a 1.7% rate of Heyde’s syndrome86. Spangenberg et al identified the presence of abnormal vWF multimers in 42% of TAVI candidates, with 18% and 3.2% of patients with bleeding episodes and proven Heyde’s syndrome, respectively87. High-molecular-weight multimers increase proportionally to the drop in the mean pressure gradient after TAVI and return to a normal value in most patients8788. Residual paravalvular leaks after TAVI, however, negatively influence the normalisation of vWF levels87. Godino et al showed that preprocedural bleeding disorders resolved in all patients after TAVI during 2 years of follow-up86. Hence, TAVI could have a positive impact on haemostasis and type 2A vWF disease. Moreover, all patients exhibiting bleeding complications during the TAVI procedure were diagnosed with subclinical vWF dysfunction88. These data suggest that vWF monitoring could be useful to predict procedural bleeding, but further studies are warranted. Ishii et al demonstrated significant decreases in total thrombogenic activity, measured by the Total Thrombus-formation Analysis System (Fujimori Kogyo Co.), and platelet count after TAVI despite the improvement in vWF multimers89. This phenomenon might explain the high risk of complications after TAVI.
PRIOR BLEEDING AND TRANSFUSION
Spontaneous (non-intracranial) bleeding requiring hospitalisation or transfusion in the past 6 months (or at any time if recurrent) is considered a major VARC-HBR criterion, and a first spontaneous (non-intracranial) bleed requiring hospitalisation or transfusion >6 and <12 months before PCI is considered a minor VARC-HBR criterion (Table 1). Information on the risk of subsequent bleeding in patients with a prior bleeding event who undergo TAVI is scarce. Long-term bleeding and related mortality after TAVI are unlikely to be related to the TAVI procedure itself but are more likely to be driven by underlying pre-existing comorbidities. Notably, bleeding beyond 30 days after the procedure was not carefully tracked nor uniformly defined in most TAVI trials. In an analysis from the NIS database from 2011 to 2018, 1% of patients undergoing TAVI had gastrointestinal bleeding, and they had higher mortality rates than those without gastrointestinal bleeding (12.1% vs 3.2%; p<0.01)63. The presence of peptic ulcer disease was associated with an 8-fold increased risk of bleeding. In patients presenting with peptic ulcer bleeding on aspirin monotherapy randomised to treatment with clopidogrel versus aspirin plus esomeprazole after confirmed ulcer healing, the respective 1-year rates of recurrent ulcer bleeding were 8.6% versus 0.7% (p=0.001)90. In another small randomised trial in patients with acute peptic ulcer bleeding on aspirin monotherapy, recurrent ulcer bleeding at 30 days occurred in 10.3% versus 5.4% of patients allocated to aspirin plus pantoprazole versus aspirin discontinuation (HR 1.9, 95% CI: 0.6-6.0; p=0.25)91. Data on the association between previous blood transfusion and subsequent bleeding risk in patients undergoing TAVI are scarce. The value of blood transfusions in managing life-threatening bleeding is undisputed. However, transfusions are used for indications with less clear benefit and are pre-emptively indicated in stable patients with low baseline Hb levels and multiple comorbidities such as chronic anaemia, older age, chronic kidney disease, malnutrition, changes in volume status, or subclinical bleeding diathesis. Zimarino et al examined the use of transfusions and outcomes in the TRITAVI registry including 2,587 patients undergoing transfemoral TAVI92. Transfusions were used in 16% of the patients. Transfusion use was associated with a 2-fold increase in 30-day mortality regardless of blood loss (Hb drop of <3 g/dL or ≥3 g/dL), or absolute Hb nadir after the procedure (<7.5 g/dL, 7.5-9.5 g/dL, or >9.5 g/dL)93. Yet, transfusions may just be a marker of multimorbidity and higher mortality risk.
NEED FOR LONG-TERM ORAL ANTICOAGULATION
The need for long-term OAC after TAVI is considered a minor VARC-HBR criterion, and the need for long-term OAC combined with an antiplatelet agent is considered a major VARC-HBR criterion (Table 1). The need for long-term OAC is more common in TAVI patients (approximately 40% of them) compared to PCI patients (<10%). The VARC-HBR participants decided to discriminate the bleeding risk associated with different intensity antithrombotic treatments. Randomised trials have consistently demonstrated the detrimental excess of bleeds in patients on oral anticoagulation with antiplatelet therapy versus anticoagulation alone (GALILEO, POPular cohort B and ENVISAGE-TAVI AF). The negative results of GALILEO highlight the challenge of antithrombotic therapy in TAVI patients who are elderly, potentially frail, or affected by multiple coexisting conditions associated with an increased risk of both bleeding and thromboembolic events26. In this context, the lack of benefit of rivaroxaban occurred despite evidence from an imaging substudy that the OAC strategy was associated with a lower incidence of subclinical valve thrombosis. The ATLANTIS study also demonstrated an unfavourable risk-benefit ratio for apixaban compared to standard antiplatelet therapy in patients without an indication for OAC27. In those with an indication for OAC, bleeding rates were higher compared to those without an indication for OAC, and the net clinical benefit of apixaban was not better than vitamin K antagonists (VKA)27. In ENVISAGE-TAVI AF, although edoxaban plus antiplatelet drugs was non-inferior for the primary efficacy outcome in approximately 60% of patients with a baseline indication to OAC, it was associated with higher rates of major bleeding, especially gastrointestinal, compared to VKA31.
DUAL ANTIPLATELET THERAPY/CONCURRENT PCI
The need for dual antiplatelet therapy (DAPT)/concurrent PCI (within 1 month of the TAVI procedure) is considered a minor VARC-HBR criterion (Table 1). Both access site and non-access site bleeding have potential adverse consequences94. The need for DAPT is no longer perceived as a major concern by most participants in the era of short (1 to 3 months) DAPT. Most randomised studies of antiplatelet regimens in TAVI included pre-TAVI loading with clopidogrel, and therefore did not specifically study the antiplatelet regimen after successful TAVI. This is the most common situation in many centres where PCI is performed upstream of TAVI, which implies a TAVI procedure under a DAPT regimen that may increase the complication rates. Several studies have shown the feasibility and safety of TAVI and concomitant PCI9596. In the POPular TAVI trial, aspirin alone was associated with a lower incidence of bleeding (HR 0.57, 95% CI: 0.42-0.77) and the composite of bleeding or thromboembolic events (HR 0.74, 95% CI: 0.57-0.95) at 1 year, compared to aspirin plus clopidogrel administered for 3 months25. Similarly, the ARTE Trial reported a higher rate of major or life-threatening bleeding events within 3 months following the TAVI procedure in patients receiving DAPT, compared to those allocated to aspirin alone (10.8% vs 3.6%, respectively; p=0.038)25. These two trials highlighted the potential role of DAPT on the consequences of bleeding after TAVI.
NON-DEFERRABLE MAJOR SURGERY
Planned non-deferrable major surgery in patients on DAPT after TAVI is considered a major VARC-HBR criterion (Table 1). After TAVI, up to 10% of patients undergo a non-cardiac surgery within 1 year, with a 30-day incidence of major or life-threatening bleeding of 11.3%97. TAVI and non-cardiac surgery have the potential to increase the risk of bleeding, especially while on antithrombotic medications. Available evidence on the safety of non-cardiac surgery after TAVI is scarce, often limited to small case series, and does not provide guidance on the timing of non-cardiac surgery or factors associated with procedural risk98. The increased risk of bleeding in a patient on antiplatelet therapy undergoing major surgery must be balanced against the potential risks of discontinuing DAPT in the potentially prothrombotic perioperative setting99. Important considerations include (i) the temporal relationship between TAVI and surgery, (ii) whether the surgery is deferrable, (iii) the anticipated bleeding risk specific to the surgical procedure, and (iv) the anticipated bleeding/thrombotic risk as defined by patient and procedural characteristics6399. Although clinical practice guidelines provide recommendations on the perioperative management of antithrombotic therapy, they do not define the perioperative bleeding risk of different surgical procedures100101. In summary, DAPT at the time of or shortly after surgery increases bleeding risk. Most elective surgery can be deferred beyond the proposed DAPT duration. For urgent or non-deferrable surgery, the risk of bleeding is much higher during the first month after TAVI compared with subsequent months.
ANATOMY-RELATED FACTORS
SHEATH-TO-FEMORAL ARTERY RATIO >1
A sheath-to-femoral artery ratio >1 is considered a major VARC-HBR criterion (Table 1). In early TAVI clinical trials with first-generation devices and 22 Fr and 18 Fr sheath calibre delivery systems, vascular complications were reported in nearly 15% of patients1418. The rates of both vascular complications and bleeding have decreased significantly since 201121. Several studies showed that larger sheath sizes are significantly associated with vascular complications (arterial rupture, perforation, or dissection) and bleeding events21102. The rates of vascular and bleeding complications seemed to decrease in the clinical trials of second- and third-generation systems that used smaller sheath sizes (18 Fr, 16 Fr, and 14 Fr)16103104. In an analysis of 34,893 patients included in the Transcatheter Valve Therapy (TVT) registry, a few important patient factors were associated with a greater risk for vascular complications and bleeding events on multivariable modelling. Female sex, a smaller common femoral artery diameter, peripheral artery disease (PAD), sheath size >17 Fr, and open surgical cutdown were independently associated with a significantly greater risk21. This is supported by studies examining vascular complications and bleeding in clinical trial populations and in observational studies105106107108. However, these complications were also associated with sheath oversizing, as measured by differences between the sheath’s outer diameter and minimal iliofemoral vessel diameters. The sheath-to-femoral artery ratio was first described in 2011102 and was shown, along with the presence of femoral calcifications and the experience of the operators, to predict the occurrence of VARC major vascular complications. Using the smallest possible delivery system may be associated with less risk for vascular complications. The sheath-to-artery ratio has not been validated in the context of expandable sheaths, whose diameters transiently exceed the nominal sheath size during delivery of the transcatheter heart valve (THV) (by up to 6 Fr).
Major and minor criteria for HBR at the time of TAVI.
SEVERELY CALCIFIED AND TORTUOUS ILIOFEMORAL ARTERIES (I.E., PERIPHERAL ARTERY DISEASE)
The association of severe arterial calcifications and tortuosity is considered a major VARC-HBR criterion (Table 1). The reported rates of major vascular complications in transfemoral TAVI range from 2% to 15% and are more frequent compared with surgery (3%)22, particularly in patients with PAD, owing to the large diameter of current devices and generalised atherosclerotic disease in patients with PAD. Indeed, multiple reports demonstrated that the prevalence of PAD in patients referred for TAVI ranged from 20% to 30% in the early 2010s and has decreased to between 10% and 20% in present practice26109110. Using the data of 2,167 patients, Yamawaki et al showed that the 2-year incidence of major or life-threatening bleeding tended to be higher in patients with PAD (p=0.06)111. In an analysis from the NIS database, 3,930/42,215 (9.3%) patients had PAD, and they had higher rates of severe bleeding requiring transfusion after transfemoral TAVI (14.2% vs 11.7%, OR 1.23, 95% CI: 1.12-1.35; p<0.001) compared to patients without PAD110. In an analysis of the Society of Thoracic Surgeons/American College of Cardiology TVT Registry, PAD was associated with increased rates of vascular (adjusted OR 1.33, 95% CI: 1.22-1.46; p<0.001) and in-hospital severe bleeding (adjusted OR 1.37, 95% CI: 1.25-1.50; p<0.001) complications21. Percutaneous closure devices are used to obtain femoral access haemostasis after large-bore arteriotomy112. Upfront combined strategies using an adjunctive non-suture-based device on top of a suture-based device may have the potential to reduce major vascular complications and major or life-threatening bleeding due to closure system failure, particularly in calcified femoral arteries113114.
PROCEDURE-RELATED FACTORS
NON-TRANSFEMORAL ROUTES
The use of non-transfemoral access is considered a minor VARC-HBR criterion (Table 1). Despite improvements in TAVI techniques and device profiles, 10% to 15% of patients are still denied transfemoral access because of unfavourable anatomy due to iliofemoral arteriopathy, tortuosity, severe calcifications, aortic aneurysm, or previous vascular surgery115116117. Several alternatives have been developed to address the limitations of transfemoral TAVI, including surgical approaches, such as transapical, transaortic and transÂcarotid, and more recently, percutaneous techniques, such as transaxillary/subclavian and transcaval. In a report from the TransCatheter Valve Treatment (TCVT) Sentinel Registry Investigators of the EURObservational Research Programme (EORP) of the European Society of Cardiology enrolling 4,571 patients from 2011 to 2012, non-femoral access was an independent predictor of 1-year mortality (HR 1.32, 95% CI: 1.04-1.66; p<0.0001 for non-femoral vs femoral access, and HR 1.64, 95% CI: 1.36-1.98; p<0.01 for apical vs femoral access)118119. In high-risk patients, non-femoral access was associated with higher rates of death up to 1 year, and this was also associated with a 2-fold increase in major and life-threatening bleeding119. In a large meta-analysis involving 49 studies and 828,528 TAVI patients, Patel et al showed that in-hospital life-threatening bleeding and non-femoral access were among the most frequent predictors for 30-day and 1-year readmission after TAVI117. More recently, a propensity-matched analysis from 3,226/21,611 patients demonstrated that non-transfemoral access for TAVI is associated with similar outcomes (including severe bleeding events) compared with transfemoral TAVI, except for 2-fold lower rates of major vascular complications and unplanned vascular repairs. Similar results were reported from a large German registry including 1,000 patients with similar rates of death and of major and life-threatening bleeding (6.1% vs 6.5%, and 10.9% vs 11.9% for apical vs femoral access, respectively)120.
CONVERSION TO OPEN HEART SURGERY
Conversion to open heart surgery during TAVI procedures is considered a major VARC-HBR criterion (Table 1). Approximately 0.2% to 2% of patients undergoing transfemoral TAVI may require immediate conversion to open heart surgery because of device embolisation, coronary obstruction, annulus rupture and ventricular perforation causing tamponade1617121122123124. Interestingly, they found that female sex was associated with more frequent conversion to surgery, which might be explained by a slightly higher risk of ventricular perforation and pericardial effusion due to smaller left ventricles. The 1-year survival of patients surviving the in-hospital period is 40% to 50%121.
The principles of trial design for VARC-HBR trials of TAVI including outcomes of interest, patient risk profile and age, medical history and endpoint definitions are provided in Supplementary Appendix 422631125126127128129130131. Several conditions were not identified as major or minor criteria by consensus and are discussed in Supplementary Appendix 5. The present article reflects the consensus views of the VARC-HBR group and does not necessarily represent the recommendations of the regulatory agencies or a regulatory requirement from the agencies (Supplementary Appendix 6).
Conclusions
The VARC-HBR group hereby provides a uniform definition of HBR for patients undergoing TAVI with the goal of guiding the assessment and reporting of current data, as well as the generation of new data. Through this effort, the VARC-HBR group aims at improving the efficiency and validity of investigations in the field of heart valve bleeding risk and ensures that interventions performed on patients undergoing transcatheter interventions are effective, safe and durable.
Supplementary data
To read the full content of this article, please download the PDF.

